Sperm protein 17 (Sp17) is thought to promote heparan sulfate–mediated cell-cell adhesion.1,2 Recent works indicated the presence of Sp17 transcripts in tumor cells.2-4 Since Sp17 mRNA is detected only within testis, Sp17 could be an ideal target for tumor vaccine. This notion is supported by our ability to generate Sp17-specific cytotoxic T-lymphocytes (CTLs) capable of lysing HLA-matched fresh tumor targets from peripheral blood mononuclear cells of healthy and cancer-bearing patients.5,6 A recent study using a Sp17 polyclonal rabbit antisera in flow cytometry suggested that Sp17 might be a ubiquitous protein expressed on both normal and malignant leukocytes.2 Further clarification into the normal tissue distribution of Sp17 protein is therefore of vital importance. To address this, we have used a recombinant Sp17 protein we produced5 to immunize a BALB/c mouse to produce Sp17 mouse monoclonal antibodies (MoAbs) from the immunized splenocytes. Successful generation of the Sp17 mouse MoAbs was first confirmed in Western blot analysis by demonstration of the ability of the antibody to bind the recombinant Sp17 protein and not a control recombinant protein produced using an identical route (Figure1A). The antibodies are mouse IgG1 in subclass.
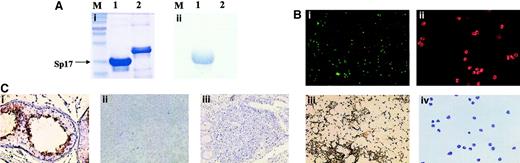
(A) Western blot analysis confirming the successful generation of Sp17 mouse monoclonal antibodies, showing binding of the antibodies to a recombinant Sp17 protein and not to a control recombinant protein. Both recombinant proteins were Escherichia coli–derived and contained a C-terminal 6-His tag. (i) Coomassie blue staining of a 10% sodium dodecyl sulfate–polyacrylamide gel. (ii) Western blot analysis demonstrating the binding of Sp17 mouse monoclonal antibodies to only recombinant Sp17 protein (M = molecular weight marker; lane 1 = recombinant Sp17 protein; lane 2 = control recombinant protein). (B) Sp17 protein was detected on normal spermatozoa (i and iii) but not on any of the peripheral blood leukocytes (ii and iv) by either immunofluorescence cytology (i and ii) or immunocytochemistry (iii and iv). (C) Immunohistochemistry showing the expression of Sp17 protein in normal testis (i). In contrast, Sp17 protein was not detected in either normal tonsils (ii) or Peyer patches (iii).
(A) Western blot analysis confirming the successful generation of Sp17 mouse monoclonal antibodies, showing binding of the antibodies to a recombinant Sp17 protein and not to a control recombinant protein. Both recombinant proteins were Escherichia coli–derived and contained a C-terminal 6-His tag. (i) Coomassie blue staining of a 10% sodium dodecyl sulfate–polyacrylamide gel. (ii) Western blot analysis demonstrating the binding of Sp17 mouse monoclonal antibodies to only recombinant Sp17 protein (M = molecular weight marker; lane 1 = recombinant Sp17 protein; lane 2 = control recombinant protein). (B) Sp17 protein was detected on normal spermatozoa (i and iii) but not on any of the peripheral blood leukocytes (ii and iv) by either immunofluorescence cytology (i and ii) or immunocytochemistry (iii and iv). (C) Immunohistochemistry showing the expression of Sp17 protein in normal testis (i). In contrast, Sp17 protein was not detected in either normal tonsils (ii) or Peyer patches (iii).
The antibodies were then applied in immunocytochemistry to determine Sp17 protein expression in normal peripheral blood cells. We found that, unlike in normal spermatozoa, Sp17 was not detected either on the cell surface or in the cytoplasm of normal peripheral blood lymphocytes, monocytes, or granulocytes (Figure 1B). This finding was further confirmed using immunofluorescence microscopy (Figure 1B) that showed the lack of Sp17 protein expression on normal peripheral blood leukocytes. These results therefore do not support the recent findings by Lacy and Sanderson,2 who used Sp17 rabbit polyclonal antisera in flow cytometry that suggested Sp17 might be a protein ubiquitously expressed in normal leukocytes. The expression of Sp17 within human Peyer patches was next investigated. A study showed the up-regulation of bovine Sp17 mRNA within the Peyer patches following bovine rotavirus infection of fetal lamb.7 This study raised the possibility that Sp17 may be expressed in small intestine, an anatomic site that was not included in the panel of normal tissue we previously studied.3 Interestingly, in contrast to the mRNA findings in fetal lamb, Sp17 protein was not detected within the human intestinal Peyer patches (Figure 1C). In addition, Sp17 protein also was not detected in any cells, including plasma cells, in the normal tonsils (Figure 1C). A control staining for Sp17 using normal testis showed strong expression of Sp17 (Figure 1C). Our results therefore indicate that Sp17 is not expressed on normal leukocytes.
There are many reasons that may account for the discrepancy between our results and those reported by Lacy and Sanderson on the expression of human Sp17 on normal leukocytes. It is well-recognized that rabbit polyclonal antisera are not always suitable for use in flow cytometry because of the difficulty in the selection of an appropriate control serum. Although preimmuned rabbit serum was used as a negative control by Lacy and Sanderson, the proportions of the different immunoglobulin isotypes within the reagents, and hence the “nonspecific stickiness” of the reagents, may not be identical. This, together with the extreme sensitivity of flow cytometry, may have resulted in positive signals observed in normal peripheral leukocytes reported by Lacy and Sanderson. Ideally, their results should have been confirmed by immunocytochemistry and not reverse transcriptase–polymerase chain reaction (RT-PCR), because RT-PCR is more suited as a rapid mRNA screening method rather than as a method for confirming protein expression.
Based on tumor specificity, Sp17 remains an ideal target for cancer immunotherapy. This is supported by our previous results indicating the restricted normal tissue expression of human Sp17 mRNA and the findings in the present study showing that Sp17 protein is not expressed in normal human leukocytes. The apparent lack of adverse reactions in healthy males who develop immunity against Sp17 following vasectomy further provides the data supporting the in vivo safety of targeting Sp17 in tumor vaccines. Our findings of the lack of expression of Sp17 in normal leukocytes are not surprising, since we previously found that Sp17-primed CTLs did not kill HLA-matched Epstein Barr-virus–transformed lymphoblastoid cells and peripheral blood monocyte–derived dendritic cells.
Expression of Sp17 on normal tissues
We agree with Grizzi et al that clarification into the normal tissue distribution of Sp17 protein is of vital importance, especially because of their interest in using Sp17 as a target for immunotherapy. We also agree that flow cytometry is extremely sensitive. We too were concerned about false-positive staining, and that is precisely why, in our published study,1-1 the appropriate controls were included and expression confirmed by reverse transcriptase–polymerase chain reaction of Sp17 on normal leukocytes. We found that levels of expression of Sp17 on normal leukocytes were relatively low but very similar to the low levels of expression seen on myeloma cells by flow cytometry as reported by both us and the authors of the above letter using the same rabbit antisera.1-1 1-2
In light of the comments by Grizzi et al, it is important to remember that the absence of detection of an antigen by immunofluorescence cytology or immunocytochemistry does not prove that the antigen is not present, only that it is not detected. Their inability to detect Sp17 on normal leukocytes could result from their monoclonal being of low affinity, or from masking of the antibody epitope at the cell surface. These possibilities are underscored by the relatively weak staining of sperm that they show in Figure 1B (panel i).
But in reference to using Sp17 as a target antigen in immunotherapy, the broader and more critical question concerns the expression of Sp17 on somatic cells. Important work regarding this issue was recently published by the laboratory of Dr Michael O'Rand, the discoverer of Sp17 and the leading authority on this molecule.1-3 They conclude, “Although Sp17 expression is highest in the testis, it is present in all of the mouse somatic tissues examined and is highly conserved throughout all mammalian species.” Although Grizzi et al are obviously aware of this work, they appear to ignore the conclusion by O'Rand's group that Sp17 is ubiquitously expressed.
Lastly, the intent of immunotherapy using Sp17 as a target antigen is to generate a massive cytotoxic T-lymphocyte response against cells that express Sp17. This intense immune response may have pathological consequences distinct from the apparent benign response of vasectomized men to circulating antibodies against Sp17. Thus, to use the finding that there is a lack of adverse reactions in healthy males who develop circulating antibodies against Sp17 as evidence supporting the in vivo safety of targeting Sp17 for immunotherapy is dubious at best.
References
Supported by the National Institute of Health/National Cancer Institute (RO1 CA 88434), the Cancer Treatment Research Foundation, and the Mary Kay Ash Charitable Foundation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal