Abstract
The idiotype protein, secreted by myeloma plasma cells, is a tumor-specific but weak antigen. Idiotype-based immunotherapy has been explored in myeloma patients with disappointing results. It is conceivable that myeloma cells contain a multitude of tumor antigens that can more effectively stimulate antitumor T cells. To explore the possibility of using whole myeloma cells as a source of tumor antigens for immunotherapy, the current study was undertaken to generate and examine the function of myeloma-specific cytotoxic T lymphocytes (CTLs) by using dendritic cells (DCs) pulsed with myeloma cell lysates as stimulating cells. After repeated stimulation, specific CTL lines, containing CD4+ and CD8+ T cells, were generated from myeloma patients. Our results show that these T cells not only recognized and lysed autologous myeloma protein–pulsed DCs, they also killed autologous primary myeloma cells. Occasionally, CTLs responded to autologous idiotype–pulsed DCs and to allogeneic primary myeloma cells. No cytolytic activity, however, was detected against autologous lymphocytes including B cells, suggesting that the T cells acted specifically against myeloma cells. Cytotoxicity against target cells was major histocompatibility complex class 1 and, to a lesser extent, class 2 restricted and was dependent mainly on the perforin-mediated pathway. CTLs secreted predominantly interferon-γ and tumor necrosis factor-α on antigenic stimulation, indicating a type 1 T-cell subset. These findings represent the first demonstration that tumor cell lysate–primed CTLs kill only myeloma cells, not autologous lymphocytes. This provides a rationale for myeloma cell–based immunotherapy in multiple myeloma.
Introduction
Multiple myeloma (MM) is a B-cell malignancy accounting for 1% of all cancers and 10% of all hematologic malignancies. With conventional chemotherapy, complete remission rates have not exceeded 5%, and median survival time has not been extended beyond 3 years.1,2 High-dose chemotherapy followed by stem cell support results in long-term disease-free survival in one third of myeloma patients.3 However, relapse of the underlying disease remains the primary cause of treatment failure.4Therefore, novel alternative therapeutic strategies aiming at improving the outcome in these patients are necessary.
Vaccines based on immunization against one or more specific tumor antigens or on adoptive transfer of ex vivo–generated lymphocytes with high levels of specific reactivity against tumor antigens represent an attractive approach.5-8 In MM, the idiotype (Id) determinant of the paraprotein can be regarded as a tumor-specific marker, and it has been used for immunotherapy.9-14Although modest and transient, Id-specific immune responses can be induced in 25% to 100% of myeloma patients. However, clinical responses, defined by significant reductions in M components, have been observed in few patients.11,13 This may be attributed to the fact that Id is a weak antigen15 and that high concentrations of circulating Id protein in myeloma patients might suppress or even deplete Id-specific T cells.16 Thus, a search for other myeloma antigens is warranted for the development of more efficient immunotherapy strategies in this disease.
Myeloma cells contain a multitude of tumor antigens that can stimulate an increased repertoire of antitumor T cells. The objectives of this study were to generate myeloma cell–specific cytotoxic T lymphocytes (CTLs) from myeloma patients and to examine the efficacy of these T cells in the lysis of their target cells, among them tumor lysate–pulsed dendritic cells (DCs), autologous primary myeloma cells, and normal blood lymphocytes including B cells. Specific CTLs were generated using autologous adherent peripheral blood mononuclear cell–derived DCs pulsed with myeloma lysates as antigen-presenting cells (APCs). We show for the first time that myeloma lysate–pulsed DCs reproducibly generate myeloma cell–specific CTLs capable of killing autologous primary myeloma cells but not autologous lymphocytes.
Materials and methods
Isolation of primary myeloma plasma cells and preparation of tumor lysate
Samples from 4 patients (2 male, 2 female) with advanced MM (stages IIB-III, 2 with IgG and 2 with IgA myeloma) were used in this study. The patients were chosen simply because enough primary myeloma plasma cells had been obtained to complete this study. Samples (bone marrow aspirates, leukapheresis of peripheral blood and serum) had been obtained before the initiation of high-dose chemotherapy and autologous transplantation. Absolute blood CD4 count in these patients was 500 to 750 cells/μL at the collection of mononuclear cells.
Fresh bone marrow aspirates from the patients were collected, and bone marrow mononuclear cells were separated using Ficoll-Hypaque (Amersham Pharmacia Biotech AB, Uppsala, Sweden) density centrifugation. Bone marrow mononuclear cells from the patients contained more than 90% CD138+CD38++ myeloma plasma cells, determined by flow cytometry analysis. Aliquots of the myeloma plasma cells were cryopreserved in −80°C until use.
Myeloma cell lysate was prepared by 5 rounds of freezing and thawing to lyse the myeloma cells. Nuclear debris was removed by centrifugation (600 rpm, 10 minutes). Cell lysate was filtered, and the protein was quantified before use.
Isolation of idiotype protein
Affinity chromatography column prepared with antihuman immunoglobulin (Ig)A (α-chain specific) monoclonal antibody–conjugated agarose (A2691; Sigma, St Louis, MO) was used to isolate and purify IgA Id protein from serum obtained from 2 patients with IgA myeloma. A Hi-Trap affinity column packed with 1 mL Protein-G Sepharose High Performance (Amersham Pharmacia Biotech AB) was used to isolate and purify IgG Id protein from serum of the other 2 patients with IgG myeloma. Protein purity was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Isoelectric focusing estimated that more than 90% of the purified IgG or IgA was monoclonal.
Immunophenotyping
Immunophenotyping was carried out on all the cultured cells using a fluorescence-activated cell sorter scan (FACScan; Becton Dickinson, San Jose, CA) and was analyzed using the Lysis II program. Briefly, cells were first washed twice in washing buffer (phosphate-buffered saline + 2% bovine serum albumin and 0.5% sodium azide). Phycoerythrin- or fluorescein isothiocyanate–conjugated monoclonal antibodies (Immunotech, Marseilles, France), at the manufacturer's recommended concentrations, were added to each cell pellet, and the cells were incubated on ice for 30 minutes before they were washed 3 times and were resuspended in phosphate-buffered saline ready for analysis. Controls consisted of cells stained with irrelevant mouse IgG antibodies.
Generation of dendritic cells
Peripheral blood mononuclear cells (PBMCs) from the same 4 patients were isolated from leukapheresis-collected blood cells using Ficoll-Hypaque (Amersham Pharmacia Biotech AB) gradient centrifugation. Aliquots of PBMCs were cryopreserved in −80°C until use. Cryopreserved PBMCs were recovered and resuspended at 5 × 106 cells/mL in RPMI 1640 supplemented with 10% fetal calf serum, 1 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (referred to as complete medium) (Gibco, Grand Island, NY). PBMCs were incubated in a humidified incubator at 37°C in 5% CO2 for 2 hours to allow cell adhesion. Nonadherent cells were removed by gentle washes. Adherent cells were then cultured in complete medium supplemented with granulocyte macrophage–colony-stimulating factor (100 ng/mL) and interleukin (IL)–4 (100 ng/mL; both from Schering-Plough Research Institute, Kenilworth, NJ) for 7 days, with the further addition of cytokines on days 3 and 5. During culture, tumor lysates of autologous or allogeneic myeloma cells (both at 100 μg/mL) were added on days 1 and 5 to pulse the cells. After 7 days of culture, cells were harvested and phenotypically characterized to assure they met the typical phenotype of immature DCs—that is, CD3−, CD4−, CD14−, CD19−, CD83−, CD1a+, CD40+, CD80+, CD86+, HLA-DR+, and HLA-ABC+.17 18
Generation of tumor lysate–specific cytotoxic T lymphocytes
CTLs were elicited from autologous PBMCs using repeated stimulation by DCs pulsed with myeloma cell lysates. Washed PBMCs (2 × 106/mL) were resuspended in complete medium containing recombinant IL-2 (30 IU/mL; Chiron, Emeryville, CA) and IL-7 (5 ng/mL; R&D Systems, Minneapolis, MN), and lysate-pulsed autologous DCs (2 × 105/mL) were added to primary cultures. Cells were incubated in 50-mL tissue culture flasks at 37°C in 5% CO2–95% air. Responder cells were restimulated with lysate-pulsed autologous DCs every 2 weeks, and the cultures were fed every 5 days with fresh medium containing recombinant IL-2 and IL-7. After 3 to 4 cycles of antigen stimulation and selection, T-cell lines were established, and cells were expanded in complete medium containing recombinant IL-2 (30 IU/mL) and IL-7 (5 ng/mL) for 2 weeks and were subjected to functional tests.
Proliferation assays
T cells (5 × 104/100 μL/well) were seeded into 96-well flat-bottomed tissue culture plates. DCs pulsed with Id protein or lysates of the autologous tumor and an allogeneic tumor from another patient or primary myeloma cells were added to cultures in different concentrations, and cultures were incubated at 37°C in 5% CO2–95% air for 4 days. T-cell proliferation was measured using overnight incubation with [3H]-thymidine (0.0185 MBq/well; Amersham Pharmacia Biotech, Piscataway, NJ). Results from triplicate cultures are given as the arithmetic means.
Enzyme-linked immunospot assay
Detailed methods of the enzyme-linked immunospot (ELISPOT) assay for the enumeration of antigen-specific cytokine-secreting cells have been described.19 20 Briefly, plates (Millititer-HAM; Millipore, Bedford, MA) were coated with mouse monoclonal antihuman IFN-γ (1 μg/mL), IL-4 (2.5 μg/mL), or TNF-α (2.5 μg/mL) (R&D Systems). Cultured T cells (104 cells/well) were added and incubated with Id- or lysate-pulsed DCs or with primary myeloma cells for 36 hours at 37°C. Cells incubated without target cells or with phytohemagglutinin (10 μg/mL; Sigma) were used as controls. After incubation, cells were detached from the plates by washing, and polyclonal antihuman IFN-γ (250 ng/mL), IL-4 (1 μg/mL) (R&D Systems), or TNF-α (4 μg/mL; Serotec, Oxford, United Kingdom) was added and incubated for 2 hours. Spots were developed by sequential incubation with biotinylated antigoat (1/500; IFN-γ and IL-4) or antirabbit (1/100; TNF-α) antibodies (Vector Laboratories; Burlingame, CA), followed by peroxidase staining, using the substrate 3-amino-9-ethyl-carbazol (Sigma). Spots corresponding to the cytokine-secreting cells were enumerated under a dissection microscope (Stemi SV6; Zeiss, Jena, Germany). All samples were run in duplicate. Data are expressed as the mean number of cytokine-secreting cells per 104 T cells.
Cytotoxicity assays
Target cells included autologous DCs pulsed with autologous or allogeneic tumor lysates or Id proteins, thawed autologous or allogeneic primary myeloma cells from another patient, and Ficoll-Hypaque centrifuged to get rid of dead cells, autologous PBMCs, purified peripheral blood B cells, or Epstein-Barr virus (EBV)–transformed B cells from the patients. Cells were labeled with chromium Cr 51 for 1 hour and with 1 × 104target cells/well mixed with various numbers of effector cells in a standard 4-hour cytotoxicity assay using 96-well round-bottomed plates. All assays were performed at least in triplicate. Results are shown as mean percentage 51Cr release calculated as follows: [(sample counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100. Spontaneous release was less than 25% of the maximum 51Cr uptake.
To determine whether cytolytic activity was restricted by major histocompatibility complex (MHC) class 1 or 2 molecules, antibodies against HLA-ABC (W6/32; Serotec) or HLA-DR (B8.12.2; Immunotech) and an isotopic control mouse IgG (Immunotech) at 10 μg/mL were added to the cultures at the initiation of the assay.
Inhibition of perforin- and Fas-mediated pathways of cytolysis
Concanamycin A (CMA; Sigma), an inhibitor of vacuolar type H+-adenosine triphosphate (ATPase), and Brefeldin A (Sigma) were used as selective inhibitors of perforin-mediated and Fas-mediated cytotoxicity,21 respectively. Effector cells were pretreated with 100 nM CMA or 10 μM Brefeldin A for 2 hours and were assayed for cytotoxicity in the presence of the drugs.
Results
Specific proliferative T-cell response against tumor lysate–pulsed dendritic cells
DCs were successfully generated from peripheral blood adherent cells of myeloma patients after 7 days of culture. Immunophenotyping showed that the cells were CD3−, CD4−, CD14−, CD19−, CD83− and CD1a+, CD40+, CD80+, CD86+, HLA-DR+, HLA-ABC+ (data not shown).
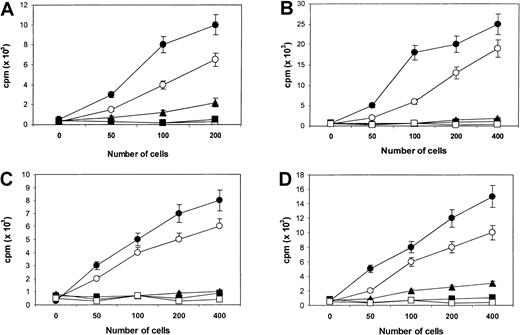
We initially examined the immune response of unstimulated autologous T cells to myeloma proteins in primary cultures. No significant proliferative response was observed in any of the repeated experiments using either freshly recovered PBMCs or IL-2 (50 IU/mL) pretreated PBMCs (data not shown). However, after repeated rounds of T-cell stimulation with myeloma lysate-pulsed DCs, specific T-cell lines were generated and propagated from PBMCs of the myeloma patients. Specific proliferative responses were observed when T cells were rechallenged with the autologous tumor lysate–pulsed DCs and, to a lesser extent, autologous primary myeloma cells (Figure1A-D). Interestingly, a weak but positive proliferative response to autologous Id-pulsed DCs was also observed in the T cells from patients 1 and 4, suggesting that the CTLs contained Id-specific T cells. No proliferative response was noted when T cells were restimulated with autologous DCs or DCs pulsed with the allogeneic tumor lysates. These results, therefore, suggested that the autologous T cells recognized patient-specific immune epitope(s) of myeloma antigens, presented by either primed DCs or primary myeloma cells.
Proliferative response (cpm) of CTL lines.
Responses generated from patients 1 (A), 2 (B), 3 (C), and 4 (D), in reply to DCs pulsed with the autologous Id protein (▴), tumor lysate (●), or autologous primary myeloma cells (○). Controls included DCs alone (▪) and DCs pulsed an allogeneic tumor lysate from another patient (■). In this study, patients 1 and 2 served as controls for each other, as did patients 3 and 4. Results are representative of 3 independent experiments.
Proliferative response (cpm) of CTL lines.
Responses generated from patients 1 (A), 2 (B), 3 (C), and 4 (D), in reply to DCs pulsed with the autologous Id protein (▴), tumor lysate (●), or autologous primary myeloma cells (○). Controls included DCs alone (▪) and DCs pulsed an allogeneic tumor lysate from another patient (■). In this study, patients 1 and 2 served as controls for each other, as did patients 3 and 4. Results are representative of 3 independent experiments.
The T-cell proliferative responses observed in the patients were partially blocked by monomorphic monoclonal antibody to MHC class 1 and 2 molecules. T-cell responses induced by DCs pulsed with tumor lysates were inhibited by MHC class 1 (HLA-ABC) and class 2 (HLA-DR) antibodies (50%-70% inhibition), whereas the response induced by primary myeloma cells was inhibited predominantly by the MHC class 1 antibody (60%-80% inhibition). A combination of MHC class 1 and 2 antibodies produced a synergistic (greater than 90%) inhibition of the proliferative response against pulsed DCs. These results indicate that the recognition of T-cell epitopes in tumor lysates presented by DCs was mediated through MHC class 1 and 2 molecules, whereas those presented by primary myeloma cells were mainly on the class 1 molecules. Consistent with these findings, flow cytometry analysis showed that the tumor lysate–primed T-cell lines consisted of approximately 45% CD8+ and 55% CD4+ T cells. No CD16+ or CD56+ NK cells were present in these cell lines.
Cytokine production of the T cells
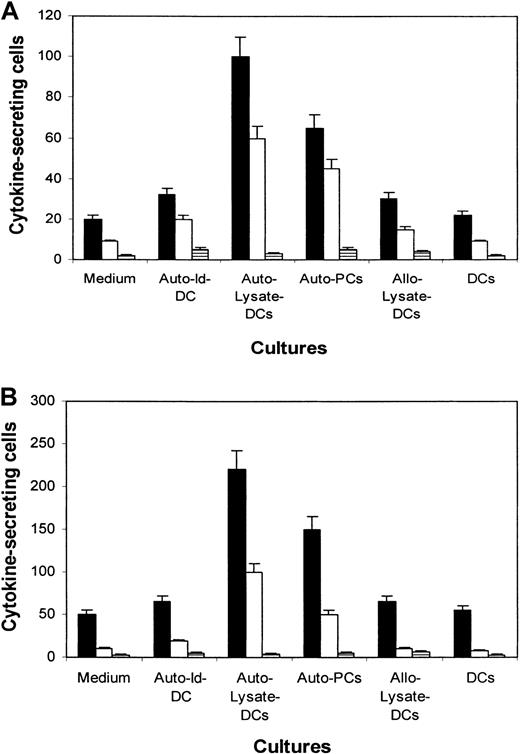
To characterize the cytokine-secretion profile and its corresponding subsets of T cells, an ELISPOT assay was used to detect cytokine secretion. As exemplified by the experiments depicted in Figure 2A,B using T cells from patients 1 and 2, small numbers of IFN-γ– and TNF-α–secreting cells were detected in the unstimulated T cells. After rechallenge with tumor lysate–pulsed autologous DCs or primary myeloma cells, elevated numbers of IFN-γ– and TNF-α–secreting cells were demonstrated. IL-4–secreting cells were rare or undetectable. T cells stimulated with autologous Id–pulsed DCs had slight increases in the numbers of IFN-γ– and TNF-α–secreting cells. Incubation with nonpulsed DCs or with DCs pulsed with the allogeneic myeloma protein did not significantly alter the number of cytokine-secreting cells. These results were reproduced in repeated experiments and with cells from the other 2 patients (data not shown), and they indicate that the effector cells were type-1 T cells.22 23
ELISPOT assay showing the number of cytokine-secreting cells per 104 CTLs.
Cells were generated from patients 1 (A) and 2 (B), in medium or induced by DCs pulsed with the autologous Id protein (Auto–Id-DC), tumor lysate (Auto–Lysate-DCs), allogeneic tumor lysate (Allo–Lysate-DCs), autologous primary myeloma cells (Auto-PCs), and unpulsed DCs (DCs). Cytokines examined are IFN-γ (▪), TNF-α (■), and IL-4 (▤).
ELISPOT assay showing the number of cytokine-secreting cells per 104 CTLs.
Cells were generated from patients 1 (A) and 2 (B), in medium or induced by DCs pulsed with the autologous Id protein (Auto–Id-DC), tumor lysate (Auto–Lysate-DCs), allogeneic tumor lysate (Allo–Lysate-DCs), autologous primary myeloma cells (Auto-PCs), and unpulsed DCs (DCs). Cytokines examined are IFN-γ (▪), TNF-α (■), and IL-4 (▤).
Cytolytic activity of the T cells against tumor lysate–pulsed dendritic cells and primary myeloma cells in vitro
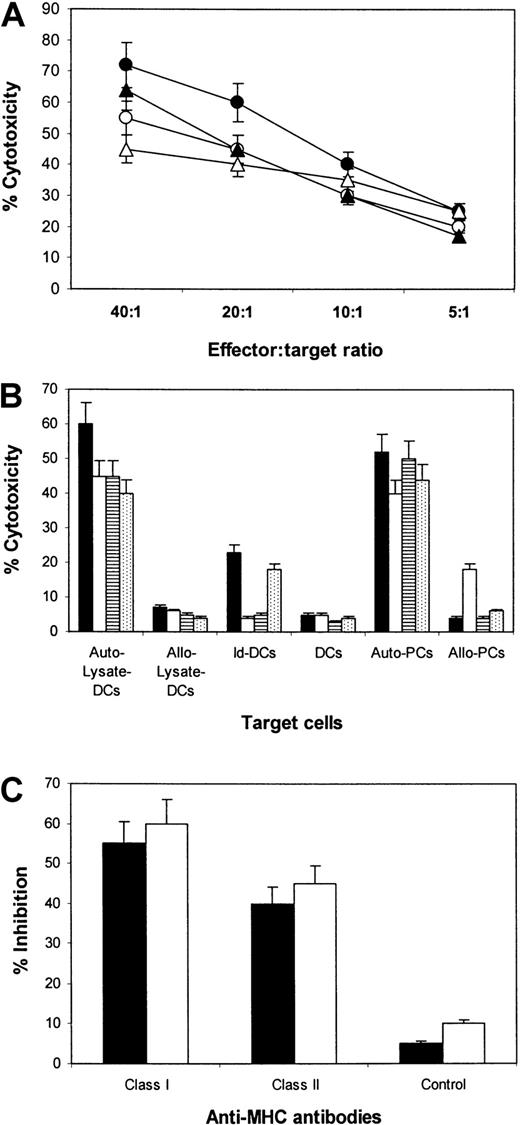
To test whether a specific CTL response could be effectively induced with tumor lysate–pulsed DCs, we assessed the cytotoxic activity of the T cells in vitro. Autologous DCs and DCs pulsed with autologous or allogeneic myeloma protein were used as target cells. As shown in Figure 3A, the CTL lines lysed the autologous DCs pulsed with myeloma lysate. No significant killing of autologous unpulsed DCs or DCs pulsed with the allogeneic myeloma cell lysates was observed (Figure 3B). Results indicate that the T cells were able to mediate tumor- and patient-specific cytotoxicity. As expected, when we used autologous Id–pulsed DCs as target cells, we noted that the CTLs from patients 1 and 4, but not patients 2 and 3, lysed this target (Figure 3B), suggesting that Id protein was part of the immune epitopes that had been presented to T cells.
Cytotoxicity of CTLs against DCs pulsed with autologous tumor lysate.
(A) Cytotoxicity of CTL lines generated from patients 1 (●), 2 (○), 3 (▴), and 4 (▵) against DCs pulsed with autologous tumor lysate at different E:T ratios. (B) Comparison of the cytotoxicity of CTLs from patients 1 (▪), 2 (■), 3 (▤), and 4 (⊡) against DCs pulsed with autologous tumor lysate (Auto–Lysate-DCs), allogeneic tumor lysate from another patient (Allo–Lysate-DCs), autologous Id protein (Id-DCs), unpulsed DCs (DCs) and autologous (Auto-PCs), or allogeneic primary myeloma cells (Allo-PCs). The E:T ratio was 20:1. (C) Inhibition by antibodies against MHC class I and class II or an isotypic control IgG on the cytotoxicity of CTLs from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 4 independent experiments.
Cytotoxicity of CTLs against DCs pulsed with autologous tumor lysate.
(A) Cytotoxicity of CTL lines generated from patients 1 (●), 2 (○), 3 (▴), and 4 (▵) against DCs pulsed with autologous tumor lysate at different E:T ratios. (B) Comparison of the cytotoxicity of CTLs from patients 1 (▪), 2 (■), 3 (▤), and 4 (⊡) against DCs pulsed with autologous tumor lysate (Auto–Lysate-DCs), allogeneic tumor lysate from another patient (Allo–Lysate-DCs), autologous Id protein (Id-DCs), unpulsed DCs (DCs) and autologous (Auto-PCs), or allogeneic primary myeloma cells (Allo-PCs). The E:T ratio was 20:1. (C) Inhibition by antibodies against MHC class I and class II or an isotypic control IgG on the cytotoxicity of CTLs from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 4 independent experiments.
To determine MHC restriction of these CTL lines, we studied the inhibitory effect of anti-MHC antibodies on their cytolysis activity. Blocking of MHC class 1 (HLA-ABC) or class 2 (HLA-DR) molecules on the target cells resulted in an inhibition of the cytotoxicity of the CTL against autologous lysate–pulsed DCs (Figure 3C; data from patients 1 and 2). The result further confirmed that the immune epitopes within myeloma proteins were presented in association with MHC class 1 and class 2 molecules.
To evaluate whether the tumor lysate–specific immune response is relevant to the clinical antimyeloma effect, we examined whether CTLs could lyse autologous primary myeloma cells. Using the CTL lines generated from the patients as effector cells, significant killing of the autologous myeloma cells was observed (Figure 3B). CTLs from patient 2 also killed allogeneic primary myeloma cells (from patient 1), though to a lesser extent, suggesting that there were shared myeloma antigens and MHC molecules between these 2 patients. Blocking of MHC class 1 but not of MHC class 2 molecules of the target cells inhibited significantly (more than 80%) the cytotoxicity of the CTLs. Thus, the results demonstrate that tumor lysate–primed CTLs are capable of mediating strong, MHC class 1–restricted cytotoxicity against autologous primary myeloma cells in vitro.
Cytolytic activity of the T cells against autologous lymphocytes
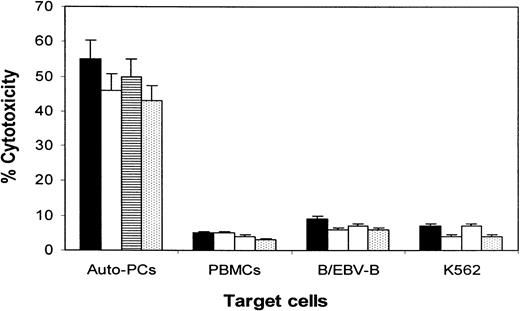
To evaluate whether tumor lysate–primed CTLs might be killing normal blood cells, especially B cells, we performed experiments to examine the cytotoxicity of CTLs against autologous PBMCs, purified B cells, and EBV-transformed B cells, and we compared the results with cytotoxicity against primary myeloma cells. As shown in Figure4, CTLs killed only the primary myeloma cells. No cytolytic activity was observed against autologous PBMCs, B cells, or NK-sensitive target cells K562. Thus, these results suggest that the CTLs specifically targeted myeloma cells.
Cytotoxicity of the CTLs against autologous primary myeloma plasma cells.
Shown are cytotoxicity of CTL lines generated from patients 1 (▪), 2 (■), 3 (▤), and 4 (⊡) against autologous primary myeloma plasma cells (Auto-PCs), PBMCs, purified B cells or EVB-transformed B cells, and K562. The E:T ratio was 20:1. Data are expressed as the mean ± SEM of 2 independent experiments.
Cytotoxicity of the CTLs against autologous primary myeloma plasma cells.
Shown are cytotoxicity of CTL lines generated from patients 1 (▪), 2 (■), 3 (▤), and 4 (⊡) against autologous primary myeloma plasma cells (Auto-PCs), PBMCs, purified B cells or EVB-transformed B cells, and K562. The E:T ratio was 20:1. Data are expressed as the mean ± SEM of 2 independent experiments.
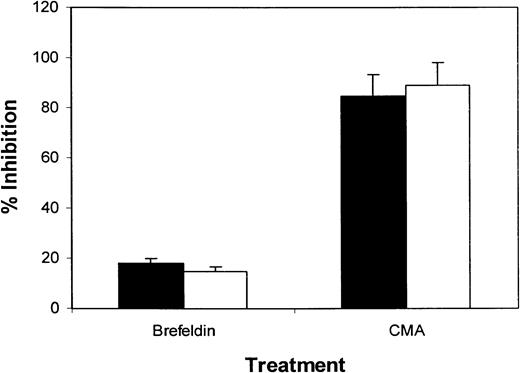
Myeloma-specific cytotoxic T-cell response is mediated by the perforin exocytosis pathway
To investigate whether the cytotoxic function of tumor lysate–primed CTLs was mediated by the FasL/Fas or perforin systems, we examined the effect of CMA and Brefeldin A, selected inhibitors of perforin-mediated and Fas-mediated cytotoxicity,21respectively. Treatment of the CTL lines with Brefeldin A induced less than 20% inhibition in the lytic activity of myeloma-specific CTLs (Figure 5; data from patients 1 and 2), indicating that the Fas/FasL system was not the main pathway of cytotoxicity mediated by these CTLs. This result was supported by flow cytometry analysis showing low or absent FasL expression by the T cells (data not shown). In contrast, when we studied the inhibition of cytotoxicity by pretreatment with CMA, cytolytic activity was almost completely abrogated (Figure 5). Treatment of the CTLs with Brefeldin A or CMA did not induce apoptosis of these CTLs (data not shown). Taken together, these results indicate that the cytotoxic function of myeloma-specific CTLs was mediated mainly by the perforin-dependent pathway.
Inhibition of CTL-mediated cytotoxicity against tumor lysate–pulsed DCs by the treatment of T cells with CMA or Brefeldin A.
CTLs tested were generated from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 3 independent experiments.
Inhibition of CTL-mediated cytotoxicity against tumor lysate–pulsed DCs by the treatment of T cells with CMA or Brefeldin A.
CTLs tested were generated from patients 1 (▪) and 2 (■). Data are expressed as the mean ± SEM of 3 independent experiments.
Discussion
Myeloma cells can be a source of tumor antigens for immunotherapy in MM. With the exception of Id protein, no other antigens have been identified yet that can be used for this purpose. In the current study, by using tumor lysate–pulsed DCs as APCs, we generated autologous tumor lysate–primed CTL lines from 4 patients with MM. These CTLs were able to recognize and lyse autologous tumor lysate–pulsed DCs and autologous primary myeloma plasma cells, justifying the exploration of myeloma cell–based immunotherapy in MM. Interestingly, in some patients the CTLs killed Id-pulsed DCs and, to a lesser extent, allogeneic myeloma cells, suggesting that Id protein might be part of the tumor antigen presented to T cells and that myeloma patients have identical antigens presented by common MHC molecules. Indeed, patients 1 and 2 did have identical MHC class 1 alleles (data not shown).
Results observed in this study indicate that myeloma cell lysates contain epitopes recognized by T cells mediating proliferative and cytotoxic responses in an MHC-restricted manner. Proliferative and cytotoxic responses of the CTLs generated from the patients were restricted by MHC class 1 and class 2 molecules, and the CTLs contained CD4+ and CD8+ T cells. Furthermore, the cytokine release by the CTLs was analyzed by the ELISPOT assay. Production of a significant amount of IFN-γ and TNF-α was observed when the CTLs were restimulated with tumor lysate–pulsed DCs. IL-4–secreting cells were rare or undetectable. Thus, these results indicate that the lysate-specific CTL lines contained type 1 CD4+ T-helper cell (Th1) and CD8+ CTL subsets,22,23 and both subsets may play important roles in the antimyeloma effects. This is true when lysate-pulsed DCs were used as APCs because DCs expressing MHC class 1 and 2 molecules can stimulate CD4+ and CD8+ T cells. However, given that primary myeloma cells express low or undetected MHC class 2 molecules,24 25 they may be less potent at activating or being recognized by CD4+ T cells. This may explain why primary myeloma cells induced weaker T-cell responses than lysate-pulsed DCs (Figures 1, 2). These observations would favor the use of tumor lysate–pulsed DCs rather than myeloma cells alone as tumor vaccines.
It is generally accepted that tumors growing in vivo naturally provide antigens to APCs either by shedding from the surfaces of viable cells or by fragmentation of dead tumor cells. Such processes elicit the induction of cellular immune responses by mechanisms that include cross-priming.26 The use of tumor cells, either as lysate or apoptotic cells,27-29 as a possible source of tumor antigens for DC pulsing has several potential advantages, among them mimicking the physiological processes by which a growing tumor induces an immune response in vivo and augmenting a broader T-cell immune response to tumor-associated antigens that would not be obtained by pulsing DCs with a single defined tumor antigen (peptides or protein). However, because tumor antigens may be shared with normal host cells, immunotherapy with tumor cell–pulsed DCs may potentially induce autoimmunity or autoimmune diseases.30 Therefore, we have examined whether myeloma cell lysate–primed CTLs lysed normal lymphocytes, especially B cells. Our results show clearly that the CTLs did not kill autologous PBMCs, purified B cells, or EBV-transformed B cells, suggesting that the CTLs primed by lysate-pulsed DCs are myeloma specific. These CTLs may be promising effector cells for immunotherapy in this disease.
Plasma cells represent the terminal differentiation stage of B-cell development. In MM, plasma cells usually constitute at least 10% and can even reach more than 90% of the total bone marrow cell count.31 One important aspect is whether the myeloma cells can be recognized and regulated by tumor-specific T cells. Our previous study has shown that freshly isolated primary myeloma cells can activate T cells; they stimulate alloreactive T cells and present recall antigens to autologous T cells,25 suggesting that myeloma cells can act as APCs. The current study demonstrates that tumor lysate–primed CTLs lyse autologous plasma cells in an MHC class 1–restricted manner, indicating that the tumor cells process tumor antigens and present the antigenic peptides in the context of MHC class 1 molecules on the cell surface. Taken together, these findings indicate that myeloma plasma cells express tumor antigens and are susceptible to tumor-specific T cell–mediated lysis.
CTL recognition of target cells through their T-cell receptor can activate 2 distinct mechanisms of cell lysis.32,33 The first is granule exocytosis, mediated by pore-forming perforin and granzymes A and B. The second involves interaction between the Fas ligand on effector cells and Fas molecules expressed on the target cells. In the current study, CTLs appeared to lyse the target cells mainly through the perforin-mediated pathway because CMA, the selective blocking agent, induced 90% of inhibition of cytotoxicity. Brefeldin A, a selective inhibitor of the Fas-mediated pathway, resulted in less than 20% inhibition. Hence, these findings are in agreement with our previous studies.34,35 This is important in view of published results on Fas expression on myeloma cells. Dalton et al36 have shown that Fas antigen point mutations were detected in 10% of patient bone marrow samples. Mutations were located in the cytoplasmic region, which is involved in the transduction of an apoptotic signal and thus renders the cells resistant to Fas-induced apoptosis. Furthermore, they found that drug-resistant myeloma cells also became resistant to Fas-mediated apoptosis.37 Thus, the use of cytotoxic CTLs through the Fas-mediated pathway may have limited benefit, but pore-forming CTLs can be used for the treatment of drug-resistant myeloma.
In conclusion, our results demonstrate that by using DCs as APCs, autologous myeloma–specific CTLs can be generated from myeloma patients. These T cells are efficient in killing lysate-pulsed autologous DCs and primary myeloma cells, but not blood lymphocytes, including B cells from patients. Thus, our study provides strong and direct evidence to support the application of tumor cell–based immunotherapies in MM.
Supported in part by grants from the Leukemia and Lymphoma Society (6548-00), the Multiple Myeloma Research Foundation and McCarty Cancer Foundation, and the National Cancer Institute (PO1-CA55819).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Qing Yi, Myeloma and Transplantation Research Center, University of Arkansas for Medical Sciences, Slot 776, 4301 West Markham St, Little Rock, AR 72205; e-mail: yiqing@uams.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal