Abstract
Chronic rejection of transplanted allografts is the major cause of graft loss after clinical solid organ transplantation. Recent data link the indirect presentation of allopeptides to chronic graft loss; thus, identification of immunodominant epitopes on major histocompatibility complex (MHC) antigens could significantly contribute to establishing novel ways for monitoring and managing chronic rejection. Here, we show that synthetic allo-MHC–derived peptides covering the polymorphic region 56 to120 of HLA-B7 modulate alloresponses. In particular, the 2 β-pleated sheet-derived peptides covering residues 91 to 105 and 96 to 120, respectively, but not sequences from the α1 helix, were presented by autologous peripheral blood lymphocytes to induce T-cell proliferation. In addition, the 2 β-pleated sheet-derived peptides and the α1-derived peptide residues 60 to 75 abrogated lysis of HLA-B7 target cells by anti–HLA-B7 cytotoxic T lymphocytes (CTLs). Although most residues between 91 and 120 are normally not directly accessible to T cells, our results indicate that peptides derived from the lower surface of the peptide-binding groove of HLA-B7 are immunodominant in HLA-B7 alloresponses. To characterize the binding and stability of allopeptides to T cells, the 62-70 peptide—derived from the 60-75 allopeptide that blocked cytotoxicity of anti–HLA-B7 CTL—was synthesized and coupled with fluorescein isothiocyanate. The peptide specifically labeled anti-B7 CTL, but not anti–HLA-A2 CTL as measured by flow cytometry. Peptide binding to CTL was specific at 4°C and remained stable for 12 hours, whereas it remained stable for less than 2 hours at 37°C. These studies allow the identification of HLA-B7 T-cell epitopes and reveal for the first time a novel, previously unrecognized application of synthetic HLA-derived allopeptides to visualize alloreactive T cells.
Introduction
Structural studies show that T-cell receptor (TCR) contacts bind peptide and large stretches of α1 and α2 major histocompatibility complex (MHC) helices.1,2 In general, endogenous peptides derived from allo-MHC are seen by T cells as foreign-eliciting alloresponses.3 T cells may recognize peptide-induced conformational changes, and they may also recognize surface MHC structures in addition to or independent of bound peptide.4 Furthermore, allospecific cytotoxic T lymphocytes (CTLs) that are peptide-dependent and peptide-specific have been described in the literature.5 In general, however, when donor and recipient MHCs are structurally similar, the endogenous peptide appears to be the dominant target of alloreactive T cells,5-7 a phenomenon backed by clinical data in graft-versus-host disease.8
Synthetic allopeptides have been used in many different models to modulate alloresponses and to study alloreactivity.9-11 In other studies, class I– and class II–derived allopeptides were used to induce tolerance.12-14 By studying a series of rat-derived synthetic allo-MHC peptides, immunodominant regions of allogeneic MHC molecules that modulate graft survival were identified.13 These studies revealed that some regions of MHC class I molecules are not immunogenic and do not modulate alloresponses. Thus, immunodominant peptides are defined here as peptides derived from an allo-MHC molecule and capable of inducing alloresponses. In human studies, peptide sequences that were able to block alloreactive human T cells were reported.15,16 Some of these peptides were found to be immunosuppressive in allogeneic murine combinations,17 though overall their efficacy in vivo has remained unclear.18 Recent studies provide evidence that indirect presentation of allopeptides after clinical organ transplantation is a major mechanism contributing to chronic rejection.19-21 In a large-animal model, Lee et al22 recently reported that indirect presentation of allopeptides promotes the development of cardiac allograft vasculopathy. Thus, better definition and identification of immunodominant sequences on the MHC molecule could significantly enhance the management of patients undergoing allograft vasculopathy.
Dimeric and tetrameric MHC antigens have been extensively used to study antigen-specific T cells.23-26 It has been possible to visualize antigen-specific T cells by flow cytometry and also to study T-cell regulation using MHC multimers.27 Here, we synthesized a series of overlapping HLA-B7–derived peptides and used them to detect the immunodominant regions of this molecule in allogeneic reactions. By directly coupling fluorescein isothiocyanate (FITC) to the synthesized peptide, we could use an immunodominant HLA-B7–derived peptide to visualize anti–HLA-B7 CTL by flow cytometry. The binding of these peptides to CTL was found to be temperature-dependent. It was stable and specific at 4°C but not at 37°C. These studies show the potency of allogeneic synthetic peptides to extend our knowledge of alloreactive T-cell epitopes and to enable the study of their role in graft rejection.
Materials and methods
Synthesis and FITC conjugation of MHC-derived allopeptides
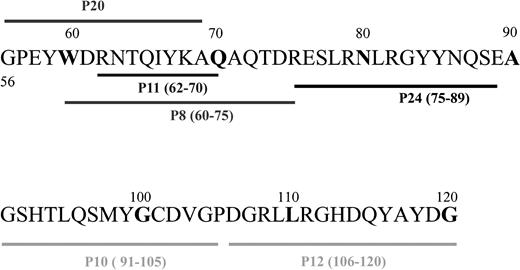
Previous data from our laboratory showed that human- and rat-derived MHC class I sequences from the α1 region (56-120) are immunogenic and are recognized after clinical renal transplantation and rat cardiac transplantation, respectively.12,13,28 On this basis, several HLA-B7–derived peptides covering residues 56 to 120 were synthesized using the FMOC technique, as we previously described in more detail.29 Peptides synthesized and used in these experiments are shown on the sequence alignment in Figure1 and Table1. After synthesis, peptides were cleaved from the resin, and all side chain–protecting groups were removed using a peptide cleavage solution constituting 8.8 mL trifluoro-acetic acid (Millipore, Bedford, MA), 0.5 g phenol (Riedel-de Haen, Seelzel, Germany), 0.2 mL tri-isopropyl silane (Sigma-Aldrich, Steinheim, Germany), and 0.5 mL distilled water. Cleavage was completed after 8 hours. Peptides were finally precipitated and washed 3 times in cooled ether (Merck, Darmstadt, Germany). Peptide analysis and purification were performed according to standard procedure using a C2C18 reversed-phase column (Amersham Pharmacia Biotech, Uppsala, Sweden) by high-pressure liquid chromatography (HPLC).
Sequence alignment of the synthetic HLA-B7–derived peptides.
Peptides covering residues 56 to 120 were synthesized using the FMOC technology.
Sequence alignment of the synthetic HLA-B7–derived peptides.
Peptides covering residues 56 to 120 were synthesized using the FMOC technology.
Sequences of synthetic peptides
| Name . | Residues . | Sequence . |
|---|---|---|
| HLA-B7 | ||
| P8 | 60 -75 | WDRNTQIYKAQAQTDR |
| P10 | 91 -105 | GSHTLQSMYGCDVGP |
| P11 | 62 -70 | RNTQIYKAQ |
| P12 | 106 -120 | DGRLLRGHDQYAYDG |
| P20 | 56 -69 | GPEYWDRNTQIYKA |
| P24 | 75 -89 | RESLRNLRGYYNQSE |
| HLA-A2 | ||
| P3 | 65 -79 | RKVKAHSQTHRVDLG |
| P4 | 75 -89 | RVDLGTLRGYYNQSE |
| P21 | 62 -70 | GETRKVKAH |
| Name . | Residues . | Sequence . |
|---|---|---|
| HLA-B7 | ||
| P8 | 60 -75 | WDRNTQIYKAQAQTDR |
| P10 | 91 -105 | GSHTLQSMYGCDVGP |
| P11 | 62 -70 | RNTQIYKAQ |
| P12 | 106 -120 | DGRLLRGHDQYAYDG |
| P20 | 56 -69 | GPEYWDRNTQIYKA |
| P24 | 75 -89 | RESLRNLRGYYNQSE |
| HLA-A2 | ||
| P3 | 65 -79 | RKVKAHSQTHRVDLG |
| P4 | 75 -89 | RVDLGTLRGYYNQSE |
| P21 | 62 -70 | GETRKVKAH |
To couple FITC to the peptides, the method by Kropshofer et al30 was modified. Peptides attached to the solid support were dissolved in 50 mM borate puffer and were mixed with FITC in a 1:3 molar ratio. In this way, it was possible to couple the peptides and FITC at a 1:1 ratio. The solution was gently shaken for 6 hours, washed 3 times with borate buffer, and cleaved. Peptide purity was verified by HPLC.
Generation and peptide inhibition of alloreactive CTL lines
Anti–HLA-B7 CTLs were raised according to the standard procedure.31 Stimulator and responder combinations are shown on Table 2. CTLs were restimulated every 7 to 10 days and were supplemented with recombinant 30 U/mL interleukin (IL)–2 (a kind gift from Martini, Division Scientifique Rousell Uclaf, Romainville, France) after the second restimulation.
HLA phenotypes and responder-stimulator cell combinations used for generating CTLs
| CTL line . | Application . | HLA phenotype . | |||
|---|---|---|---|---|---|
| JRB7.2 | Responder | A2,26 | B38,56 | C1,− | DR7,11 |
| Stimulator | A3,− | B7,− | DR2,− | ||
| AFB7.2b | Responder | A1,24 | B8,35 | C4,− | DR2,3 |
| Stimulator | A24,− | B7,− | Cw7,− | DR1,− | |
| HNB7.1 | Responder | A26,− | B41,60 | C3,− | DR3,13 |
| Stimulator | A2,3 | B7,− | Cw7,− | DR2,− | |
| AMB7.1 | Responder | A1,2 | B8,57 | C6,− | DR7,12 |
| Stimulator | A1,2 | B7,37 | C6,w7 | DR2,− | |
| UWB7 | Responder | A1,24 | B57,62 | Cw3,6 | DR6,7 |
| Stimulator | A3,− | B7,14 | Cw8/− | DR1,11 | |
| UWA2 | Responder | A1,24 | B57,62 | Cw3,6 | DR6,7 |
| Stimulator | A2,24 | B51,62 | Cw1,3 | DR4,11 | |
| ALA2.1 | Responder | A1− | B8,− | DR3,− | |
| Stimulator | A2,− | B57,− | Cw6 | DR7,− | |
| BCA2 | Responder | A1,− | B8,− | DR3 | |
| Stimulator | A2,24 | B51,62 | Cw1,3 | DR4,11 | |
| BCB7 | Responder | A1,− | B8,− | DR3 | |
| Stimulator | A3,− | B7,14 | Cw8 | DR1,11 | |
| CTL line . | Application . | HLA phenotype . | |||
|---|---|---|---|---|---|
| JRB7.2 | Responder | A2,26 | B38,56 | C1,− | DR7,11 |
| Stimulator | A3,− | B7,− | DR2,− | ||
| AFB7.2b | Responder | A1,24 | B8,35 | C4,− | DR2,3 |
| Stimulator | A24,− | B7,− | Cw7,− | DR1,− | |
| HNB7.1 | Responder | A26,− | B41,60 | C3,− | DR3,13 |
| Stimulator | A2,3 | B7,− | Cw7,− | DR2,− | |
| AMB7.1 | Responder | A1,2 | B8,57 | C6,− | DR7,12 |
| Stimulator | A1,2 | B7,37 | C6,w7 | DR2,− | |
| UWB7 | Responder | A1,24 | B57,62 | Cw3,6 | DR6,7 |
| Stimulator | A3,− | B7,14 | Cw8/− | DR1,11 | |
| UWA2 | Responder | A1,24 | B57,62 | Cw3,6 | DR6,7 |
| Stimulator | A2,24 | B51,62 | Cw1,3 | DR4,11 | |
| ALA2.1 | Responder | A1− | B8,− | DR3,− | |
| Stimulator | A2,− | B57,− | Cw6 | DR7,− | |
| BCA2 | Responder | A1,− | B8,− | DR3 | |
| Stimulator | A2,24 | B51,62 | Cw1,3 | DR4,11 | |
| BCB7 | Responder | A1,− | B8,− | DR3 | |
| Stimulator | A3,− | B7,14 | Cw8 | DR1,11 | |
In addition, anti–HLA-B7 CTLs were raised using allopeptides to pulse autologous antigen-presenting cells (APCs). Peripheral blood lymphocytes (PBLs) were isolated by Ficoll-Hypaque density gradient centrifugation from 5 non–HLA-B7 blood donors. Phytohemagglutinin blasts were grown from the PBLs of each individual and, after 5 days, were pulsed for 1 hour at 37°C with 10 μg/mL peptide compound made by mixing 50 μg each of the HLA-B7–derived peptides shown in Table 1. After irradiation at 20 Gy, the blast cells were cocultivated with autologous PBLs at a 1:1 cell ratio. Cell cultures were supplemented with 30 U/mL recombinant IL-2 and were restimulated every 5 to 7 days.
Cytotoxicity was tested in a standard chromium-release assay.32 To test the effect of various allopeptides on the cytotoxicity of alloreactive CTLs, CTL–target cell ratios of 10:1 were plated on 96-well round-bottomed microtiter plates, and serial dilutions of the peptides were added, respectively. Chromium Cr 51 release was measured after 4-hour incubation.
Peptide pulsing and stimulation of peripheral blood lymphocytes
PBLs (2 × 105) were seeded in 96-well round-bottomed microtiter plates (Life Technologies, Paisley, United Kingdom) in RPMI 1640 medium (Life Technologies) supplemented with 15% AB-serum, 125 μg/mL penicillin, 250 μg/mL streptomycin, and 2 mM glutamine. An equal number of irradiated HLA-B7 stimulator cells (PBL or tumor cell lines) was added, and the cell mixture was left untreated or was supplemented with varying concentrations of peptide P3, P8, P10, P11, or P12. After 4 days of cocultivation, the cells were pulsed with3H-thymidine for 18 hours. Stimulator and responder combinations used are shown in Table 2.
Kinetics of peptide binding to CTL
Serially diluted FITC-labeled peptides 11 and 21 were incubated with anti–HLA-B7–specific CTLs at 4°C for 60 minutes. The cells were washed 3 times with cold phosphate-buffered saline, and cell fluorescence was measured using a FACScan (Becton Dickinson, Mountain View, CA). To test the binding kinetics of the peptides used, peptide-labeled cells were further cultivated at either 4°C or 37°C in culture medium, aliquots of the cells were removed, and cell fluorescence was measured at various times.
Results
Allogeneic immunodominant allopeptides are recognized by indirect presentation
Allopeptides used in these studies ranged between 9 and 16 residues in length. They were synthesized and analyzed after purification by HPLC. Purity of the peptides was greater than 98%. To test whether the peptides were immunogenic in vitro, PBLs from non–HLA-B7 blood donors were gradient-separated and pulsed with serial dilutions of peptide P3, P8, P10, P11, P12, or P20. Cells were irradiated with 20 Gy and mixed with nonirradiated autologous PBLs at a 1:1 cell ratio. PBLs pulsed with P10 (91-105) and P12 (106-120) presented these peptides to autologous T cells, inducing proliferation. A representative assay performed with PBLs from a blood donor whose HLA phenotype was HLA-A2,-B57,-Cw6/-, DR7/- is shown in Figure2A. Proliferation of these 2 peptides was significantly higher than that of untreated cells (P < .0001). As a positive control, Epstein-Barr virus–transformed B cells (HLA-A3,-B7,-C-,-DR-,-) were used as stimulator cells without the addition of peptides. P3 is an HLA-A2–derived peptide (residues 65-79) used as an irrelevant control peptide. Four of 5 blood donors responded to P10 and P12 by T-cell proliferation. None of the other peptides induced T-cell proliferation. To confirm that the peptides were presented indirectly by APCs, PBLs were depleted of APCs as we recently described,28 pulsed with the peptides, and used to stimulate nondepleted autologous PBLs. APC-depleted PBLs failed to present the allopeptides, indicating a requirement for indirect peptide presentation (Figure 2B). Furthermore antibodies against CD4, HLA-DR, and HLA-DP—but not against CD8 or HLA-A, HLA-B, or HLA-C—blocked peptide-induced T-cell proliferation (data not shown).
Allopeptides were immunogenic.
(A) Irradiated autologous PBLs were pulsed with allopeptides and cocultured with nonirradiated autologous PBLs. After 4 days,3H-thymidine uptake was measured. P10 and P12 induced significant cell proliferation (P > .0001). Cells incubated with allogeneic stimulator cells (MLC) but no peptides showed, as expected, the highest proliferation. (B) To test whether APCs were required for peptide presentation, PBLs, purified T cells, APCs, and a mixture of reconstituted APCs and T cells were incubated with P10, and 3H-thymidine uptake was measured. Proliferation was only measured in cells containing APCs, suggesting the requirement of indirect presentation through the APCs. (C) Allopeptides augment allostimulation. Responder PBLs were stimulated in a 4-day mixed-day culture with irradiated allogeneic REH stimulator cells. Cell cultures were supplemented with various peptides, and [3H-thymidine uptake was measured. P10 and P12 induced elevated T-cell proliferation over the control cultures (Med) containing no peptides.
Allopeptides were immunogenic.
(A) Irradiated autologous PBLs were pulsed with allopeptides and cocultured with nonirradiated autologous PBLs. After 4 days,3H-thymidine uptake was measured. P10 and P12 induced significant cell proliferation (P > .0001). Cells incubated with allogeneic stimulator cells (MLC) but no peptides showed, as expected, the highest proliferation. (B) To test whether APCs were required for peptide presentation, PBLs, purified T cells, APCs, and a mixture of reconstituted APCs and T cells were incubated with P10, and 3H-thymidine uptake was measured. Proliferation was only measured in cells containing APCs, suggesting the requirement of indirect presentation through the APCs. (C) Allopeptides augment allostimulation. Responder PBLs were stimulated in a 4-day mixed-day culture with irradiated allogeneic REH stimulator cells. Cell cultures were supplemented with various peptides, and [3H-thymidine uptake was measured. P10 and P12 induced elevated T-cell proliferation over the control cultures (Med) containing no peptides.
To test whether allopeptides modulate mixed lymphocyte reactions, PBLs from a non–HLA-B7 donor (HLA-A2,-B51,60 Cw3,-DR8,12) were cocultivated with irradiated stimulator cells derived from tumor cell line REH (HLA-A23,32 B35,50 Cw4,-DR3,5) at a 1:1 cell ratio. In addition, 50 μg/mL each peptide was added. The control coculture that was incubated with no peptide showed [3H]-thymidine uptake values of 28.500 ± 5.450 cpm. Proliferative changes that occurred after peptides were added to the cultures were expressed as percentages. Results of this responder–stimulator combination are shown in Figure 2C. Cells incubated with either P10 or P12 showed augmented proliferation, suggesting enhanced antigen presentation. However, of the 7 stimulator–responder combinations tested with these peptides, 6 showed strong responses to P10 and only 3 showed strong responses to P12. The results show peptide-induced enhanced proliferation, allowing speculation about whether indirect presentation of donor-derived MHC molecules shed during rejection episodes33 exacerbate rejection by enhancing T-cell sensitization.
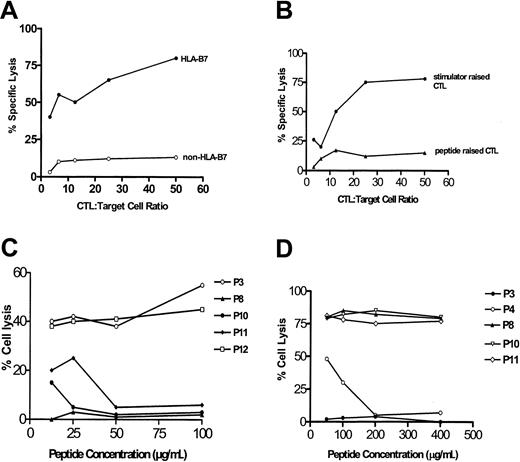
Allopeptides abrogate target cell lysis by anti–HLA-B7 alloreactive T cells
Cytotoxic T cells were generated in vitro according to standard procedures. These CTLs were cytotoxic to HLA-B7 target cells, but not to control cells (Figure 3A). As we reported previously, Epstein-Barr virus–transformed cells, tumor cells, and PHA-blast cells expressing B7 were lysed by a single CTL line or clone, indicating that the specificity of the CTLs was peptide-dependent31 but not peptide-specific. However, allo-MHC–restricted CTLs have been well described and characterized in the literature,34,35 indicating heterogeneity in the specificity of polyclonal alloreactive CTLs.36 We further attempted to raise long-term T-cell cultures using autologous PBLs pulsed with a mixture of the B7-derived peptides. Responder cells used were from non-B7 blood donors. Three CTL lines were raised after repetitive restimulation with peptide-pulsed autologous PBLs. These cell lines were found to be more than 85% CD4+ and showed low cytotoxicity to B7 target cells, as would be expected (Figure 3B). To test whether allopeptides interfered with CTL target cell lysis, CTLs were incubated with serially diluted peptides and51Cr-labeled target cells. P8 (60-75), P10 (91-105), and P11 (62-80) blocked CTL cytotoxicity (Figure 3C). Interestingly, P12 (106-120), which was readily presented in the proliferation assays leading to T-cell proliferation, failed to modulate CTL cytotoxicity in repetitive inhibition assays. In contrast, P8 was not presented in the proliferation assay, but it successfully blocked T-cell lysis of target cells, suggesting that the peptides presumably interacted with different T-cell populations in the 2 assays. HLA-A2–derived control peptide P3 (65-79) did not interfere with T-cell cytotoxicity. Conversely, when anti–HLA-A2 CTL line UWA2 was tested, only the A2-derived peptides P3 (65-79) and P4 (75-89), but not the B7-derived peptides, blocked CTL cytotoxicity, again indicating allospecificity of the peptides rather than their binding to the MHC alleles of the CTLs.
Allopeptides abrogate target cell lysis by anti–HLA-B7 CTLs.
(A) Anti–HLA-B7 CTLs lysed HLA-B7 target cells but not non–HLA-B7 control cells. (B) CTLs raised against stimulator cells efficiently lysed target cells, whereas CTLs raised against HLA-B7–derived synthetic peptides poorly lysed target cells.(C) P8, P10, and P11, but not P3 or P12, blocked cytolysis of target cells by anti–HLA-B7 CTL UWB7 in a concentration-dependent manner. (D) In contrast, P3 and P4—both HLA–A2-derived peptides—blocked target cell lysis by an anti–HLA-A2 CTL line, UWA2. P8, P10, and P11 (HLA–B7-derived peptides) did not modulate target cell lysis.
Allopeptides abrogate target cell lysis by anti–HLA-B7 CTLs.
(A) Anti–HLA-B7 CTLs lysed HLA-B7 target cells but not non–HLA-B7 control cells. (B) CTLs raised against stimulator cells efficiently lysed target cells, whereas CTLs raised against HLA-B7–derived synthetic peptides poorly lysed target cells.(C) P8, P10, and P11, but not P3 or P12, blocked cytolysis of target cells by anti–HLA-B7 CTL UWB7 in a concentration-dependent manner. (D) In contrast, P3 and P4—both HLA–A2-derived peptides—blocked target cell lysis by an anti–HLA-A2 CTL line, UWA2. P8, P10, and P11 (HLA–B7-derived peptides) did not modulate target cell lysis.
Visualization of alloreactive CTLs using FITC-labeled allopeptides
Peptides P11 and P21, covering residues 62 to 70 of HLA-B7 and HLA-A2, respectively, were FITC-labeled, purified, and analyzed. Unlabeled P11 had a retention time of 35.46 minutes, whereas the labeled peptide had a delayed retention time of 44.65 minutes as analyzed by HPLC. The difference in retention times allowed efficient purification of the labeled peptides by HPLC.
To test whether alloreactive CTLs can be labeled by allopeptides, an anti–HLA-B7 CTL line, HNB7.1, was incubated with various concentrations of the FITC-labeled B7 peptide P11 at 4°C. Optimal staining of the cells was achieved with 250 μg/mL peptide (Figure4). Control peptide P21 failed to stain the CTL at this concentration, indicating that P11 was specific for anti–HLA-B7 CTL. However, at 37°C, P21 stained 23% of the CTLs; in contrast, it stained less than 1% at 4°C. This showed a temperature-dependent increase in unspecific binding.
FITC-conjugated allopeptides enable visualization of alloreactive CTLs.
Anti–HLA-B7 CTLs HNB7.1 were incubated with FITC-conjugated P11 or control peptide P21 at 4°C, and fluorescence was measured by flow cytometry. CTLs were successfully labeled by P11, but not by P21. To rule out peptide binding to the MHC alleles on the surfaces of CTLs rather than to the TCR, 2 CTL lines against A2 and B7, respectively, were raised from the same individual. P11 successfully labeled anti-B7 UWB7, whereas P21 labeled anti-A2 CTL only, indicating allospecific binding by the peptides.
FITC-conjugated allopeptides enable visualization of alloreactive CTLs.
Anti–HLA-B7 CTLs HNB7.1 were incubated with FITC-conjugated P11 or control peptide P21 at 4°C, and fluorescence was measured by flow cytometry. CTLs were successfully labeled by P11, but not by P21. To rule out peptide binding to the MHC alleles on the surfaces of CTLs rather than to the TCR, 2 CTL lines against A2 and B7, respectively, were raised from the same individual. P11 successfully labeled anti-B7 UWB7, whereas P21 labeled anti-A2 CTL only, indicating allospecific binding by the peptides.
To further test whether peptide binding to T cells was influenced by CTL phenotype rather than by TCR binding, 2 CTL lines from the same responder (UW) were raised against HLA-A2 and HLA-B7. Peptide binding was tested by flow cytometry. Only UWB7 was labeled by P11 but not by P21. Conversely, UWA2 was labeled by P21 but not by P11, clearly confirming that peptide interaction with CTL was specificity regulated and was not determined by the HLA antigens present on the CTL lines. However, peptide binding could not be blocked by anti-TCR antibodies. This can be explained by the small molecular size of the peptides, which appeared not to be affected by possible steric conformational changes that occur after antibody binding to the TCR.
To test the efficiency and specificity of P11 to label different CTL lines, 6 CTL lines were generated in vitro. All CTLs were cytotoxic to HLA-B7 target cells, as tested in a 4-hour 51Cr release assay. In addition, 3 anti–HLA-A2 CTL lines were used as control CTLs. Staining of the CTLs with the FITC-labeled peptide varied among the different CTL lines and ranged from 21.2% to 97%, as shown on Table3. This indicates that binding of alloreactive CTLs to allopeptides is variable, presumably determined by apparent affinities of the particular CTL lines studied. In contrast, the anti–HLA-A2 CTL lines were poorly stained by P11.
Comparison of the peptide-binding capacities of different CTL lines to FITC-labeled P11 and P21
| CTL line . | Cells positive for P11 (%) . | Cells positive for P21 (%) . |
|---|---|---|
| Anti-HLA-B7 | ||
| AMB7.1 | 70.2 | 2.1 |
| HNB7.1 | 97.3 | 4.4 |
| JRB7.2 | 36.8 | 12.4 |
| AFB7.2b | 21.2 | 6.5 |
| UWB7 | 90.5 | 2.5 |
| BCB7 | 80.0 | 1.5 |
| Anti-HLA-2 | ||
| ALA2.1 | 1.2 | 2.9 |
| UWA2 | 0.1 | 8.4 |
| BCA2 | 0.5 | 15.5 |
| CTL line . | Cells positive for P11 (%) . | Cells positive for P21 (%) . |
|---|---|---|
| Anti-HLA-B7 | ||
| AMB7.1 | 70.2 | 2.1 |
| HNB7.1 | 97.3 | 4.4 |
| JRB7.2 | 36.8 | 12.4 |
| AFB7.2b | 21.2 | 6.5 |
| UWB7 | 90.5 | 2.5 |
| BCB7 | 80.0 | 1.5 |
| Anti-HLA-2 | ||
| ALA2.1 | 1.2 | 2.9 |
| UWA2 | 0.1 | 8.4 |
| BCA2 | 0.5 | 15.5 |
Extent of T-cell binding by peptides P11 and P21 was compared using 6 anti-HLA-B7 and 3 anti-HLA-A2 CTL lines by flow cytometry. Anti-HLA-B7 CTLs differentially bound P11, whereas recognition of P21 was significantly low in all cases. However, P11 failed to recognize the anti-HLA-A2 CTL lines.
Several factors govern the binding of any ligand to its receptor, including affinity and temperature, which regulate binding-off rates and specificity. HNB7.1 CTLs were labeled with P11 as described above, and the cells were further incubated at 4°C or at 37°C. Cells were removed at various time points, and cell fluorescence was measured by flow cytometry (Figure 5). At 4°C, binding intensity of the peptide to the CTL remained stable up to at least 12 hours. In these experiments, cell vitality was greater than 95% at each time point. In contrast, at 37°C, cell fluorescence had declined by more than 45% after 2 hours and by 60% after 5 hours. Thus, the off-rates at 37°C appeared to be much higher than they did at 4°C.
CTL binding to allopeptides is temperature dependent.
Anti–HLA-B7 CTLs were labeled by P11 and were incubated at either 4°C or 37°C for 24 hours. At 4°C, the CTL binding to P11 was fairly stable for 12 hours but was much less stable at 37°C. Control peptide P21 remained low at both temperatures.
CTL binding to allopeptides is temperature dependent.
Anti–HLA-B7 CTLs were labeled by P11 and were incubated at either 4°C or 37°C for 24 hours. At 4°C, the CTL binding to P11 was fairly stable for 12 hours but was much less stable at 37°C. Control peptide P21 remained low at both temperatures.
Discussion
Alloreactivity may result from the large number of MHC–peptide complexes found in normal cells, estimated as greater than 2000,37 to which a responding allogeneic T-cell population is not tolerant. The additive frequency of T cells reactive with such complexes on the allogeneic cell may be high.38
The complexity of the molecular requirements of alloreactivity have been described in great depth.6,7,39 A high proportion of alloreactive T cells reacts with antigenic sequences on allogenic MHC rather than with the endogenous peptide.36,40 These T cells are peptide-dependent but not peptide-specific. However, a subpopulation of alloreactive T cells is peptide-dependent and peptide-specific.35,36,41 This specificity is often driven by the molecular differences between donor and responder cells. When the structural differences between stimulator and responder MHC antigens are significant, T cells are often peptide-dependent but not peptide- specific. However, when responder and donor MHCs are structurally identical or similar, the endogenous peptide often dominates as the antigenic structure against which alloreactive T cells develop.6 Clinical evidence of the existence of peptide-specific alloreactive T cells is best illustrated in graft-versus-host disease,8 in which T cells react to minor histocompatibility antigens presented by MHC antigens. Unlike studies using mutagenesis to define epitopes of alloreactive T cells on the MHC molecule,4 we show here that synthetic peptides are useful candidates for defining MHC allospecificity. Interestingly, despite the differences in approach, both methods identify similar regions as immunogenic.
The polymorphic MHC region covering residues 56 to 120 was chosen on the basis of our own previous data and those of others.9,13,28 In these earlier experiments, it was demonstrated that peptides derived from the α1 region were immunogenic in allogeneic combinations. For example, Fangmann et al42 successfully demonstrated that animals presensitized by peptides from the α1 region of a class I molecule sensitized the animals to reject skin allografts more rapidly than did nonsensitized animals or those sensitized by a peptide derived from the α2 region. This result indicated immunogenic differences between the α1 and α2 helices of MHC class I molecules. Our current data demonstrate that peptide sequences 91 to 105 and 106 to 120 were presented and led to T-cell proliferation. This result is interesting because this region is normally not accessible to alloreactive T cells in conformationally folded membrane-bound MHC antigens. Therefore, the result suggests that the immune system warrants the recognition of those regions of allogeneic MHC molecules normally not directly accessible to T cells by indirect presentation to T cells. In contrast, the α-helices that directly come in contact with T cells immunize by direct recognition, indicating a dichotomy in antigen recognition of peptides derived from different regions of allo-MHC molecules.
Indirect presentation of MHC molecules during graft rejection is an important mechanism that occurs during acute rejection and particularly during chronic rejection.43,44 Recently, Lee et al22 demonstrated that indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy in an inbred miniature swine model. Pigs immunized with swine leukocyte class I allopeptides showed in vitro proliferative responses and in vivo delayed-type hypersensitivity responses to the allogeneic peptides. More important, donor MHC class I disparate hearts transplanted into peptide-immunized cyclosporine-treated pigs not only were rejected more quickly than in unimmunized cyclosporine-treated controls, they developed obstructive fibroproliferative coronary artery lesions much earlier than did controls. These results for the first time link indirect presentation of alloantigen to clinical vasculopathy in a large animal model. Thus, the elevated donor-derived soluble MHC measured during rejection episodes and inflammation in general33 may contribute to the sensitization of even more T cells, exacerbating chronic rejection.
Of all the peptides used in this study, P10 (residues 91-105) was best presented by HLA-A2 responder APCs to autologous T cells. This is interesting because this peptide contains the anchor positions for HLA-A2, leucine (residue 95) and valine (residue 103). Sequence alignment of P10 to the hepatitis B T-cell epitope described by Bertoletti et al45 shows similarity of these 2 peptides to each other at 3 positions, including the anchor residues. The fact that this peptide was well presented to T cells and successfully blocked alloreactive T cells clearly confirms the relevance of this sequence as an immunogenic T-cell epitope.
MHC tetramers have been extensively used to identify antigen-specific T cells23 and, more recently, peptide-specific alloreactive T cells.35 However, synthetic peptides have virtually never been used to visualize alloreactive T cells. We therefore developed a novel method for labeling synthetic peptides with FITC. Our data show that the peptide derived from residues 62 to 70 sufficiently visualized anti–HLA-B7 CTLs in specific fashion but did not visualize irrelevant anti–HLA-A2 CTLs. There was low background staining at 4°C. However, the binding capacity of CTLs was highly variable among the anti–HLA-B7 CTLs studied (range, 13.5%-97%). This variance might be attributed to several factors. First, we used polyclonal CTL lines in these experiments, implying that the CTL population in these cultures may be heterogenous and may display different specificities on the HLA-B7 molecule. A CTL line with a high proportion of peptide-specific CTLs would stain less than T cells that predominantly recognize the α-helices. Second, it has recently been shown that the antigen-binding abilities of naive, memory, and activated T cells vary greatly.46 Activated T cells have a 50-fold higher affinity for antigen than do naive T cells. Although the cells used here were long-term CTLs, possible differences in their affinities for alloantigen cannot be ruled out, explaining the varying binding differences of the FITC-labeled peptide. In addition, because different responder–stimulator combinations were used to raise these CTLs, it is probable that the CTLs recognize different epitopes on HLA-B7. In spite of this it cannot be ruled out that some peptides may bind to the HLA molecules on the CTLs, even though our data suggest that this may not be a major problem.
The ability of the peptide-stained T cells to maintain their fluorescence was temperature-dependent. As observed recently with MHC–immunoglobulin complexes,46 the specificity of peptide binding was significantly reduced at 37°C. At this temperature, half the cells lost their fluorescence after 2 hours, possibly because of internalization, whereas fluorescence remained stable for 12 hours at 4°C. The affinities and off-rates of allopeptides are, therefore, highly temperature-dependent. Altogether, the current data indicate that it is feasible to identify CTL epitopes using synthetic peptides and that immunodominant peptides can be applied to visualize alloreactive T cells. In addition, allopeptides have the potential to regulate T cells by blocking their cytotoxicity against target cells.
We thank Kyoung-Ae Yoo Ott for her tremendous technical support in generating CTL lines and performing flow cytometry during the final stages of manuscript completion.
Supported by a grant from the Deutsche Forschungsgemeinschaft ZA (131/7-2), Bonn, and a generous grant from the Volkswagen Stiftung (AZ:I/72 735), Hannover, Germany. This study was performed at the Institute of Immunology at the University of Kiel, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicholas Zavazava, Department of Internal Medicine, University of Iowa Hospitals and Clinics, C51-F, 200 Hawkins Dr, Iowa City, IA 52242; e-mail: nicholas-zavazava@uiowa.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal