Abstract
Stem cell factor (SCF) has crucial roles in proliferation, survival, and differentiation of hematopoietic stem cells and mast cells through binding to c-Kit receptor (KIT). Chemotaxis is another unique function of SCF. However, little is known about the intracellular signaling pathway of SCF/KIT-mediated cell migration. To investigate the signaling cascade, we made a series of 22 KIT mutants, in which tyrosine (Y) residue was substituted for phenylalanine (F) in the cytoplasmic domain, and introduced into BAF3 cells or 293T cells. On stimulation with SCF, BAF3 expressing KITWT(WT) showed cell migration and Ca2+ mobilization. Among 22 YF mutants, Y567F, Y569F, and Y719F showed significantly reduced cell migration and Ca2+ mobilization compared to WT. In Y567F, Lyn activation on SCF stimulation decreased and C-terminal Src kinase (Csk) suppressed KIT-mediated Ca2+ influx and cell migration, suggesting that Y567-mediated Src family kinase (SFK) activation leads to Ca2+ influx and migration. Furthermore, we found that p38 mitogen-activated protein kinase (p38 MAPK) and Erk1/2 were also regulated by Y567/SFK and involved in cell migration, and that p38 MAPK induced Ca2+ influx, thereby leading to Erk1/2 activation. In Y719F, the binding of phosphatidylinositol 3′-kinase (PI3K) to KIT was lost and KIT-mediated cell migration and Ca2+ mobilization were suppressed by PI3K chemical inhibitors or dominant-negative PI3K, suggesting the involvement of Y719-mediated PI3K pathway in cell migration. Combination of Csk and the PI3K inhibitor synergistically reduced cell migration, suggesting the cooperation of SFK and PI3K. Taken together, these results indicate that 2 major KIT signaling pathways lead to cell migration, one is Y567-SFK-p38 MAPK-Ca2+ influx-Erk and the other is Y719-PI3K-Ca2+ influx.
Introduction
c-Kit is a receptor tyrosine kinase (RTK), which constitutes a type III RTK subfamily with the receptors for platelet-derived growth factor (PDGF), colony-stimulating factor 1 (CSF-1), and flt-3 ligand.1,2 The type III RTKs are characterized by an extracellular domain with 5 immunoglobulinlike domains and a cytoplasmic domain consisting of a kinase domain that is interrupted by a kinase insert. c-Kit (KIT) and its ligand stem cell factor (SCF) play an important role in hematopoiesis, melanogenesis, and gametogenesis,3 as has been clearly shown by loss of function mutations of c-kit gene. In addition, c-kit gene product has been associated with various forms of neoplasms. Activating mutants of KIT, either in the juxtamembrane domain or the catalytic domain, were identified as the cause for transformation of hematopoietic stem cells, mast cells, and gastrointestinal stromal cells.4-10 Thus, KIT/SCF has pleiotropic functions such as proliferation, survival, differentiation, and transformation. In this report, we focus on SCF/KIT-mediated cell migration, which is also a characteristic function of SCF in hematopoietic stem cells and mast cells,11-13 and has critical roles in immunity, metastasis, and development.
On ligand stimulation, KIT receptors dimerize, activate its intrinsic tyrosine kinase, and autophosphorylate. The phosphorylated KIT receptor generates binding sites for SH2 domain-containing proteins, which include proteins of the p21Ras–mitogen-activated protein kinase (MAPK) pathway,14 the p85 subunit of phosphatidylinositol 3′ kinase (PI3K),15 phospholipase C-γ1, the Grb2 adaptor protein,16 the Src family kinases (SFKs),17 Cbl, CRKL,18p62Dok-1,19 SHP1, and SHP2.20 Those proteins are subsequently activated or phosphorylated and further transduce signaling cascades that lead to various cellular responses. However, little is known about which signaling is essential for SCF-mediated migration. Recently, a few reports indicated that Lyn or p38 MAPK plays an important role,21 22 but no comprehensive investigation has been done in which the tyrosine residue of KIT is involved in signal transduction, which is required for cell migration. In this study, we have converted all possible tyrosine (Y) residues on KIT cytoplasmic domain to phenylalanine (F) and introduced these YF substitute mutants on 293T cells or murine interleukin 3 (IL-3)–dependent BAF3 cells. We used these cell lines to elucidate signaling cascades that are important for SCF-mediated cell migration. We found the critical roles of Y567 and Y719 in KIT and defined 2 distinct pathways, SFK and PI3K, initiating from respective tyrosine residues.
Materials and methods
Reagents and antibodies
Highly purified recombinant murine (rm) SCF and rmIL-3 were provided by Kirin Brewery (Tokyo, Japan). Antiphosphotyrosine antibody, a murine monoclonal antibody generated against phosphotyrosine, was generously supplied by Dr B. J. Drucker (Oregon Health Science University, Portland). Antibodies against mouse KIT, Lyn, and C-terminal Src kinase (Csk) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and anti-MEK1/2, antiphospho MEK1/2, anti-p38 MAPK, antiphospho p38 MAPK, anti-Akt, antiphospho Akt (pSer473), and antiphospho Src (pY416) were purchased from New England Biolabs (Beverly, MA); the anti-PI3K p85α antibody from Upstate Biotechnology (Lake Placid, NY); PI3K inhibitor, wortmannin and LY294002, p38 MAPK inhibitor, SB203850, and MEK inhibitor, U0126 from Calbiochem (San Diego, CA); BAPTA-AM and Indo 1-AM from Dojin Chemical (Kumamoto, Japan); and G418 sulfate (geneticin) from Gibco BRL (Grand Island, NY).
Construction of expression plasmids
The mammalian expression vector pEF-BOS was donated by Dr S. Nagata (Osaka University, Osaka, Japan). A dominant-negative mutant of bovine p85 complementary DNA (cDNA), which lacks a binding site for the catalytic 110-kd subunit of PI3K, was kindly provided by Dr M. Kasuga (Kobe University, Kobe, Japan). The gene encoding murine c-kit WT cDNA was cloned into an EcoRV site of Bluescript I KS (−). BluntedHindIII-EcoRI fragment of Bluescript I KS (−) containing c-kit WT cDNA was introduced into the blunted XbaI site of pEF-BOS. To convert tyrosine residues (at codons 544, 546, 552, 567, 569, 577, 608, 645, 671, 674, 702, 719, 728, 745, 772, 821, 844, 853, 868, 878, 898, and 934) to phenylalanine in the cytoplasmic domain of KIT, point mutations were generated by overlap extension polymerase chain reaction with synthetic oligonucleotides encoding the desired amino acid substitutions using c-kit WT as a template. The digested fragments of amplified products were exchanged by the corresponding fragment of c-kit WT cDNA in pEF-BOS. The resulting plasmids were sequenced to confirm the mutations and named as Y544F, Y546F, Y552F, and so on. The cDNAs of rat WT-Csk, a kinase-defective csk[csk(−)] in which Lys222 is replaced to arginine, and a membrane-anchoring csk mutant (mcsk) possessing c-src myristoylation signal sequence, were subcloned into an expression vector, pCXN2 harboring neomycin-resistant gene.
Cells and transfection
The IL-3–dependent murine pro-B cell line, BAF3, was maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 1 ng/mL rmIL-3. Transfection of BAF3 cells was performed by electroporation. Following gene transfer, cells were cultured in IL-3–containing medium for 24 hours and then selected in G418 at a concentration of 1 mg/mL. Multiple clones were expanded for further analysis. Human embryonic kidney 293T cell was transfected by the calcium phosphate method as described previouly.23 Two days after transfection, the cells were used for further analysis. To detect KIT proteins on the cell surface, cells were treated for 30 minutes at 4°C with biotin-conjugated rat antimurine KIT antibody (Immunotech, Marseille, France). After washing, cells were incubated for 30 minutes at 4°C with a 1:40 dilution of avidin–fluorescein isothiocyanate (FITC; Becton Dickinson, San Jose, CA). Samples were analyzed using a FACSCalibur (Becton Dickinson).
Immunoprecipitation and Western blot analysis
BAF3 cells transfected with c-kit constructs (BAF3/KIT) were starved from IL-3 and serum for 12 hours in RPMI 1640 medium supplemented with 0.1% FCS. Subsequently, cells were resuspended in 1 mL medium for 5 minutes at 37°C with or without 100 ng/mL SCF, washed twice with ice cold phosphate-buffered saline (PBS), and lysed with buffer containing 50 mM HEPES (pH 7.4), 10% glycerol, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 50 μM ZnCl2, 25 mM NaF, proteinase inhibitors (Complete, Boehringer Mannheim, Mannheim, Germany), 1 μM pepstatin, and 1 mM sodium orthovanadate. Cell lysates were clarified at 20 000g for 20 minutes. For immunoprecipitation, cell lysates were incubated with rabbit polyclonal antibody to murine KIT and with Protein A/G-Plus-Sepharose (Santa Cruz). The immunoprecipitates were washed 3 times with lysis buffer. Immunoprecipitates and total lysates were resuspended in sodium dodecyl sulfate (SDS) sample buffer, heated and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Gels were blotted on Immobilon P membrane (Millipore, Bedford, MA) and stained with the indicated antibody. Antibody binding was detected by incubation with a horseradish peroxidase (HRP)–labeled secondary antibody followed by chemiluminescence detection (ECL-Plus, Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
In vitro 2–chamber migration assay
The modified Boyden chamber assay in a 24-well chemotaxis chamber (5-μm pore size, Kurabow, Osaka, Japan) was used to measure SCF-induced cell migration. The upper and lower chambers were filled with RPMI 1640 medium supplemented with 10% FCS and various concentrations of SCF. A total of 3 × 105 IL-3–starved BAF3/KIT cells were plated in each of the upper chamber. Chambers were incubated for 6 hours at 37°C in a CO2 incubator. Cells migrating into the lower chamber were counted in the flow cytometry by passing each sample at the same predetermined time and flow condition as previously described.11
Intracellular Ca2+ measurement
The measurements of intracellular Ca2+ was as done as described previously.24 In short, cells were washed twice in Hepes-buffered Krebs solution, which consisted of 124 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 10 mM glucose, and 25 mM Hepes (pH 7.4; HBKS). Then, a total of 107 cells were incubated in 1 mL HBKS containing 5 μM Indo 1-AM for 30 minutes at 37°C in the dark. The cells were subsequently washed twice with HBKS and resuspended at 2.5 × 106 cells/mL. The cell suspension was plated in a continuously stirred cuvette at 37°C in a CAF-110 fluorometer (Jasco, Tokyo, Japan). Fluorescence was monitored at an excitation wavelength of 355 nm and emission wavelengths of 405 nm and 485 nm. The data are presented as the relative ratio of fluorescein excited at 405 to 485 nm.
Statistical analysis
Comparisons between other groups were made using an unpaired Student t test (2-tails).
Results
SCF induces cell migration and increases in the intracellular Ca2+
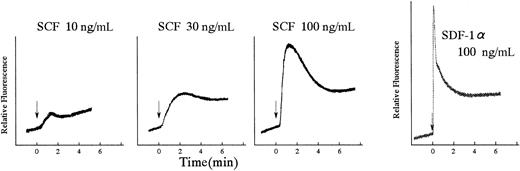
The chemotactic property of BAF3/KITWT (BAF3/WT) against SCF is presented as a checkerboard diagram (Table1). Maximal response was obtained with a concentration of 30 ng/mL SCF in the lower chamber. Because Ca2+ is the key mediator of cell movement, we measured SCF-induced intracellular change of Ca2+ in BAF3/WT cells using Indo1-AM. Following SCF stimulation, BAF3/WT cells exhibited a prompt peak and subsequently sustained increase of Ca2+. The Ca2+ influx was dependent on SCF concentration within a range from 10 to 100 ng/mL. This Ca2+ influx peak was gradual as compared with stromal cell-derived factor–1α (SDF-1α) (Figure 1). Then, we examined the effect of the intracellular Ca2+ chelator BAPTA-AM on SCF-induced Ca2+ mobilization and cell migration. The pretreatment of cells with BAPTA-AM also inhibited SCF-induced cell migration in a dose-dependent manner (Figure2A). The pretreatment of cells with BAPTA-AM dose dependently attenuated SCF-induced intracellular Ca2+ mobilization as shown in Figure 2B.
Checkerboard analysis of the SCF-induced cell migration of BAF3/KITWT cells
| Chemotaxis of BAF3/KITWT cells (% cells in lower chamber/whole cells) . | ||||||
|---|---|---|---|---|---|---|
| SCF concentration (ng/mL) in lower chamber . | SCF concentration (ng/mL) in upper chamber . | |||||
| 0 . | 1 . | 3 . | 10 . | 30 . | 100 . | |
| 0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 3 | 1.4 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| 10 | 17.9 ± 1.3 | 14.8 ± 0.7 | 13.3 ± 0.9 | 5.4 ± 0.3 | 3.6 ± 0.4 | 1.2 ± 0.1 |
| 30 | 37.2 ± 3.6 | 32.2 ± 1.9 | 22.7 ± 2.5 | 14.0 ± 1.1 | 7.6 ± 0.5 | 1.2 ± 0.1 |
| 100 | 10.5 ± 0.9 | 8.5 ± 0.8 | 8.5 ± 0.7 | 8.5 ± 0.4 | 3.2 ± 0.3 | 0.5 ± 0.0 |
| Chemotaxis of BAF3/KITWT cells (% cells in lower chamber/whole cells) . | ||||||
|---|---|---|---|---|---|---|
| SCF concentration (ng/mL) in lower chamber . | SCF concentration (ng/mL) in upper chamber . | |||||
| 0 . | 1 . | 3 . | 10 . | 30 . | 100 . | |
| 0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 3 | 1.4 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| 10 | 17.9 ± 1.3 | 14.8 ± 0.7 | 13.3 ± 0.9 | 5.4 ± 0.3 | 3.6 ± 0.4 | 1.2 ± 0.1 |
| 30 | 37.2 ± 3.6 | 32.2 ± 1.9 | 22.7 ± 2.5 | 14.0 ± 1.1 | 7.6 ± 0.5 | 1.2 ± 0.1 |
| 100 | 10.5 ± 0.9 | 8.5 ± 0.8 | 8.5 ± 0.7 | 8.5 ± 0.4 | 3.2 ± 0.3 | 0.5 ± 0.0 |
Data are shown as the percentages of cells in the lower chamber to whole applied cells. Results presented the mean ± SD of triplicate cultures. The experiments were repeated at least 3 times with similar results.
SCF induced the increase of the intracellular Ca2+concentration.
Ca2+ influx in BAF3/KIT cells induced by SCF or SDF-1α. Cells were loaded with Indo 1-AM for 30 minutes at 37°C and stimulated by SCF at the indicated concentrations or SDF-1α (100 ng/mL). The intracellular Ca2+ concentration was monitored by measuring the relative fluorescence using a spectrofluorometer. Similar results were obtained from 3 independent experiments.
SCF induced the increase of the intracellular Ca2+concentration.
Ca2+ influx in BAF3/KIT cells induced by SCF or SDF-1α. Cells were loaded with Indo 1-AM for 30 minutes at 37°C and stimulated by SCF at the indicated concentrations or SDF-1α (100 ng/mL). The intracellular Ca2+ concentration was monitored by measuring the relative fluorescence using a spectrofluorometer. Similar results were obtained from 3 independent experiments.
Inhibition of Ca2+ mobilization suppresses SCF-induced cell migration.
(A) BAF/WT cells were preincubated with the indicated concentrations of BAPTA-AM for 30 minutes. Then, cell migration was induced by 30 ng/mL SCF in the presence of BAPTA-AM in both chambers. Data are shown as percentages of cells in lower chamber to the whole applied cells. The results are shown as the means ± SD of triplicate cultures. (B) Inhibition of Ca2+ influx of BAF3/KIT cells with BAPTA-AM. The cells were preincubated with BAPTA-AM for 30 minutes at 37°C. The intracellular Ca2+ concentration was measured as described above. Similar results were obtained from 3 independent experiments.
Inhibition of Ca2+ mobilization suppresses SCF-induced cell migration.
(A) BAF/WT cells were preincubated with the indicated concentrations of BAPTA-AM for 30 minutes. Then, cell migration was induced by 30 ng/mL SCF in the presence of BAPTA-AM in both chambers. Data are shown as percentages of cells in lower chamber to the whole applied cells. The results are shown as the means ± SD of triplicate cultures. (B) Inhibition of Ca2+ influx of BAF3/KIT cells with BAPTA-AM. The cells were preincubated with BAPTA-AM for 30 minutes at 37°C. The intracellular Ca2+ concentration was measured as described above. Similar results were obtained from 3 independent experiments.
Reduced Ca2+ mobilization and cell migration in Y567F, Y569F, and Y719F cells
As shown in Figure 3A, KIT contains 22 tyrosine (Y) residues in the cytoplasmic domain. To determine which tyrosine residue(s) is involved in Ca2+ mobilization and cell migration, we constructed a series of 22 single YF mutants for all these tyrosine residues. On transient transfection into 293T cells, Y567F, Y569F, and Y719F, respectively, showed 73%, 60%, and 54% reduction of Ca2+ mobilization when compared with WT (Figure 3B). To further examine the effects of these YF conversions in stable cell clones, we transfected these constructs into murine IL-3–dependent BAF3 cells that had no intrinsic KIT expression. The surface expressions and ligand-induced autophosphorylations of KIT were observed at the same level in BAF3 clones expressing WT or each mutant (data not shown). Similar to the results of 293T cells, Ca2+ influx was suppressed in BAF3 expressing Y567F (BAF3/Y567F), BAF3/Y569F and BAF3/Y719F (data not shown). SCF-induced cell migration was decreased for 85%, 70%, and 58% in BAF3/Y567F, BAF3/Y569F, and BAF3/Y719F mutants, respectively, as compared to BAF3/WT, which were similar to the reduction rate of Ca2+mobilization (Figure 3C).
Reduced Ca2+ mobilization and cell motility in Y567F, Y569F, and Y719F mutant cell lines.
(A) The schematic structure of murine KIT is shown with all tyrosine residue sites in the cytoplasmic region. The cytoplasmic domain contains 22 tyrosine residues. Extracellular indicates extracellular domain; Juxta, juxtamembrane domain; TK, tyrosine kinase domain; KI, kinase insert. (B) SCF-stimulated Ca2+ influx in 293T cells expressing WT or YF mutants. Cells were stimulated by 100 ng/mL SCF. Ca2+ influx was measured as described in Figure1. (C) SCF-stimulated cell migration in BAF3 cells expressing WT or YF mutants. Cell migration was induced by 30 ng/mL SCF. Data are shown as percentages of cells in lower chamber to the whole applied cells. The results are shown as the means ± SD of triplicate cultures. All experiments were repeated at least 3 times with similar results.
Reduced Ca2+ mobilization and cell motility in Y567F, Y569F, and Y719F mutant cell lines.
(A) The schematic structure of murine KIT is shown with all tyrosine residue sites in the cytoplasmic region. The cytoplasmic domain contains 22 tyrosine residues. Extracellular indicates extracellular domain; Juxta, juxtamembrane domain; TK, tyrosine kinase domain; KI, kinase insert. (B) SCF-stimulated Ca2+ influx in 293T cells expressing WT or YF mutants. Cells were stimulated by 100 ng/mL SCF. Ca2+ influx was measured as described in Figure1. (C) SCF-stimulated cell migration in BAF3 cells expressing WT or YF mutants. Cell migration was induced by 30 ng/mL SCF. Data are shown as percentages of cells in lower chamber to the whole applied cells. The results are shown as the means ± SD of triplicate cultures. All experiments were repeated at least 3 times with similar results.
SCF-induced Ca2+ influx and cell migration is regulated by SFK
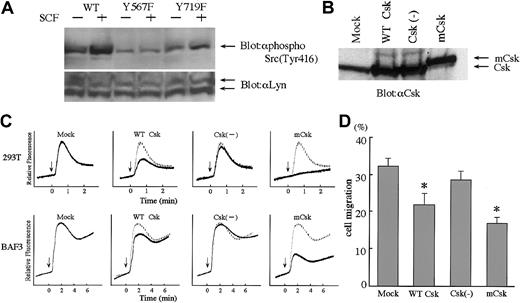
Consistent with previous reports that indicated the close relationship between Y567 and SFK, we found that in 293T cells expressing Y567F mutant (293T/Y567F), SCF-induced Lyn activation was remarkably reduced as compared to 293T/WT (Figure4A). To determine if SFK activity is involved in SCF-stimulated Ca2+ mobilization and cell migration, SFK activity was inhibited specifically by expression of Csk, which is the negative regulator of SFK. We transfected WT-Csk, Csk(−) (kinase dead), or mCsk (constitutive active) into BAF3 cells and 293T cells, with c-kit WT. Csk proteins were highly expressed as shown in Figure 4B. In both cell lines, WT-Csk inhibited SCF-induced Ca2+ influx significantly. The suppression of Ca2+ influx was more prominent in mCsk transfectants. In contrast, expression of Csk(−) did not suppress Ca2+influx, indicating that Csk activity plays an important role in Ca2+ influx in the signaling cascades mediated by c-Kit (Figure 4C). We next examined the effect of WT-Csk, Csk(−), and mCsk on cell migration by using stably transfected BAF3 cells. WT-Csk inhibited cell migration and mCsk inhibited more profoundly than WT-Csk (Figure 4D). These findings suggested that SFK may regulate SCF-induced Ca2+ influx and cell migration.
Involvement of SFK in SCF-induced migration and Ca2+influx. (
A) SCF-induced activation of Lyn. 293T cells were transiently cotransfected with Lyn and c-Kit constructs. After 18 hours of starving from serum, cells were stimulated with SCF (100 ng/mL) for 10 minutes and lysed. Total cell lysates were blotted with antiphospho Src antibody, which detects activated forms of SFK. Stripped membrane was reblotted with anti-Lyn antibody to prove the same loading. (B) Expression of Csk constructs. BAF3/KIT cells were stably transfected with WT-Csk, kinase dead Csk (Csk(−)) and constitutive active Csk (membrane-associated Csk; mCsk). The amounts of Csk proteins in total cell lysates were detected by blotting with anti-Csk. (C) Inhibition of Ca2+ influx by Csk. SCF-induced Ca2+ influx was measured in 293T cells transiently cotransfected with c-kit and Csk constructs BAF3/KIT cells stably transfected with Csk constructs. (D) Inhibition of cell migration by Csk. Cell migration toward 30 ng/mL SCF was analyzed in BAF3/KIT-expressing Csk constructs. Data are shown the same as Figure 3C. The asterisk represents a significant difference from a control (Mock) value (P < .01). Similar results were obtained from 3 independent experiments.
Involvement of SFK in SCF-induced migration and Ca2+influx. (
A) SCF-induced activation of Lyn. 293T cells were transiently cotransfected with Lyn and c-Kit constructs. After 18 hours of starving from serum, cells were stimulated with SCF (100 ng/mL) for 10 minutes and lysed. Total cell lysates were blotted with antiphospho Src antibody, which detects activated forms of SFK. Stripped membrane was reblotted with anti-Lyn antibody to prove the same loading. (B) Expression of Csk constructs. BAF3/KIT cells were stably transfected with WT-Csk, kinase dead Csk (Csk(−)) and constitutive active Csk (membrane-associated Csk; mCsk). The amounts of Csk proteins in total cell lysates were detected by blotting with anti-Csk. (C) Inhibition of Ca2+ influx by Csk. SCF-induced Ca2+ influx was measured in 293T cells transiently cotransfected with c-kit and Csk constructs BAF3/KIT cells stably transfected with Csk constructs. (D) Inhibition of cell migration by Csk. Cell migration toward 30 ng/mL SCF was analyzed in BAF3/KIT-expressing Csk constructs. Data are shown the same as Figure 3C. The asterisk represents a significant difference from a control (Mock) value (P < .01). Similar results were obtained from 3 independent experiments.
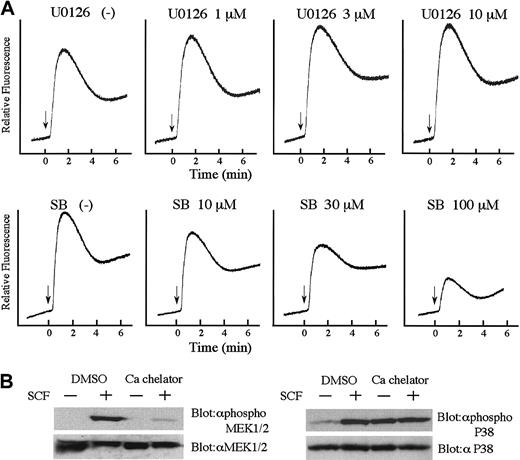
SFK regulates cell motility through Erk and p38 MAPK
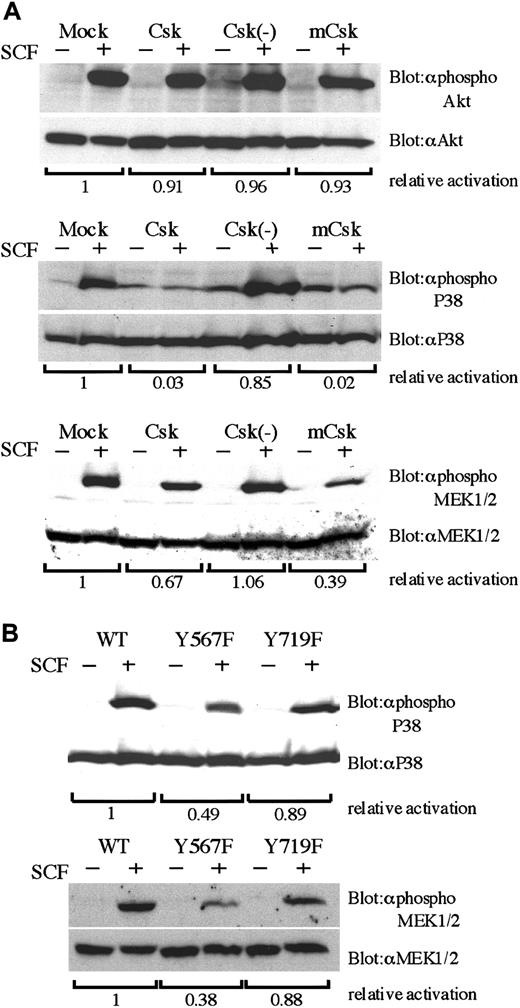
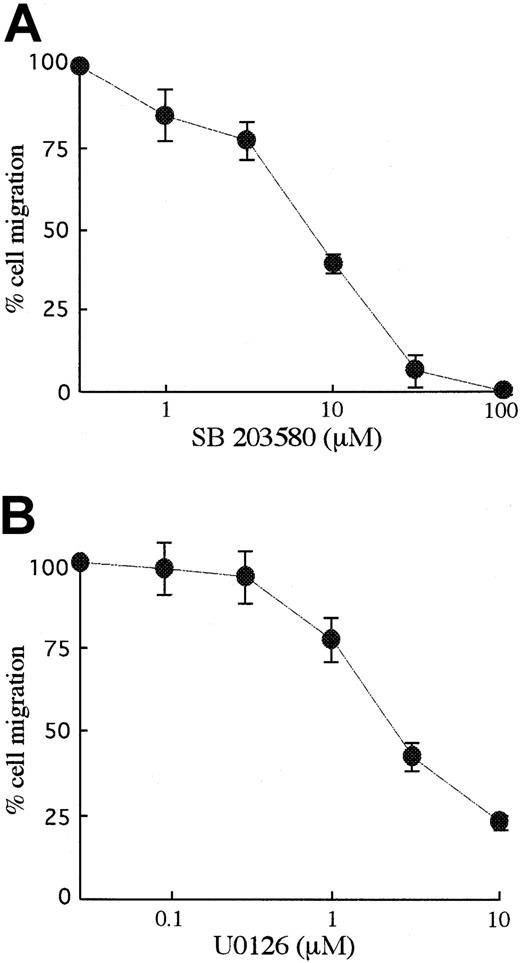
We next explored how Csk affects SCF-mediated signal transduction, thereby leading to cell migration. As shown in Figure5A, in any Csk transfectant there was no difference in the activation of Akt. On the other hand, Csk and mCsk inhibited SCF-mediated activation of p38 MAPK and MEK1/2 (Figure 5A). SCF-mediated activations of MEK1/2 and p38 MAPK were also significantly reduced in BAF3/Y567F, where SCF-induced SFK activation was suppressed (Figure 5B). Moreover, MEK1/2 inhibitor U0126 and p38 MAPK inhibitor SB203580 suppressed cell migration dose dependently as shown in Figure6. Both U0126 and SB203580 did not affect cell viability at these concentrations. These results indicate that Y567 of KIT mediates MEK/Erk and p38 MAPK signaling through SFK and regulates SCF-induced cell migration.
Impaired activation of p38 MAPK and MEK1/2 by SCF in Y567F and Csk-expresssing cells.
(A) BAF3/KIT-expressing Csk constructs were starved from IL-3 for 12 hours, stimulated with 100 ng/mL SCF, and lysed. Total cell lysates were analyzed by Western blotting with indicated antibodies. Antiphospho Akt, antiphospho p38, and antiphospho MEK1/2 are antibodies specific for the activation state of respective proteins (upper lanes). Each membrane was stripped and reblotted with antibodies detecting total amount of respective proteins (lower lanes). The relative activation was quantitated by densitometric scanning of transblot bands. (B) Total cell lysates were prepared from BAF3 cells expressing WT KIT or YF mutants and analyzed by Western blotting with indicated antibodies as described in panel A. Three independent experiments were performed with comparable results.
Impaired activation of p38 MAPK and MEK1/2 by SCF in Y567F and Csk-expresssing cells.
(A) BAF3/KIT-expressing Csk constructs were starved from IL-3 for 12 hours, stimulated with 100 ng/mL SCF, and lysed. Total cell lysates were analyzed by Western blotting with indicated antibodies. Antiphospho Akt, antiphospho p38, and antiphospho MEK1/2 are antibodies specific for the activation state of respective proteins (upper lanes). Each membrane was stripped and reblotted with antibodies detecting total amount of respective proteins (lower lanes). The relative activation was quantitated by densitometric scanning of transblot bands. (B) Total cell lysates were prepared from BAF3 cells expressing WT KIT or YF mutants and analyzed by Western blotting with indicated antibodies as described in panel A. Three independent experiments were performed with comparable results.
The effects of MAPK inhibitors on SCF-induced migration.
BAF3/KIT cells were preincubated with p38 MAPK inhibitor, SB203580, or MEK1/2 inhibitor, U0126 for 30 minutes and induced for migration with 30 ng/mL SCF. Results were calculated as percentages to the migration without inhibitor. The results are shown as the means ± SD of triplicate cultures. Similar results were obtained from 3 independent experiments.
The effects of MAPK inhibitors on SCF-induced migration.
BAF3/KIT cells were preincubated with p38 MAPK inhibitor, SB203580, or MEK1/2 inhibitor, U0126 for 30 minutes and induced for migration with 30 ng/mL SCF. Results were calculated as percentages to the migration without inhibitor. The results are shown as the means ± SD of triplicate cultures. Similar results were obtained from 3 independent experiments.
p38 MAPK induced Ca2+ mobilization
We next examined the relationship between Ca2+mobilization, Erk, and p38 MAPK. After treatment of BAF3/WT cells with U0126 or SB203580, Ca2+ influx on SCF stimulation was measured. As shown in Figure 7A, p38 MAPK inhibitor SB203580 suppressed Ca2+ influx, but MEK inhibitor U0126 did not. Furthermore, we examined the effect of the intracellular Ca2+ chelator, BAPTA-AM on SCF-induced activation of MEK1/2 and p38 MAPK. As shown in Figure 7B, treatment of the cells with BAPTA-AM remarkably reduced the activation of MEK1/2, but did not suppress the activation of p38 MAPK. These data indicated that in SCF signaling, SFK-activated P38 MAPK may induce Ca2+ mobilization, resulting in the activation of MEK/ Erk pathway.
Interaction of MAPKs and Ca2+ influx in KIT signal transduction.
(A) Effects of MEK1/2 inhibitor and p38 MAPK inhibitor on SCF-induced Ca2+ influx. BAF3/KIT cells were loaded with Indo 1-AM and incubated with or without U0126 or SB203580 at the indicated concentration for 30 minutes at 37°C. The intracellular Ca2+ concentration was monitored by measuring the relative fluorescence using a spectrofluorometer. (B) Effects of BAPTA-AM on SCF-induced MEK1/2 and p38 MAPK phosphorylation by Western blot analysis. BAF3/KIT cells were incubated with or without 10 μM BAPTA-AM for 30 minutes and then stimulated with 100 ng/mL SCF in the presence of the inhibitors. Similar results were obtained from 3 independent experiments.
Interaction of MAPKs and Ca2+ influx in KIT signal transduction.
(A) Effects of MEK1/2 inhibitor and p38 MAPK inhibitor on SCF-induced Ca2+ influx. BAF3/KIT cells were loaded with Indo 1-AM and incubated with or without U0126 or SB203580 at the indicated concentration for 30 minutes at 37°C. The intracellular Ca2+ concentration was monitored by measuring the relative fluorescence using a spectrofluorometer. (B) Effects of BAPTA-AM on SCF-induced MEK1/2 and p38 MAPK phosphorylation by Western blot analysis. BAF3/KIT cells were incubated with or without 10 μM BAPTA-AM for 30 minutes and then stimulated with 100 ng/mL SCF in the presence of the inhibitors. Similar results were obtained from 3 independent experiments.
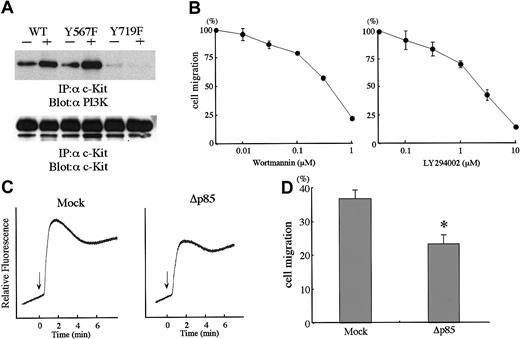
PI3K regulates SCF-induced Ca2+ influx and cell migration
Y719F abolished the binding of PI3K to KIT (Figure8A). In BAF3/Y719F, Ca2+influx and cell migration were impaired about 50% as compared with WT. To explore the involvement of PI3K in SCF-induced cell migration, we tested specific PI3K inhibitors, wortmannin and LY294002, both of which inhibited cell migration in a concentration-dependent manner (Figure8B). Furthermore, we introduced an expression vector of dominant-negative PI3K or an empty control vector into BAF3/WT cells. Dominant-negative PI3K expression inhibited SCF-induced PI3K activity by about 85% (data not shown). BAF3/WT cell expression of dominant-negative PI3K inhibited significantly Ca2+ influx and cell migration in BAF3/WT cells (Figure 8C,D).
Involvement of PI3K pathway in SCF-induced cell migration.
(A) Association of PI3K p85 subunit with KIT was lost in Y719F. Total cell lysates prepared as described in Figure 4 were immunoprecipitated with anti-KIT antibody and blotted with anti-p85 antibody. The membrane was reblotted with anti-KIT antibody. (B) Effects of PI3K inhibitors on SCF-induced cell migration. Two different inhibitors, wortmannin or LY294002, were preincubtaed with BAF3/KIT cells and induced for migration with 30 ng/mL SCF. Migration without inhibitors was calculated as 100%. The results are shown as the means ± SD of triplicate cultures. (C) Dominant-negative PI3K inhibits SCF-induced Ca2+ influx. BAF3/KIT cells were stably transfected with dominant-negative PI3K (Δp85) and analyzed for SCF-induced Ca2+ influx (SCF; 100 ng/mL). (D) Dominant-negative PI3K inhibits SCF-induced cell migration. BAF3/KIT cells stably transfected with Δp85 or mock vector were analyzed for SCF-induced cell migration (SCF; 30 ng/mL). The asterisk represents a significant difference from a control (Mock) value (P < .01). Similar results were obtained from 3 independent experiments.
Involvement of PI3K pathway in SCF-induced cell migration.
(A) Association of PI3K p85 subunit with KIT was lost in Y719F. Total cell lysates prepared as described in Figure 4 were immunoprecipitated with anti-KIT antibody and blotted with anti-p85 antibody. The membrane was reblotted with anti-KIT antibody. (B) Effects of PI3K inhibitors on SCF-induced cell migration. Two different inhibitors, wortmannin or LY294002, were preincubtaed with BAF3/KIT cells and induced for migration with 30 ng/mL SCF. Migration without inhibitors was calculated as 100%. The results are shown as the means ± SD of triplicate cultures. (C) Dominant-negative PI3K inhibits SCF-induced Ca2+ influx. BAF3/KIT cells were stably transfected with dominant-negative PI3K (Δp85) and analyzed for SCF-induced Ca2+ influx (SCF; 100 ng/mL). (D) Dominant-negative PI3K inhibits SCF-induced cell migration. BAF3/KIT cells stably transfected with Δp85 or mock vector were analyzed for SCF-induced cell migration (SCF; 30 ng/mL). The asterisk represents a significant difference from a control (Mock) value (P < .01). Similar results were obtained from 3 independent experiments.
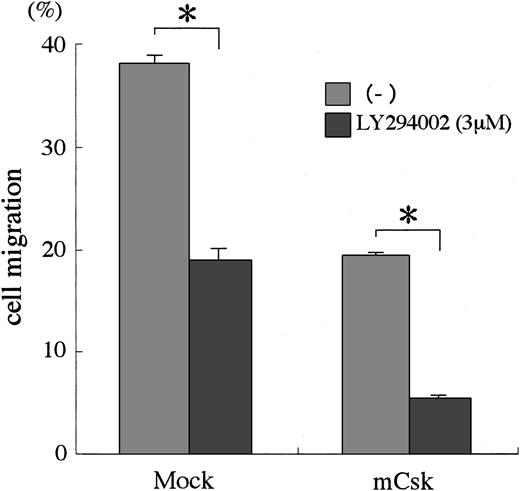
SFK and PI3K cooperate for cell migration
To examine the interaction between SFK and PI3K, we analyzed the migration of BAF3/WT expressing mCsk (BAF3/WT/mCsk) in the presence of the PI3K inhibitor LY294002. The migration of BAF3/WT/mCsk was reduced to about 50% of the BAF3/WT cells. When BAF3/WT/mCsk cells were treated with LY294002, the migration response was further inhibited and reduced to about 15% of BAF3/WT (Figure9). These results suggested that both SFK and PI3K cooperate for SCF-mediated cell migration.
SFKs and PI3K cooperate on SCF signaling for cell migration.
BAF3/KIT cells expressing WT Csk or control vector were untreated or preincubated with 3 μM LY294002 for 30 minutes. Cells were then tested for migration in the presence of 30 ng/mL SCF in the lower chamber. Cells that migrated into the lower chamber were counted and expressed as a percentage for the whole applied cells. The results are shown as the means ± SD of triplicate cultures. The asterisk represents a significant difference from a control value (P < .01). Similar results were obtained from 3 independent experiments.
SFKs and PI3K cooperate on SCF signaling for cell migration.
BAF3/KIT cells expressing WT Csk or control vector were untreated or preincubated with 3 μM LY294002 for 30 minutes. Cells were then tested for migration in the presence of 30 ng/mL SCF in the lower chamber. Cells that migrated into the lower chamber were counted and expressed as a percentage for the whole applied cells. The results are shown as the means ± SD of triplicate cultures. The asterisk represents a significant difference from a control value (P < .01). Similar results were obtained from 3 independent experiments.
Discussion
Migration is one of the unique and important cell functions undertaken by SCF/KIT system. Much effort has been done to clarify signal transduction leading to SCF/KIT-mediated proliferation and survival, but the signaling mechanism in SCF-mediated cell migration has not been clarified yet. In this study, we determined several critical signaling molecules in SCF-induced cell migration, using a series of 22 YF mutants of KIT.
First, we found that intracellular Ca2+ mobilization plays a pivotal role in SCF-mediated cell migration. It has been reported that Ca2+ mobilization plays a critical role in cell migration of keratinocytes and neutrophils.25 26SCF-induced Ca2+ mobilization was not significantly impaired by most single YF substitution mutants, but it was remarkably abrogated by Y567F and moderately by Y569F and Y719F. In Y567F, Y569F, and Y719F mutants, cell motilities were also inhibited similarly, corresponding to the reduction levels of Ca2+ mobilization. Moreover, Ca2+ chelator completely abolished the migratory activity. These results indicated the importance of Ca2+mobilization in SCF-mediated cell migration, which are largely mediated by Y567, Y569, and Y719 of KIT.
Second, we found that Y567/SFK play an important role in SCF-mediated Ca2+ mobilization and cell migration. The previous study showed that Y567 of KIT is the binding site of SFK.27-29It was also shown that SFK plays an important role in PDGF-induced cell migration.30,31 Therefore, we investigated the roles of SFK on SCF/KIT-mediated Ca2+ mobilization and cell migration. In disagreement with previous reports,27-29 we could not find the direct binding of Lyn or Fyn with KIT after SCF stimulation in either BAF3/KIT cells or 293T/KIT cells (data not shown). However we could show the activation of SFK, Lyn, in 293T/KIT cells after SCF stimulation; this ligand-dependent activation of Lyn was remarkably reduced by the Y567F substitution, indicating that Y567 is critical for SFK activation. Although previous reports determined the importance of SFK by so-called specific SFK inhibitors PP1 or PP2, they might not be suitable for analyzing KIT signaling, because these inhibitors abolished ligand-dependent KIT autophosphorylation and kinase activity at the concentration of 10 to 30 μM in BAF3 cells (data not shown). This observation was supported by the finding that PDGF receptor activation was inhibited by PP1.32Therefore, we further investigated the role of SFK in cells overexpressing Csk. In the previous study, we showed that Csk and mCsk suppressed the activation of SFKs specifically.33,34 In this study, overexpression of Csk or mCsk inhibited SCF-induced Ca2+ mobilization and cell migration in BAF3/KIT cells. These results strongly suggested that SFK is a critical signaling pathway for SCF-mediated cell migration and Ca2+mobilization. In Y567F cells, the activations of p38 MAPK and Erk1/2 were significantly impaired. Expressions of Csk or mCsk also inhibited the activation of p38 MAPK and Erk1/2, suggesting the involvement of SFK in their activations. Furthermore, p38 MAPK inhibitor, SB203580 or MEK1/2 inhibitor, U0126, suppressed cell migration significantly. These data suggested that SFK regulates cell migration through p38 MAPK and Erk1/2. Regarding the relationship between p38 MAPK and Ca2+ mobilization, SB203580 impaired SCF-induced Ca2+ influx, but intracellular Ca2+ chelator BAPTA-AM did not suppress p38 MAPK activation. On the other hand, U0126 did not impair Ca2+ influx, but Ca2+ chelator suppressed MEK1/2 activation. These data indicate that p38 MAPK induces Ca2+ mobilization, thereby resulting in Erk1/2 activation. Previous reports showed that Ca2+ influx activates p38 MAPK and Erk1/2 in human neutrophils or PC12 cells.35,36Recently, it was reported that Y568 of human KIT (corresponding to murine Y567) is important of SFK activation and Shc phosphorylation resulting in Ras/Erk2 activation,37 and p38 MAPK was involved in SCF-mediated cell migration.22 In this report, for the first time, we showed that not only p38 MAPK but also Erk1/2 was involved in SCF-mediated cell migration, and that both MAPKs differentially transduce the signaling from Y567-induced SFK activation to Ca2+ influx and cell migration.
Finally, we showed the Y719/PI3K pathway also regulates SCF-mediated cell migration. Consistent with the previous report,15 we confirmed that Y719 was the binding site for PI3K. In PDGF-induced chemotaxis, PI3K is regarded as a major signaling pathway of Ca2+ influx leading to cell migration.38-40 In our study, Y719F mutant exhibited less effective inhibition of Ca2+ influx and cell migration than Y567F, suggesting that SFK has a more essential role than PI3K. The importance of PI3K was confirmed by the inhibition studies with chemical inhibitors and dominant negative PI3K. BecauseY719F did not show the impaired activation of p38 MAPK or Erk1/2, which were suppressed by the inhibition of SFK, PI3K may act by a different pathway from SFK, for the cell migration followed by Ca2+ mobilization. Furthermore, the PI3K inhibitor and Csk showed synergistic suppression of cell migration, suggesting the cooperation of SFK and PI3K in SCF-mediated migration.
In summary, this study has provided evidence that SFK and PI3K cooperatively contribute to SCF-mediated cell migration through Ca2+ mobilization, and the signaling of SFK is transduced sequentially from p38 MAPK, Ca2+ influx, and then to Erk1/2. Many kinds of growth factors can activate SFK, Erk1/2, p38 MAPK, or PI3K, but this does not always lead to the cell motility. Therefore, the coordination of this signaling by scaffolding proteins could be important for SCF-mediated chemotaxis. Future studies focused on this point will further clarify the signaling mechanism of SCF/KIT-mediated cell migration.
We thank Dr Koji Hashimoto for great help of making YF substitution mutants and their expression vectors, and we thank Noriko Kikunaga for her excellent experimental assistance.
Supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology, the Japanese Ministry of Health, Labor and Welfare, and the Japan Society for Promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yuzuru Kanakura, Department of Hematology and Oncology, Osaka University Graduate School of Medicine, 2-2, Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail:kanakura@bldon.med.osaka-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal