Abstract
Population size is governed through cells reacting to a variety of intrinsic and extrinsic cues. Tumors, while liberated from many of the homeostatic constraints placed on physiologic counterparts, can nonetheless remain subject to both social and environmental control. Burkitt lymphoma cells faithful to the biopsy phenotype were used to model the reliance of the colony, if any, on an inbuilt population sensor. Below a normally suicidal threshold number of cells, low picomolar quantities of exogenous CD40 ligand (CD40L/CD154) were found to sustain the clone without the discernible shift in phenotype that accompanies high CD40L encounter. Although CD154 was undetectable in populous cultures, message was induced as numbers became limiting. Correspondingly, attempts to neutralize endogenous CD40L activity failed to perturb cells at optimal densities but resulted in their marked decline as the critical threshold was approached. These data reveal an auto-inducible survival mechanism seemingly regulated through the monitoring of population size, a process somewhat akin to that of “quorum sensing” among gram-negative bacteria in which diffusible molecules provide a means of communication to coordinate gene expression with population density. This process could be activated as cells discern depletions in their community or when deprived of signals otherwise furnished within an appropriate environmental niche.
Introduction
Although progressive liberation from homeostatic control is a feature of tumor development, malignant cells are rarely emancipated completely from the multitude of environmental constraints that are placed on normal counterparts.1 Indeed, before any progression toward metastasis, a dependency on specific tissue microenvironment is often observed in malignancy. For example, follicular lymphoma, one of the most common nonleukemic lymphoid tumors, develops within and is primarily limited to the lymphoid follicles that harbor their normal equivalents, the germinal center (GC) B cells.2-6
Another tumor bearing a GC B-cell phenotype is Burkitt lymphoma (BL), although here development is not constrained to conventional follicles.7-10 Nevertheless, BL presents with a classical “starry sky” histology reflecting the presence of large infiltrating macrophages that are there to scavenge tumor cells entering apoptosis.9,11,12 This feature of spontaneous apoptosis is a vestige of the tumor's GC origin in which the constitutive B cells need to be selected by antigen during the development of a memory response.6,13 14 Unlike their normal equivalents, however, the maintenance of the BL population proceeds independently of discernible antigen input and, despite their residual propensity for apoptosis, the balance is clearly in favor of survival.
BL cells established in culture and kept at early passage remain remarkably faithful to their GC origins.7,12,15,16 The L3055 cell line, derived from an Epstein-Barr virus (EBV)–negative case of sporadic BL, has been used extensively to model both GC B cells and BL itself.17-24 These “biopsylike” cultures display a low background of apoptosis that can be accelerated by a number of factors, including B-cell receptor engagement, exposure to transforming growth factor β, cold shock, and serum deprivation.17-20,24 With regards to survival factors, roles for both autocrine and paracrine factors have been described in the maintenance of BL populations as modeled by the L3055 cell line.24
As with normal GC B cells, the most potent signal for rescuing biopsylike BL cells from either spontaneous or induced apoptosis is provided by interaction of the CD40 receptor with exogenous CD40 ligand (CD154).12,14,19,21,22,24,25 For GC B cells in vivo, the selection of centrocytes by virtue of their ability to bind antigen with high affinity reflects subsequent presentation of processed antigenic peptides to helper T cells with a resulting display of CD40L and its engagement by B-cell CD406 14; thus, a need for antigen to effect survival could theoretically be bypassed by providing CD40L directly.
Despite its potent antiapoptotic activity, an argument for CD40L having a role in the maintenance of BL in vivo has not received favor.24 This argument is based on the observation that CD154-carrying membranes, or high concentrations of soluble trimeric CD40L (sCD40L), promote a dramatic shift of BL cells away from their in situ phenotype.22,24 Thus, in keeping with their GC origin,6,26 biopsylike BL cell lines are CD95−/CD54low/CD77hi and lack Bcl-2 protein. However, CD40 engagement drives Bcl-2 expression and encourages a CD95+/CD54hi/CD77lophenotype.22 24
Observations indicate that B cells, under some circumstances, may themselves produce low amounts of CD40L,27,28 a contention supported by the finding that the AT-hook transcription factor AKNA can coordinately regulate CD40 and CD154 expression.29Moreover, it has been demonstrated both in hybridoma lines and in normal subsets that quite diverse and contrary outcomes of CD40 ligation on human B cells can arise, depending on the degree of receptor occupancy involved with low-level occupancy being stimulatory and high-level occupancy being inhibitory.30 31 These considerations led us to reexamine a possible contribution of particularly small quantities of CD40L to the maintenance of BL populations. Our findings reported herein demonstrate that CD40L at low picomolar concentration is capable of suppressing the apoptosis that follows precipitous reductions in cell number and, importantly, that this occurs without any discernible change of phenotype. At a critical population threshold, the cells were seen to initiate autonomous CD154-dependent survival that allowed for their continuous propagation in the absence of exogenous signaling.
Materials and methods
Reagents
sCD40L was generated as an isoleucine zipper construct as described by Morris et al.32 The mouse immunoglobulin (Ig)G1 monoclonal antibody (mAb) interacting with the CD40-binding site of CD154 has been described previously33; the OKT3 isotype-matched CD3 mAb was purified from hybridoma supernatant that was generated in-house. Rat IgM anti-CD77 mAb (38.13)34and control ascitic fluid containing rat IgM anti-DNP was obtained from Serotec (Oxford, United Kingdom) as was fluorescein isothiocyanate (FITC)-conjugated mouse antirat IgM and FITC-conjugated CD80 (DAL-1) and CD40 (LOB7/6) mAbs. Mouse antihuman Bcl-2 oncoprotein conjugated to FITC was from DAKO (Ely, United Kingdom), phycoerythrin (PE)-conjugated mouse IgG3 antihuman Bcl-xL from Southern Biotechnology Associates (Birmingham, AL), rabbit (sc-526) polyclonal IgG anti-Bax (P-19) from Santa Cruz (Santa Cruz, CA), and FITC-conjugated goat F(ab)2 antirabbit (H+L) from Caltag Laboratories (Burlingame, CA). Unconjugated mouse monoclonal anti–Bcl-2 (clone 124) was obtained from DAKO as was FITC-labeled CD2 (MT910) and CD54 (6.5B5) mAbs. FITC-conjugated CD27 (M-T2 71) and CD95 (DX2) mAbs were from PharMingen (San Diego, CA). Moloney murine leukemia virus reverse transcriptase and DNaseI (amplification grade) were from Gibco (Paisley, United Kingdom). RNAzol reagent was from Biogenesis (Poole, United Kingdom).
Cell lines
The biopsylike, EBV-negative, BL cell line L3055 was maintained in early passage (< 60) as previously described.19 Mutu I and Elijah, both EBV genome–positive but retaining their biopsy phenotype, were also used.15 Cells were maintained in continuous culture with RPMI 1640 supplemented with 10% prescreened fetal calf serum (FCS; Bio-Whittaker, Wokingham, United Kingdom), 5000 IU/mL penicillin, 5 mg/mL streptomycin (Gibco/BRL, Paisley, United Kingdom), and 200 mM glutamine (Gibco/BRL). Stable bcl-2 transfectants of L3055 cells and subclones carrying the empty vector were obtained as described previously.20 21
Flow cytometric and immunoblotting analysis of Bcl-2 family protein expression
For flow cytometric analysis of intracellular proteins, 106 cells were taken from culture, washed in phosphate-buffered saline (PBS), and resuspended in 50 μL FCS, and they were incubated with 500 μL Ortho Permeafix for 45 minutes at room temperature. Cells were then washed 3 times in PBS and resuspended in 100 μL FCS, left for 15 minutes at room temperature before another wash in PBS, and then incubated with 50 μL primary antibody (diluted in 2% bovine serum albumin [BSA]/PBS) for 30 minutes on ice. Following 2 washes in PBS, cells were incubated with 50 μL secondary antibody (diluted in 2% BSA/PBS) for 30 minutes on ice then washed twice and resuspended in 400 μL 5% BSA/PBS and analyzed on an EPICS XL Flow cytometer (Beckman Coulter, Miami, FL). Primary antibodies were FITC-labeled mouse antihuman Bcl-2 (1/100), PE-labeled mouse antihuman Bcl-xL (1/100), and unconjugated rabbit polyclonal IgG anti-Bax (1/100). The secondary antibody for Bax staining was FITC-conjugated goat F(ab)2 antirabbit IgG (H+L) at 1/20.
For Western blotting, 107 cells were lysed in 0.2 mL of 1% Triton X-100 lysis buffer (25 mM Tris, pH 7.6, 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethyl sulfonyl fluoride, 1 μg/mL aprotinin, and 1 μg/mL leupeptin). Protein concentrations of each sample were determined. After adding 40 μL of 5× gel sample buffer (500 mM Tris [pH 6.8], 12% sodium dodecyl sulfate [SDS], 25% glycerol, 5 μM EDTA, 0.01% bromophenol blue) supplemented with 10% 2-mercaptoethanol, samples were boiled for 5 minutes. Samples were fractionated after loading equal protein concentrations onto 12% SDS-polyacrylamide gel electrophoresis. Proteins were transferred to Immobilon P membranes (Millipore, Watford, United Kingdom) as described previously,22 and specific proteins were detected by using mouse antihuman-Bcl-2 mAb (100; Santa Cruz). Immunoblots were visualized by using the enhanced chemiluminescence kit (Amersham, Amersham, United Kingdom) according to the manufacturer's instructions.
Flow cytometric analysis of surface antigen expression
Cells were harvested after culture under the conditions indicated in the text. Cells were washed once in PBS with 0.1% BSA and 0.01% sodium azide (FACS buffer) before direct or indirect immunofluorescence analysis was performed. Briefly, cells were labeled by a 30-minute incubation on ice with either directly conjugated mAb or, for CD77, unconjugated rat 38.13 second-stage mAb that was then detected by an additional second-stage incubation with FITC-conjugated antirat IgM antibody; in this case, rat IgM anti-DNP was used as the primary control. Cells labeled either directly or indirectly were washed once and resuspended in FACS buffer before flow cytometric analysis. Viable cells were gated according to forward (FSC) and side (SSC) light scatter settings. Data were processed and analyzed by using WinMDI software (Scripps Research Institute, La Jolla, CA.).
Measurement of DNA synthesis
DNA synthesis was determined by 3H-thymidine incorporation.19 After culture of cells at numbers indicated per 200 μL with additions as specified, wells were pulsed for the final 4 hours with 3H-thymidine (Amersham; 0.37 MBq [10 μCi]/mL in culture medium, 50 μL per well) and harvested on a Skatron cell harvester (Helis Bio, Newmarket, United Kingdom). All assays were performed in quadruplicate with replicates usually being within 10% and always within 15% of each other.
Cell viability and apoptosis assays
Cell viability was assessed by a standard trypan blue dye exclusion assay.35 Apoptosis was determined by staining treated cells with acridine orange and visualizing nuclear morphology exactly as described previously.35 Viable cells display a homogeneous chromatin staining pattern, whereas apoptotic cells show characteristically condensed and fragmented chromatin. Each determination was carried out by scoring 200 cells in duplicate.
Reverse transcription–polymerase chain reaction for CD154
Total RNA was isolated from cells by using RNAzol. After treatment with DNase to remove possible DNA contaminants, complementary DNA synthesis was performed in 3 μg total RNA as previously described.34 Primers for CD40L were as in Grammer et al27: 5′-AGAATCCTCAAATTGCGGC-3′ (CD40L-forward), 5′-TGTGGGTATTTGCAGCTCTG-3′ (CD40L-reverse), and 5′-ATGCCCAAGTCACCTTCTGT-3′ as a probe. Conditions for 50 cycles of polymerase chain reaction (PCR) amplification were 94°C for 45 seconds, 49°C for 60 seconds, and 72°C for 60 seconds. PCR products (40 μL) were analyzed on a 1.5% agarose gel before transfer onto nylon membrane (Hybond N+; Amersham) and hybridization. Glyceraldehyde phosphate dehydrogenase PCR primers and probe were as described by Eliopoulos et al,36 and CD40L-transfected mouse L cells were used as positive controls.
Results
Low-level sCD40L rescues BL cells dying in response to population depletion
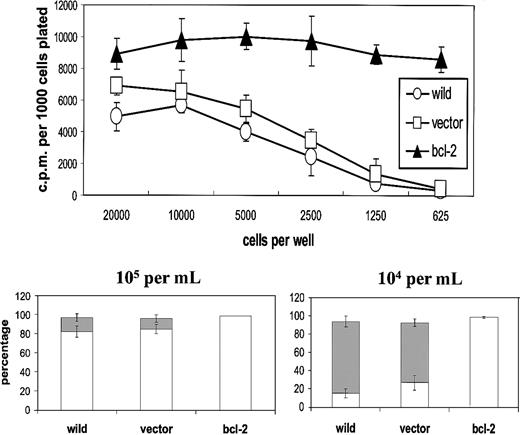
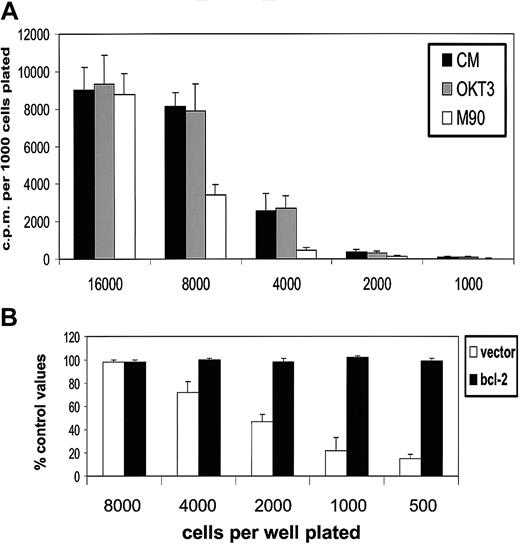
First, we established the influence of population depletion on the proliferation and survival of L3055 biopsylike BL cells cultured under conditions otherwise considered optimal for their growth. It can be seen from Figure 1 (upper panel) that below 5000 per microwell (2.5 × 104/mL), cells exhibited an increasingly marked reduction in their ability to proliferate as assessed by DNA synthesis. The same cells that had been transfected with bcl-2 showed no such diminution in their proliferation capacity: L3055 cells transfected with and selected for empty vector displayed the same sensitivity to reduction in number as wild-type parental cells. These findings indicate that the reduction in DNA synthesis observed as cell number decreased reflected a failure to survive rather than simple growth cycle arrest. Analysis of nuclear morphology in acridine orange–stained cells confirmed that at low seeding densities, cells were being lost because of their entering apoptosis (Figure 1, lower panel).
L3055 cells succumb to apoptosis at low plating densities.
(Upper panel) L3055 cells (wild-type; empty vector controls;bcl-2 transfected) were plated at numbers indicated in flat-bottom microwells containing 200 μL culture medium. DNA synthesis was assessed 5 days later by 3HTdr incorporation during a 4-hour pulse. Results represent the mean (± SE) of 3 separate experiments and are expressed as cpm per 1000 cells plated. (Lower panel) L3055 cells (wild-type; empty vector controls; bcl-2 transfected) were cultured at 105 or 104 per mL as indicated for 3 days and then stained with acridine orange to assess nuclear morphology and trypan blue to assess viability. Results are expressed as the mean percentage (± SE) apoptotic (filled bars) and viable cells (open bars) of 3 separate experiments.
L3055 cells succumb to apoptosis at low plating densities.
(Upper panel) L3055 cells (wild-type; empty vector controls;bcl-2 transfected) were plated at numbers indicated in flat-bottom microwells containing 200 μL culture medium. DNA synthesis was assessed 5 days later by 3HTdr incorporation during a 4-hour pulse. Results represent the mean (± SE) of 3 separate experiments and are expressed as cpm per 1000 cells plated. (Lower panel) L3055 cells (wild-type; empty vector controls; bcl-2 transfected) were cultured at 105 or 104 per mL as indicated for 3 days and then stained with acridine orange to assess nuclear morphology and trypan blue to assess viability. Results are expressed as the mean percentage (± SE) apoptotic (filled bars) and viable cells (open bars) of 3 separate experiments.
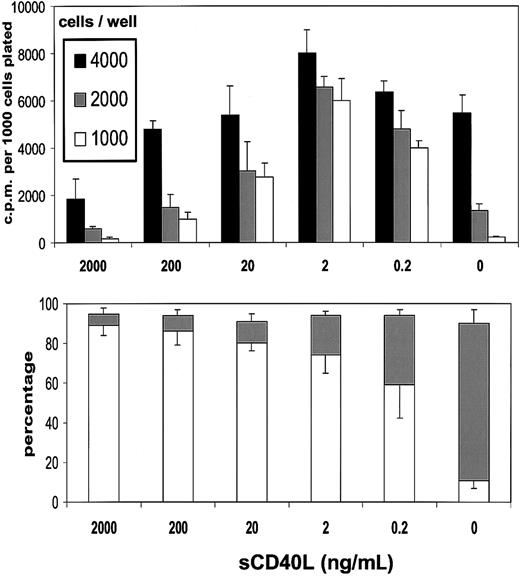
In the presence of sCD40L, there was a concentration-dependent restoration in the ability of L3055 cells to proliferate at below what was otherwise a critical density threshold (Figure2, upper panel). Optimal recovery was achieved with around 2 ng/mL sCD40L; however, even concentrations as low as 0.2 ng/mL provided a level of rescue from the proliferation drop equivalent to some 60% to 70% of this optimum. With increasing concentrations of sCD40L, cultures began to exhibit a falloff from the levels of DNA synthesis obtained with 2 ng/mL material, and at 2 μg/mL, as reported previously,22 an inhibition on control values of proliferation was noted. Results presented in Figure2 (lower panel) illustrate that, at all concentrations of sCD40L studied, there was substantial rescue of cells from the apoptosis to which they otherwise succumbed on cell dilution. An approximate 50% rescue was observed with 0.2 ng/mL sCD40L, a figure that progressively increased to more than 90% by 200 ng/mL and above of sCD40L. Thus, by keeping subthreshold density cells alive, low concentrations of sCD40L allowed the expression of their proliferation potential. Higher sCD40L concentrations, while also antiapoptotic, additionally promoted growth inhibition as described previously.22 BL cell behavior in vitro thus appears to mimic that of B-cell hybridomas and some normal B subsets in that low levels of CD40L can be stimulatory, whereas high levels are inhibitory, although, by contrast with other examples, the inhibitory actions of high-level CD40 engagement on BL cells do not reflect the induction of cell death.30 31
Low concentrations of sCD40L rescue L3055 cells from death arising from population depletion.
(Upper panel) L3055 cells (wild-type) were plated at the cell numbers indicated in flat-bottom microwells containing 200 μL culture medium in the presence of concentrations of sCD40L indicated. DNA synthesis was assessed 5 days later by 3HTdr incorporation during a 4-hour pulse. Results represent the mean (± SE) of 3 separate experiments and are expressed as cpm per 1000 cells plated. (Lower panel) L3055 cells (wild-type) were cultured at 104 per mL for 3 days with concentrations of sCD40L indicated and then stained with acridine orange to assess nuclear morphology and trypan blue to assess viability. Results are expressed as the mean percentage (with SE indicated) apoptotic (filled bars) and viable (open bars) cells of 3 separate experiments.
Low concentrations of sCD40L rescue L3055 cells from death arising from population depletion.
(Upper panel) L3055 cells (wild-type) were plated at the cell numbers indicated in flat-bottom microwells containing 200 μL culture medium in the presence of concentrations of sCD40L indicated. DNA synthesis was assessed 5 days later by 3HTdr incorporation during a 4-hour pulse. Results represent the mean (± SE) of 3 separate experiments and are expressed as cpm per 1000 cells plated. (Lower panel) L3055 cells (wild-type) were cultured at 104 per mL for 3 days with concentrations of sCD40L indicated and then stained with acridine orange to assess nuclear morphology and trypan blue to assess viability. Results are expressed as the mean percentage (with SE indicated) apoptotic (filled bars) and viable (open bars) cells of 3 separate experiments.
Although analyzed in less detail, 2 other biopsylike BL lines held in early passage were also found to respond to low quantities of sCD40L. Thus, when plated at 1000 cells per well for 5 days, Mutu I and Elijah showed a mean times-fold increase (with SE over 3 experiments) in DNA synthesis because of the presence of 0.2 ng/mL sCD40L of 2.8 (0.7) and 3.5 (1.2), respectively.
Low-level exogenous CD40L maintains low-density L3055 cultures without altering levels of Bcl-2, Bcl-xL, or Bax
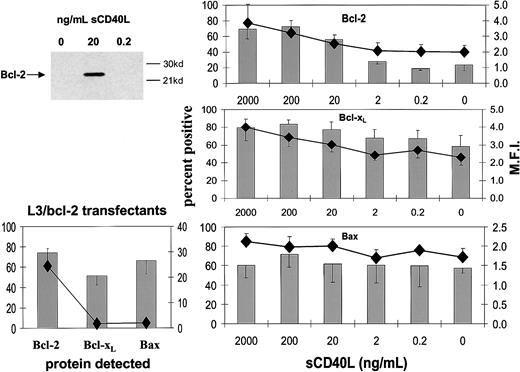
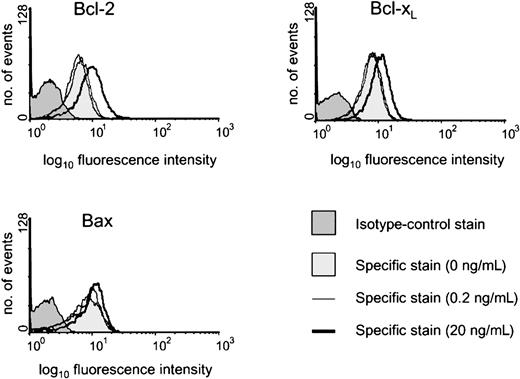
The constituent cells of BL share with their GC cell equivalents the unusual feature for B cells of being negative or low for Bcl-2 protein.20,25 It has been shown previously that both CD154-carrying membranes and high concentrations of sCD40L (1 μg/mL) drive biopsylike BL cells to express Bcl-2 at high levels.22,25 Here, we asked whether low sCD40L concentrations capable of promoting survival in subthreshold density cultures evoked a similar induction of Bcl-2 in L3055 cells. Western blotting in Figure 3 (upper left panel) shows that, although L3055 cells appearing negative for Bcl-2 protein in control cultures become clearly positive in response to 20 ng/mL sCD40L, they remain negative on culture with 0.2 ng/mL ligand. To examine this further and to assess possible changes in 2 otherbcl-2 family members potentially involved in CD40-dependent survival,37-39 we turned to FACS-based analysis of the proteins. As can be seen from Figure 3 (upper right panel), this approach confirmed the relative lack of Bcl-2 induction in L3055 cells with 0.2 ng/mL sCD40L, whereas concentrations of 20 ng/mL and above led to an increase in both the number of Bcl-2+ cells and in the level of Bcl-2 expression within the positive fractions. Bcl-xL, another CD40-regulated antiapoptotic protein, already present at low level in 50% to 60% of L3055 cells, showed slightly augmented expression with increasing sCD40L concentrations. Once more, however, no significant change in Bcl-xL levels was evident on culture of L3055 cells with 0.2 ng/mL sCD40L (Figure 3; middle right panel). The proapoptotic protein Bax, again present in some 50% to 60% of L3055 cells under basal conditions, neither increased nor decreased in response to any concentration of sCD40L offered to the cells (Figure 3; lower right panel). Analysis of the L3055 bcl-2 transfectants confirmed the expected high-level expression of Bcl-2 protein, whereas Bcl-xL and Bax were present in amounts essentially identical to those of wild-type cells (Figure 3; lower left panel). Figure 4illustrates representative histograms of Bcl-2 family member staining for cells left untreated or cultured with 0.2 or 20 ng/mL sCD40L. It can be seen that, consistent with Western blot data, the level of Bcl-2 in cells cultured with 0.2 ng/mL sCD40L remains unaltered by comparison with control cultures, whereas 20 ng/mL sCD40L promotes a significant shift in the level of intracellular Bcl-2 immunostaining; a similar pattern is observed for Bcl-xL, whereas the lack of change in Bax levels even with 20 ng/mL sCD40L is confirmed. The data reveal that with regards to the 3 Bcl-2 family members studied, low concentrations of sCD40L rescue L3055 cells from cell depletion-mediated apoptosis without altering the steady-state level of these proteins.
Influence of sCD40L at different concentrations on the expression of Bcl-2 family members.
L3055 (wild-type) cells at 2 × 105 per mL were cultured for 3 days with concentrations of sCD40L indicated. (Upper left panel) Lysates were prepared and equal protein loadings (50 μg) subjected to Western blotting for Bcl-2 protein on 12.5% gels as described in “Materials and methods”; molecular weight markers are indicated to the right. (Right panels) Cells were harvested and stained for the expression of Bcl-2 (upper), Bcl-xL (middle), Bax (lower) and analyzed by FACS as described in “Materials and methods.” Results are given as means (with SE) of 3 separate experiments with percentage of cells positive shown as histograms and the mean fluorescence intensities (MFI) of positively gated cells as symbols. (Lower left panel) The levels of Bcl-2, Bcl-xL, and Bax expression in L3055 cells transfected with bcl-2 as determined by FACS analysis.
Influence of sCD40L at different concentrations on the expression of Bcl-2 family members.
L3055 (wild-type) cells at 2 × 105 per mL were cultured for 3 days with concentrations of sCD40L indicated. (Upper left panel) Lysates were prepared and equal protein loadings (50 μg) subjected to Western blotting for Bcl-2 protein on 12.5% gels as described in “Materials and methods”; molecular weight markers are indicated to the right. (Right panels) Cells were harvested and stained for the expression of Bcl-2 (upper), Bcl-xL (middle), Bax (lower) and analyzed by FACS as described in “Materials and methods.” Results are given as means (with SE) of 3 separate experiments with percentage of cells positive shown as histograms and the mean fluorescence intensities (MFI) of positively gated cells as symbols. (Lower left panel) The levels of Bcl-2, Bcl-xL, and Bax expression in L3055 cells transfected with bcl-2 as determined by FACS analysis.
Representative histograms of intracellular staining for Bcl-2 family members.
L3055 (wild-type) cells at 2 × 105 per mL were cultured for 3 days with sCD40L at 0, 0.2, or 20 ng/mL before analyzing by FACS for intracellular levels of Bcl-2, Bcl-xL, or Bax, as indicated. Histograms shown are as follows: 0 ng/mL (lighter shaded histogram), 0.2 ng/mL (light line), 20 ng/mL (heavy line). Appropriate isotype-matched control staining is indicated by darker shaded histogram for each protein detected and was essentially identical for cells harvested from each of the culture conditions.
Representative histograms of intracellular staining for Bcl-2 family members.
L3055 (wild-type) cells at 2 × 105 per mL were cultured for 3 days with sCD40L at 0, 0.2, or 20 ng/mL before analyzing by FACS for intracellular levels of Bcl-2, Bcl-xL, or Bax, as indicated. Histograms shown are as follows: 0 ng/mL (lighter shaded histogram), 0.2 ng/mL (light line), 20 ng/mL (heavy line). Appropriate isotype-matched control staining is indicated by darker shaded histogram for each protein detected and was essentially identical for cells harvested from each of the culture conditions.
Low-level exogenous CD40L maintains low-density L3055 cultures without altering cell surface phenotype
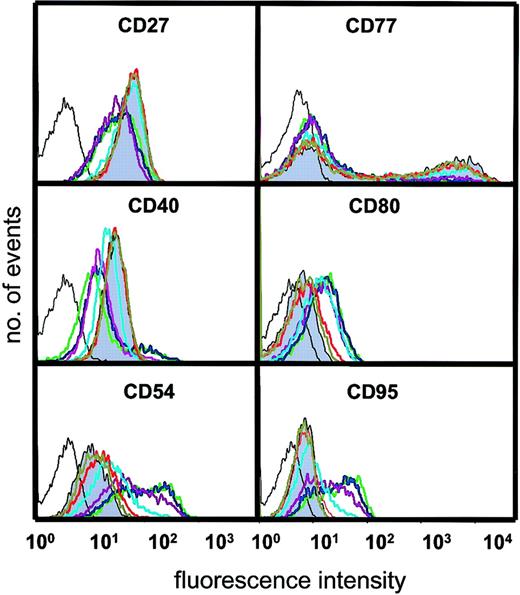
We next asked about the influence of varying amounts of sCD40L on the surface phenotype that characterizes BL cells and their normal GC equivalents. It is important to stress that these analyses were prompted not to demonstrate that sCD40L could promote phenotypic change (as this is already well documented14,22,24,27,31,35,40) but, rather, to ask whether the survival engendered by very low amounts of CD40L was accompanied by alteration in the biopsylike status of the BL population. Changes in the expression of CD27, CD40, CD54, CD77, CD80, and CD95 were observed when cells were cultured with certain concentrations of sCD40L. CD27, considered to be a marker of memory B cells but also found on a subset of GC B cells,41 was clearly expressed on L3055 cells but decreased on culture with sCD40L in a dose-dependent manner (Figure 5). Maximal down-regulation was observed at 50 ng/mL ligand; however, no change occurred with sCD40L at 10 ng/mL or below. Down-regulation of CD40 itself, however, was increasingly enhanced in cultures with sCD40L concentrations of 10 ng/mL and above. Conversely, the expression of the adhesion molecule CD54, the costimulatory CD80 molecule, and CD95/Fas by L3055 cells increased with a discernible sCD40L dose dependence at 10 ng/mL and above. As reported previously,35 the centroblast-restricted CD77 antigen (globotriaosylceramide) displays a bimodal distribution on L3055 cells, with levels decreasing as cells are cultured with sCD40L. In this study, CD77 expression was down-regulated only with sCD40L concentrations of 10 ng/mL and higher. Thus, to achieve a perceptible CD40-dependent shift in BL cells away from the biopsy phenotype, a concentration of 10 ng/mL sCD40L is needed. Concentrations below that level, although capable of promoting survival in subthreshold density cultures, fail to alter the population's phenotype. The observed increases in CD54, CD80, and CD95, promoted by higher amounts of sCD40L in the BL cells, is fully in keeping with previous studies on the CD40-dependent modulation of these surface molecules on a range of B-cell types (for review, see Grammer and Lipsky40) as is the down-regulation of CD40 and CD7722 35; to the best of our knowledge, the description of a CD40-dependent decrease in CD27 is entirely novel.
Influence of sCD40L at different concentrations on surface phenotype.
L3055 (wild-type) cells were cultured for 3 days as for Figure 3 with concentrations of sCD40L shown and stained and analyzed by FACS for surface antigens indicated as described in “Materials and methods.” Negative (control) staining is indicated by unshaded black line. Shaded histogram is basal level of antigen expression for cells cultured in medium alone; with concentrations of sCD40L in culture: olive green = 0.4 ng/mL; red = 2 ng/mL; light blue = 10 ng/mL; purple = 50 ng/mL; blue = 250 ng/mL; green = 1250 ng/mL. Histograms shown are representative of at least 5 similar experiments.
Influence of sCD40L at different concentrations on surface phenotype.
L3055 (wild-type) cells were cultured for 3 days as for Figure 3 with concentrations of sCD40L shown and stained and analyzed by FACS for surface antigens indicated as described in “Materials and methods.” Negative (control) staining is indicated by unshaded black line. Shaded histogram is basal level of antigen expression for cells cultured in medium alone; with concentrations of sCD40L in culture: olive green = 0.4 ng/mL; red = 2 ng/mL; light blue = 10 ng/mL; purple = 50 ng/mL; blue = 250 ng/mL; green = 1250 ng/mL. Histograms shown are representative of at least 5 similar experiments.
L3055 cells activate an endogenous CD154-dependent survival pathway in response to population depletion
The ability of picomolar quantities of exogenous CD40L to sustain L3055 cells below a critical population density without a discernible shift in phenotype raised the possibility that a low-level production of endogenous CD40L might contribute to their maintenance at higher cell numbers. This possibility was initially examined by attempting to block any endogenously produced CD40L with the neutralizing anti-CD154 mAb M90; isotype-matched OKT3 was used as control. Preliminary experiments failed to demonstrate a reduction in the growth of L3055 cells by this approach (data not detailed). As a variety of autocrine factors have been implicated in the maintenance of BL cell lines,24 we reasoned that any putative contribution from CD154 to autonomous growth might not be manifested until conditions became limiting. It can be seen from Figure6 (upper panel) that, as cells were plated at or below their threshold density for optimal growth, the presence of neutralizing M90 effected a profound reduction in their proliferation potential. No such inhibition was seen when M90 was included in cultures of L3055 cells overexpressing the bcl-2 gene and exhibiting optimal proliferation rates that continued at all cell concentrations studied; cells carrying vector alone showed the same inhibition of growth in response to M90 as the parental wild-type cultures (Figure 6, lower panel).
M90 neutralizing antibody to CD40L inhibits proliferation of L3055 cells at limiting population densities.
(A) L3055 cells (wild-type) were plated at numbers indicated in flat-bottom microwells containing 200 μL culture medium (CM) with OKT3 or M90 added at 5 μg/mL as indicated. DNA synthesis was assessed 5 days later by 3HTdr incorporation during a 4-hour pulse. Results represent the mean (with SE) of 3 separate experiments and are expressed as cpm per 1000 cells plated. (B) L3055 cells (empty vector controls or bcl-2 transfected as indicated) were plated and cultured as for upper panel. Here, the results are given as percentage of control values as a result of M90 addition and expressed as mean (with SE) of 3 separate experiments.
M90 neutralizing antibody to CD40L inhibits proliferation of L3055 cells at limiting population densities.
(A) L3055 cells (wild-type) were plated at numbers indicated in flat-bottom microwells containing 200 μL culture medium (CM) with OKT3 or M90 added at 5 μg/mL as indicated. DNA synthesis was assessed 5 days later by 3HTdr incorporation during a 4-hour pulse. Results represent the mean (with SE) of 3 separate experiments and are expressed as cpm per 1000 cells plated. (B) L3055 cells (empty vector controls or bcl-2 transfected as indicated) were plated and cultured as for upper panel. Here, the results are given as percentage of control values as a result of M90 addition and expressed as mean (with SE) of 3 separate experiments.
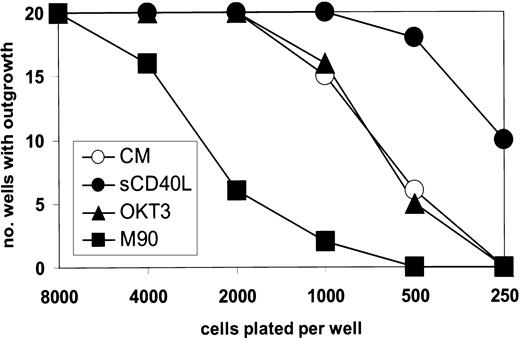
These changes monitored by DNA synthesis were mirrored by results obtained from experiments in which the outgrowth of cells plated at limiting number was studied. Thus, from Figure7 it can be seen that at low-density plating, the M90 antibody was substantially inhibitory to successful outgrowth, as compared with wells containing no or control antibody. Moreover, the presence of 0.2 ng/mL sCD40L increased the culture outgrowth when cells were plated below their critical threshold density. For L3055 cells carrying bcl-2, all the wells established successful growth irrespective of cell number or antibody addition (data not detailed).
M90 inhibits the outgrowth of L3055 cells plated at limiting population densities.
L3055 cells (wild-type) were plated in 20 flat-bottom microwells containing 200 μL culture medium for each number indicated. After 7 days, the wells were scored for those with successful outgrowth. M90 and OKT3 were present at 5 μg/mL and sCD40L at 0.2 ng/mL as indicated
M90 inhibits the outgrowth of L3055 cells plated at limiting population densities.
L3055 cells (wild-type) were plated in 20 flat-bottom microwells containing 200 μL culture medium for each number indicated. After 7 days, the wells were scored for those with successful outgrowth. M90 and OKT3 were present at 5 μg/mL and sCD40L at 0.2 ng/mL as indicated
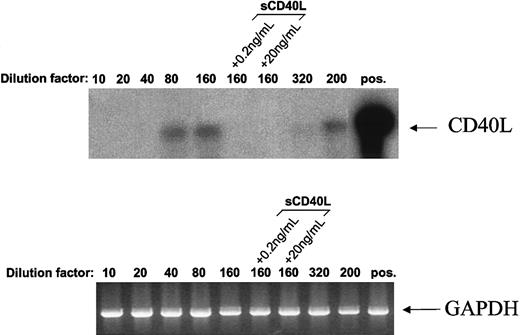
When populous cultures were analyzed for CD40L transcripts by PCR, no detectable band was generated even after 50 cycles of amplification. However, establishing cultures for 2 days at seeding concentrations of 2.5 × 104 cells/mL and below resulted in the appearance of detectable CD40L transcripts (Figure8). For a seeding concentration of 1.25 × 104 cells/mL, it was found that the presence of either 0.2 ng/mL or 20 ng/mL sCD40L during culture suppressed the appearance of CD40L messenger RNA. Although CD40L transcripts were clearly demonstrable in cultures established at concentrations as low as 1 × 104/mL (dilution factor = 200), they were barely discernible when cells were plated at even lower densities (dilution factor = 320), a condition that resulted in a precipitous decline in viability at these cell numbers. A similar induction of CD154 message was seen for both Mutu I and Elijah cells in response to seeding at decreasing cell numbers (not detailed).
Population depletion leads to induction of CD40L transcripts in L3055 cells.
CD40L expression was assessed in L3055 (wild-type) cells cultured at various densities by using reverse transcriptase-PCR. Cells (2 × 106) were plated out in increasing volumes of culture medium (10, 20, 40, 80, 160, 320, and one of 200 mL), in the absence or presence of sCD40L as indicated and cultured for 48 hours. Complementary DNAs were synthesized from total RNA isolated from these cultures and subjected to PCR by using primers specific for CD40L (upper panel) or glyceraldehyde phosphate dehydrogenase (lower panel). To increase the sensitivity of the detection, the CD40L PCR products were transferred onto nylon membrane and hybridized with a32P-labeled CD40L-specific oligo-probe. As a positive control for CD40L expression, RNA from CD40L-transfected mouse L cells was used (upper panel, pos). Data shown are representative of 4 similar experiments.
Population depletion leads to induction of CD40L transcripts in L3055 cells.
CD40L expression was assessed in L3055 (wild-type) cells cultured at various densities by using reverse transcriptase-PCR. Cells (2 × 106) were plated out in increasing volumes of culture medium (10, 20, 40, 80, 160, 320, and one of 200 mL), in the absence or presence of sCD40L as indicated and cultured for 48 hours. Complementary DNAs were synthesized from total RNA isolated from these cultures and subjected to PCR by using primers specific for CD40L (upper panel) or glyceraldehyde phosphate dehydrogenase (lower panel). To increase the sensitivity of the detection, the CD40L PCR products were transferred onto nylon membrane and hybridized with a32P-labeled CD40L-specific oligo-probe. As a positive control for CD40L expression, RNA from CD40L-transfected mouse L cells was used (upper panel, pos). Data shown are representative of 4 similar experiments.
The data presented herein collectively indicate that at high cell number there is no requirement for endogenous CD154 to sustain survival (and thereby growth) of biopsylike BL cells. However, the cells have the capacity to express CD154, such that, when the population becomes limiting, CD40L transcription can be induced with an ensuing utilization of the product to promote cell viability.
Discussion
BL is an aggressive malignancy of B cells with a GC phenotype.7-10 It is invariably associated with a translocation of the c-myc proto-oncogene into either one of the 3 immunoglobulin gene loci.42,43 Although the tumor is capable of doubling in size within 24 hours, BL is nevertheless characterized by high-rate spontaneous apoptosis11,12; this paradoxical behavior probably reflects the dichotomous property of the deregulated c-myc gene to drive both proliferation and cell death.44,45 Clearly, for the tumor to be “successful” the balance between growth and death must be in favor of the former. Although a myriad autocrine growth factors have been described for BL cells,46 little attempt has been made to unravel whether these operate directly on the growth cycle or, instead, work indirectly through the enhancement of survival, thereby allowing proliferation potential to be realized. Here, we have shown that, in response to depletion in number, biopsylike BL cells activate an autonomous CD154-dependent survival pathway that allows continued propagation of the dividing population.
Efficient promotion of survival by low picomolar concentrations of exogenous CD40L could be encouraged without discernible change in the basal phenotype of biopsylike BL cells. A contribution from CD40L to the in vivo maintenance of BL had been previously argued against, as high-level engagement of CD40 results in a profound shift of the cells away from their GC phenotype24; among other changes, they up-regulate CD54 and CD95 while down-regulating CD40 and CD77.22,24,35 Here, we have additionally shown that CD80 is induced in BL cells on high-level CD40 engagement with a corresponding reduction in the otherwise strong expression of the CD27 “memory” marker.41 Moreover, the Bcl-2 negativity that characterizes both BL cells and their normal GC equivalents is lost as cells become Bcl-2+, a change that correlates with a tendency to growth arrest, in response to high levels of soluble CD40L.22 In the present study, we confirmed both the acquisition of Bcl-2 and growth arrest. In addition, we showed an increase in the level of Bcl-xL expressed in the biopsylike BL cells on high-level CD40 engagement. These increases in the 2 prosurvival proteins with large amounts of CD40L may explain why in BL cells, by contrast to some other B-cell types, the CD40-dependent growth arrest is not accompanied by cell death.30 31 The finding that neither of the antiapoptosis molecules (nor proapoptotic Bax) changed in BL cells in response to soluble CD40L, at concentrations that are effective in halting the demise of low-density cultures, indicates that in vivo, low-level CD40 signaling could contribute to the maintenance of the BL population in a native state.
In populous cultures, CD154 was neither needed nor produced. Only as critically low cell numbers were reached did the appearance of CD154 messenger RNA and a use of its product to maintain viability become evident. Consistent with this, we have been able to detect in the biopsylike BL cells used in the present study high levels of AKNA, the AT-hook transcription factor that coordinates CD40/CD154 expression in normal GC B cells29 (our own unpublished observations, 2001). The trigger for activating autonomous CD40L-dependent survival seemed to be population depletion itself. It was not merely a response to stress because cells cultured in low serum failed to initiate this pathway (our own unpublished observations, 2001). This finding suggests that the population is equipped to sense a deprivation in survival factors as numbers are reduced. Such factors could be cytokines or other soluble activities produced by companion cells. For example, Falk et al47 have demonstrated that, when seeded at low density, BL cells require cysteine to survive, an activity that can be provided by feeder cells. Interestingly, within the context of the present study, the survival and growth-promoting activity of thiol compounds was not associated with induction of Bcl-2 or with alterations in the levels of Bax. It is thus conceivable that a change in the redox status of the cell may provide the trigger for the initiation of autonomous CD40L-dependent survival.
An alternative explanation is that CD154 transcription is the default option of the BL cell and that as numbers increase an accumulation of secreted products repress this pathway. In this regard, it is of interest that the provision of exogenous CD40L suppressed the appearance of CD154 transcripts in low-density cultures. Such behavior is reminiscent of quorum sensing among gram-negative bacteria in which diffusible molecules provide a means of communication to coordinate gene expression with population density.48
It remains to be determined whether the mechanism described here for BL reflects similar behavior in normal GC cell equivalents. Grammer et al49 have reported on CD40L expression by subsets of tonsillar B cells with GC markers, although, when sought immunohistochemically, readily detectable staining for CD40L in situ appears to be restricted to a subset of CD45R0 T cells located primarily in the light and outer zones of GCs.50 However, as indicated here, the amounts concerned may be extremely low and consequently missed when sought on B cells by relatively insensitive techniques. A physiologic role for B-cell–produced CD40L would, teleologically, make most sense within the dividing centroblast-rich dark zone of the GC, where there is a paucity of not only T cells but also other non–B-cell elements.14 Thus, it could be envisaged that, deprived of exogenous survival signals, centroblasts (whose phenotypic profile most resemble that of BL cells) supply their own in the form of endogenous CD154. Rationally, this should be limiting and transient, as T-cell–expressed CD40L needs to be the arbiter of selection as rapidly cycling CD77+ centroblasts differentiate to nondividing CD77−centrocytes.6,14 Consistent with these notions, we have shown that the survival of GC B cells ex vivo can be maintained by monovalent CD40 ligand and does not require the high-level multivalent engagement that is a prerequisite for the development of CD40-dependent phenotypes associated with differentiation.33 51
The conclusion that small amounts of soluble CD40L can promote growth in a B-lymphoma population finds precedent in the study of Andersen et al52 in which as little as 1 ng/mL of the ligand resulted in significant stimulation of primary mantle cell lymphoma cells cultured ex vivo. It is important to place these findings in the perspective of in vivo models in which targeting CD40 by cross-linking reagents has provided a seemingly contradictory outcome: namely, the eradication of a tumor, including the specific example of BL.53-56 Thus, Funakoshi et al53 demonstrated that administration of anti-CD40 antibody resulted in a significant increase in the survival of severe combined immunodeficient mice carrying a variety of inoculated human B-cell lymphomas. This finding was shown, in vitro, to correlate with growth inhibition that could be elicited with either anti-CD40 or soluble CD40 ligand; the inhibition achieved with antibody was markedly increased with a further cross-linking step.53-55 Heath et al57 have reported that murine B-lymphoma cells similarly growth arrest on in vitro exposure to anti-CD40, contrasting with the growth stimulatory outcome of engaging CD40 on normal B cells from mice. For lymphoma B cells, the resolution of these apparently paradoxical consequences of CD40 engagement—either growth stimulation or arrest—appears to lie firmly within the context of the strength of signal being delivered. Thus, even within the same population of cells, as we have shown here and consistent with studies on B-cell hybridomas and normal B subsets,30,31 whereas high-level CD40-engagement can promote substantial growth arrest, low-level engagement can favor proliferation albeit indirectly by encouraging lymphoma cell survival. An efficient engagement of the CD40 signaling machinery can further encourage the in vivo elimination of lymphoma cells through the recruitment of appropriate immune effectors58 59; again, our findings reported herein of high concentrations of CD40L up-regulating both CD95, the target for Fas ligand, and CD80, an important costimulatory molecule, are wholly compatible with this and fully support the aspirations of CD40-targeted therapies.
The differential promotion of survival, phenotypic change, and growth arrest in relation to increasing levels of CD40 engagement (both for the BL cells described here and other systems) presumably relates, in turn, to the quality and quantity of the signal transduction machinery that is activated in response to varying amounts of CD40L.48 The precise set of signaling pathways engaged on CD40 ligation depends on a host of factors of which the degree of CD40 occupancy is only one; for example, the activation, differentiation, and tumorigenic status of the cell are also contributory. Such considerations have recently been covered in a comprehensive review by Grammer and Lipsky.40 The pattern of recruitment of TRAF (tumor necrosis factor receptor-associated factor) adaptor molecules41 is likely to be play a key role in determining the diverse outcomes of high- and low-level CD40 engagement in the BL cell, an issue we are currently exploring.60 In addition, some of the effects may be indirect: it is interesting to note the study of Baccam and Bishop61 in which membrane-bound CD154 (but not sCD40L) was capable of provoking interleukin 6 (IL-6) production in murine B cells.61 IL-6 itself can be either growth stimulatory or inhibitory to B cells, and differential cytokine production and/or responsiveness could be a factor underlying the assorted manifestations of CD40 engagement in BL cells and other B-cell subtypes.46
In summary, we have demonstrated that low picomolar quantities of sCD40L are capable of sustaining a viable BL cell population at numbers below which it would normally succumb to apoptosis. This situation occurs without discernible change in the hallmark GC phenotype that characterizes BL in situ. As the population reaches its critical threshold density, there is turn on of an autonomous CD154-dependent pathway that permits survival at what would otherwise be disastrously low cell numbers. By suppressing the apoptotic tendencies of the cells, propagation is sustained. Such a reaction to population depletion could allow the continuance of a malignant clone in which numbers were being compromised by, for example, migration from an environmental niche or immune attack. Whether the findings modeled here on BL cells in vitro find parallels among neoplastic populations in vivo now needs to be established but, if so, would represent an important new paradigm in tumor cell behavior.
We thank Darrel Pilling for advice and expertise on FACS analysis of Bcl-2 family members.
Supported by a Program Grant from the Medical Research Council (United Kingdom) and by Cancer Research United Kingdom (grant number SP2584/0101). A.E. is in receipt of an MRC Senior Fellowship. J.G. is an MRC Non-Clinical Research Professor.
One author (R.J.A.) is employed by Immunex Corporation.
A. Challa and A.G. Eliopoulos contributed equally.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John Gordon, MRC Centre for Immune Regulation, The University of Birmingham, Vincent Dr, Birmingham B15 2TT, United Kingdom; e-mail: j.gordon@bham.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal