Abstract
Resting dendritic cells (DCs) are resident in most tissues and can be activated by environmental stimuli to mature into potent antigen-presenting cells. One important stimulus for DC activation is infection; DCs can be triggered through receptors that recognize microbial components directly or by contact with infection-induced cytokines. We show here that murine DCs undergo phenotypic maturation upon exposure to type I interferons (type I IFNs) in vivo or in vitro. Moreover, DCs either derived from bone marrow cells in vitro or isolated from the spleens of normal animals express IFN-α and IFN-β, suggesting that type I IFNs can act in an autocrine manner to activate DCs. Consistent with this idea, the ability to respond to type I IFN was required for the generation of fully activated DCs from bone marrow precursors, as DCs derived from the bone marrow of mice lacking a functional receptor for type I IFN had reduced expression of costimulatory and adhesion molecules and a diminished ability to stimulate naive T-cell proliferation compared with DCs derived from control bone marrow. Furthermore, the addition of neutralizing anti–IFN-α/β antibody to purified splenic DCs in vitro partially blocked the “spontaneous” activation of these cells, inhibiting the up-regulation of costimulatory molecules, secretion of IFN-γ, and T-cell stimulatory activity. These results show that DCs both secrete and respond to type I IFN, identifying type I interferons as autocrine DC activators.
Introduction
Dendritic cells (DCs) are recognized as the key antigen-presenting cells (APCs) controlling the initiation of T cell–dependent immune responses.1 DCs not only are the most potent APCs for activation of resting T cells, but can also regulate the type of response made, dictating the cytokines expressed by responding T cells.2 Furthermore, DCs have the ability to down-regulate T-cell activation and may play a role in the induction of tolerance.3-5 The type of response elicited by particular DCs is likely to reflect their developmental origin, anatomical location, and state of activation.6
Immature (resting) DCs reside in most tissues and are efficient in binding and internalizing antigens.1 However, these cells are relatively poor at presenting antigen and inducing T-cell activation, owing at least in part to their low cell surface expression of costimulatory and adhesion molecules. In response to a variety of stimuli (see below), DCs undergo a process that has been variably termed activation or maturation, during which they lose their capacity for antigen uptake and acquire potent T-cell stimulatory ability. This is associated with up-regulation of cell surface major histocompatibility complex (MHC) class II, costimulatory molecules (eg, CD80, CD86, CD40), and adhesion molecules (eg, CD54).1 DC activation can be induced by signals that have been described as indicative of “danger,” such as heat shock proteins,7mechanical manipulation,8 or exposure to necrotic cells,8,9 as well as components of the extracellular matrix,10 interaction with activated (CD40 ligand–expressing)T cells,11-14 and infection. DCs are particularly tuned to recognition of infection, as DC activation can be stimulated by exposure to whole pathogens (viruses or bacteria15-17), components of micro-organisms (eg, lipopolysaccharide [LPS], double-stranded RNA, CpG DNA, and toxins15,18-21), and cytokines induced by infection (reviewed in Banchereau et al22).
One family of infection-induced cytokines that can activate DCs is the type I interferons (type I IFNs). Type I IFNs include a number of evolutionarily conserved proteins encoded by closely related and linked genes, the major species of which are IFN-α (including multiple subtypes) and IFN-β23; all type I IFNs exert their activity by binding to a common cell surface receptor.24Type I IFNs are expressed at low levels in normal or axenic (germ-free) mice, but are induced to high levels by viral or bacterial infection.25,26 Type I IFNs are also elicited by components of infectious agents, such as LPS, bacterial DNA, and double-stranded RNA.15 27-29
Evidence that type I IFNs can activate DCs has come from studies on DCs generated in vitro from peripheral blood or bone marrow precursor cells. In these models, the general finding has been that addition of type I IFNs to mouse8 or human30-34 DCs enhanced their expression of costimulatory molecules and ability to stimulate T cells, although there is also a report that type I IFNs can have the opposite effect.35 The ability of type I IFNs to activate DCs is interesting given that these cells can also produce type I IFNs in response to infection. Thus, it has been reported that type I IFNs are secreted by human DC precursors,28,36 in vitro–derived human DCs,15 and murine splenic DCs37 in response to pathogen-associated signals. In addition, there is some evidence that the DC-secreted type I IFNs can act in an autocrine manner, promoting survival of DC precursors29 and simulating expression of type I IFN–induced genes in activated human DCs.15 38 These findings therefore raise the question of whether type I IFN can also serve as an autocrine factor for DC activation.
In this study, we have examined the ability of in vivo– or in vitro–generated DCs to secrete and respond to type I IFN. We report that murine splenic DCs (sDCs), like in vitro–derived DCs, undergo maturation after treatment with type I IFN in vivo or in vitro. Furthermore, both sDCs assayed immediately ex vivo and DCs generated from bone marrow precursors in vitro (BM-DCs) express IFN-α and IFN-β. BM-DCs derived from the bone marrow of type I IFN-receptor–deficient mice (type I IFN-R KO) had lower cell surface expression of CD40, CD80, CD86, and MHC class II and were less efficient at presenting peptides to CD4+ or CD8+ T cells than DCs grown from control bone marrow under the same conditions. Similarly, the “spontaneous” activation of sDCs that occurs when these cells are placed in vitro, which includes increased production of IFN-γ and up-regulation of costimulatory molecules, was partially blocked by neutralizing anti–IFN-α/β antibodies; treatment of sDCs with anti–IFN-α/β also reduced their ability to stimulate proliferation of CD4+ and CD8+ peptide-specific T cells. These results show that type I IFNs are produced by BM-DCs and sDCs and act in an autocrine manner to activate these cells.
Materials and methods
Mice
C57Bl/6 (B6) mice were obtained from Charles River–UK (Margate, Kent) or from the SPF unit at the Institute for Animal Health (Compton, United Kingdom). From the SPF unit at the Institute for Animal Health, we obtained 129SvEv(129) mice, 129 background mice that were type I IFN-R KO,39 (originally purchased from B&K Universal, North Humberside, United Kingdom), 2C T-cell receptor (TCR) transgenic mice40 (originally obtained from J. Sprent, The Scripps Research Institute, La Jolla, CA), and DO11.10 TCR transgenic mice41 (originally obtained from Dr Fiona Powrie, Nuffield Department of Surgery, Oxford, United Kingdom). All mice were used at 6 to 12 weeks of age.
DC isolation and culture
DCs were generated from cultures of bone marrow cells as described previously.42 43 Briefly, bone marrow was extracted from the tibia and femur, and cell suspensions were cultured for 6 days in Iscoves modified Dulbecco medium (IMDM) (Life Technologies, Paisley, United Kingdom) containing 10% heat-inactivated fetal calf serum (FCS), 50 μM 2-ME, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 U/mL polymyxin B, and 10 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) (R&D Systems, Abingdon, Oxon, United Kingdom). Fresh medium was given every other day. On day 6, loosely adherent cells were harvested, washed, and replated in fresh medium. Phenotypic analysis and functional assays were performed between days 10 and 14. CD11c+ranged between 95% and 98% without any further sorting or treatment (see “Results”).
The sDCs were obtained with the use of a method based on that described by Vremec et al.44 In brief, spleens from 6 to 8 mice were pooled and cut into small fragments. The homogenate was digested in RPMI 1640 medium containing 10% heat-inactivated FCS, 1 mg/mL Type III collagenase (Worthington Biochemical, Lorne Laboratories, Twyford, Reading, United Kingdom), and 325 U/mL DNase I (Sigma-Aldrich) by mixing for 25 minutes at room temperature. Then 0.6 mL of 0.1 M EDTA, pH 7.2, was added for an additional 5 minutes, to allow disruption of DC–T-cell complexes. Cells were pelleted, resuspended in 1.077 g/mL Nycodenz (Life Technologies), layered on Nycodenz, and centrifuged in a Sorval RT7plus centrifuge at 2000g for 20 minutes. The low-density fraction was collected and incubated on ice with anti-CD11c fluorescein isothiocyanate (FITC) monoclonal antibody (Pharmingen, Becton Dickinson UK, Cowley, Oxford) followed by incubation with anti-FITC Microbeads (Miltenyi Biotech, Bisley, Surrey, United Kingdom) for 20 and 15 minutes, respectively. The positive fraction was recovered by passing over a MACS separation column (Miltenyi Biotech) and checked on a FACSCalibur (Becton Dickinson UK) for purity; 93% to 98% purity was routinely attained. Cells were then plated at 1 × 106/mL in IMDM containing 10% heat-inactivated FCS, 50 μM 2-ME, 100 U/mL penicillin, 100 μg/mL streptomycin, and 100 U/mL polymyxin B in the presence or absence of 1000 neutralizing units of antimouse IFN-αβ sheep immunoglobulin45 (or, as a control, normal sheep immunoglobulin45), with or without 12 ng/mL rmIFN-γ (R&D Systems), or with or without 5000 U/mL IFN-α/β (see below) as indicated. At 18 hours later, DCs were harvested for fluorescence-activated cell sorter (FACS) analysis and proliferation assays.
Flow cytometric analysis
The following monoclonal antibodies (all from Pharmingen) were used: anti-CD54 (ICAM-1) biotin (3E2), anti-CD40 biotin (HM40-3), anti-CD80 (B7-1) biotin (16-10A1), anti-CD87 (B7-2) biotin (GL1), anti-H2Db-biotin (28-14-8), anti-I-Ad/I-Ed-biotin (2G9), anti-CD11c (HL3), used as FITC or biotin. Biotinylated monoclonal antibodies were detected with streptavidin-Red 670 (Life Technologies) or streptavidin-Cy5 (TCS Biologicals, Botolph Claydon, Buckinghamshire, United Kingdom). Aliquots of 2 to 5 × 105 cells were stained in phosphate-buffered saline (PBS), 2% FCS, and 0.1% NaN3 and analyzed on a FACScalibur with the use of the CellQuest software (Becton Dickinson UK).
Reverse transcriptase–polymerase chain reaction and analysis of amplified products
Messenger RNA (mRNA) was purified from 2 × 106CD11c+ sDCs or BM-DCs by means of a Quickprep Micro mRNA purification kit (Amersham Pharmacia Biotech UK). Then, 500 ng mRNA was incubated for 10 minutes with Oligo-p(dT)15 primers (Boehringer Mannheim UK, Lewes, East Sussex) at 25°C in the presence of 50 U RNAse inhibitors (Boehringer Mannheim UK) and reverse transcribed with 20 U AMV reverse transcriptase (Boehringer Mannheim UK) for 1 hour at 42°C in a final volume of 20 μL (10 mM Tris, 50 mM KCl, 5 mM MgCl2, 1 mM deoxynucleoside-nucleoside 5′-triphosphates [dNTPs]; pH 8.3). In some experiments, total RNA was extracted by means of a miniprep total RNA purification kit (Qiagen, Crawly, West Sussex, United Kingdom). In this case, an “on-column” digestion with DNase I (Qiagen) was performed. RNA so obtained was then reverse transcribed by means of Oligo-p(dT)15 primers to select polyadenylated RNA (ie, mRNA). Polymerase chain reaction (PCR) was performed on 2 μL each complementary DNA (cDNA) sample or, as controls, either 2 μL sterile water or 2 μL total RNA that had not been reverse transcribed, by means of 1.25 U Thermoprime Plus DNA polymerase (Advanced Biotechnologies, Epson, Surrey, United Kingdom) in a final volume of 50 μL containing 75 mM Tris-HCl, 20 mM ammonium persulfate, 0.1% Tween 20, 1.5 mM MgCl2, 0.2 mM dNTPs, 10 pmol sense primer, and 10 pmol antisense primer at pH 8.8. Primers used were the following: murine IFN-α1/IFN-α2, 5′-TGTCTGATGCAGCAGGTGG-3′ (sense), 5′-AAGACAGGGCTCTCCAGAC-3′ (antisense); murine IFN-β, 5′-CCATCCAAGAGATGCTCCAG-3′ (sense), 5′-GTGGAGAGCAGTTGAGGACA-3′ (antisense); β-actin, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ (sense), 5′-CTAGAAGCATTGCGGTGGAGCATGGAGGG-3′ (antisense). All primers were obtained from Life Technologies. Samples were amplified under the following PCR conditions: 40 seconds denaturation at 94°C, 40 seconds annealing at 62°C, and 1 minute extension at 72°C (40 cycles for IFN-α1/IFN-α2 and IFN-β, 25 to 30 cycles for β-actin). Samples were then further incubated at 72°C for 5 minutes. Amplified products (10 μL) were then separated by agarose gel electrophoresis on a 1.2% Tris-acetate ethylenediamine-tetraacetic acid gel and visualized by ethidium bromide staining and UV transillumination.
IFN bioassay
First, 106 DCs were plated in each well of a 96-well culture plate in a volume of 200 μL, in the presence or absence of 1000 neutralizing units of antimouse IFN-αβ sheep immunoglobulin. Cultures were incubated at 37°C in 5% CO2 for 18 hours, after which the supernatant was harvested. We assayed 50 or 100 μL of each sample for IFN-αβ biological activity by measuring its ability to confer resistance to encephalomyelocarditis virus infection upon L929 cells as described elsewhere.46 The activity of the supernatants was determined by comparison with that of rIFN-α (ICN Pharmaceuticals, Basingstoke, Hampshire, United Kingdom). Each unit, as expressed in the text, represents 4 international IFN units.
Proliferation assays
DCs were irradiated (2500 radions) and plated in RPMI 1640 medium supplemented with 7% heat-inactivated FCS, 50 μM 2-ME, 100 U/mL penicillin, 100 μg/mL streptomycin, and 100 U/mL polymyxin B at the indicated numbers per well in 96-well flat-bottomed culture plates. Then, 2 × 105 responder cells were added per well. CD4+ responder T cells were positively selected from the lymph nodes of DO11.10 mice.41 Briefly, lymph nodes were pooled, gently cut into small fragments, and digested in collagenase/DNase as described above. Cells were washed and incubated with anti-CD4 microbeads (Miltenyi Biotech), and the positive fraction was collected by passing cells through a MACS column (Miltenyi Biotech). This population was subsequently incubated with a monoclonal antibody to mouse TCR clonotype (KJ1-26-FITC) (Caltag Laboratories, TCS Biologicals), and the labeled cells were sorted on a MoFlo flow cytometer (Cytomation, Fort Collins, CO). CD8+ responder T cells were isolated from 2C TCR transgenic mice40by positive selection with antimouse CD8α microbeads (Miltenyi Biotech). The purity of the responder cell populations was checked by FACS analysis and ranged from 90% to 98%. Agonistic peptides (synthesized in the Institute for Animal Health) were added at the indicated concentrations. For DO11.10 responder cells, the peptide corresponding to amino acids (aa) 323 through 339 of ovalbumin, which can be presented by I-Ad or I-Ab, was used,47 while for 2C responder cells, the synthetic peptide Ser-Ile-Tyr-Arg-Tyr-Tyr-Gly-Leu, which is presented on Kb (Kb = H − 2Kb),48 was used. At 4 days after the cells were placed in culture, plates were pulsed for 16 hours with 3H-thymidine (1 μCi [37 KBq] per well). Incorporation of 3H-thymidine into DNA was analyzed following cell harvesting by liquid scintillation counting.
Interferons
High-titer IFN-αβ was prepared in the C243-3 cell line following a method adapted from Tovey et al46 Briefly, confluent cells were primed by the addition of 10 U/mL IFN in Eagle minimum essential medium (MEM) enriched with 10% FCS and 1 mM sodium butyrate. After 16 hours of culture at 37° C, C243-3 cells were infected by Newcastle disease virus (multiplicity of infection of 1) in MEM plus 0.5% FCS plus 5 mM theophylline. At 18 hours after infection, culture supernatant was collected and centrifuged at 1500 rpm for 10 minutes. The supernatant was adjusted to pH 2.0 and kept at 0°C for 6 days. IFN was concentrated and partially purified by ammonium sulfate precipitations and dialysis against PBS, followed by further dialysis for 24 hours at 4°C against 0.01 M percloric acid and then against PBS, before being tested for any possible residual toxicity on a line of L1210 cells resistant to IFN. These partially purified IFN preparations had a titer of at least 2 × 107 U/mg of protein and were endotoxin free, as assessed by the Limulusamebocyte assay.
For injections, either 1 × 105 U IFN-α/β diluted in 200 μL PBS or 200 μL PBS alone was injected intravenously into B6 mice. Mice were killed 4 hours later and sDCs isolated as described above.
Cytokine detection assays
First, 106 purified sDCs cells were plated in each well of a 96-well culture plate in a volume of 200 μL, in the presence or absence of 1000 neutralizing units of antimouse IFN-αβ sheep immunoglobulin.
Cultures were incubated at 37°C, in 5% CO2 for 18 hours, after which supernatant was collected and titers of IFN-γ, interleukin (IL)–4, and IL-18 were measured by means of Quantikine M enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems).
Confocal analysis
Splenic DCs were purified from B6 mice, incubated for 1 hour at 37° C in IMDM containing GolgiPlug (Pharmingen), placed on microscope slides, and fixed with an acetone-to-ethanol ratio of 1:1. Fixed cells were stained primarily with biotin-conjugated hamster antimouse CD11c (HL3) and rat antimouse IFN-α (Alexis, Nottingham, United Kingdom) or with isotype-matched controls. Primary antibodies were detected with streptavidin-Cy5 and FITC-conjugated antirat immunoglobulin κ chain (MRK-1, Pharmingen), respectively. Cells were analyzed on a Leica TCS-NT confocal system (Milton Keynes, United Kingdom).
Results
Role of type I IFN in DCs maturation from bone marrow precursors
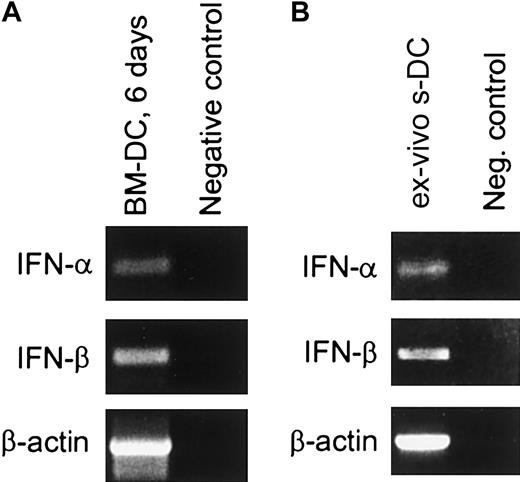
Cells exhibiting the surface markers and functional properties of mature DCs can be generated in vitro by culturing mouse bone marrow cells in the presence of GM-CSF.42,43 This in vitro culture system was used as an initial approach both to assess the expression of type I IFN in DCs and to determine whether type I IFNs play a role in DC maturation. Cells expressing the CD11c marker indicative of DCs were present as early as 6 days after initiating the culture. Approximately 60% to 72% of the cells were CD11c+ at this time (data not shown), and this increased to about 90% by day 14 (Figure 1). To determine whether these BM-DCs were expressing type I IFN, CD11c+ cells from day-6 cultures were purified by means of MACS beads, and mRNA was extracted for reverse transcriptase (RT)–PCR analysis. With the use of primers specific for IFN-α (subtypes 1 and 2) and IFN-β, it was apparent that both IFN-α and IFN-β were expressed in these cells (Figure2A). Expression of type I IFN at the protein level was also assessed by means of a bioassay that detects the presence of antiviral activity.46 In this experiment, day-12 CD11c+ BM-DCs were washed and incubated for 18 hours, after which the supernatant was collected. As shown in Table1, this supernatant contained IFN-α/β activity, demonstrating that BM-DCs were both producing and secreting type I IFN.
Phenotype of BM-DCs unable to respond to type I IFN.
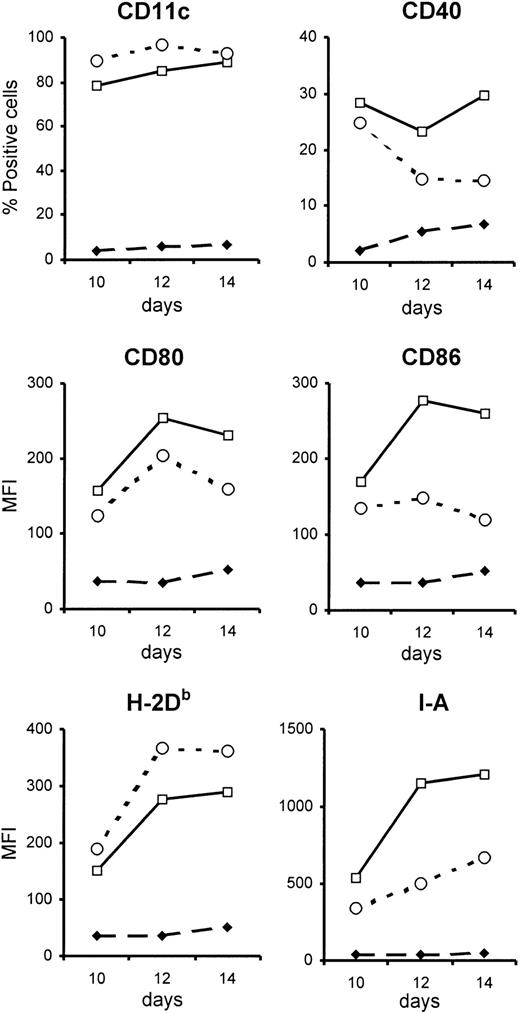
BM-DCs unable to respond to type I IFN have a less activated phenotype than control BM-DCs. BM-DCs were generated from the bone marrow of type I IFN-R KO mice (open circles) or control mice (open squares). Phenotypic analysis was done every 2 days from day 6 after initiation of in vitro culture, but differences between the 2 types of BM-DCs were evident only from day 10 onwards (closed diamonds represent staining with isotype control antibodies). Expression of the various surface markers (except CD11c) was evaluated after gating on CD11c+cells. These data are from 1 representative experiment of 3. MFI indicates mean fluorescence intensity.
Phenotype of BM-DCs unable to respond to type I IFN.
BM-DCs unable to respond to type I IFN have a less activated phenotype than control BM-DCs. BM-DCs were generated from the bone marrow of type I IFN-R KO mice (open circles) or control mice (open squares). Phenotypic analysis was done every 2 days from day 6 after initiation of in vitro culture, but differences between the 2 types of BM-DCs were evident only from day 10 onwards (closed diamonds represent staining with isotype control antibodies). Expression of the various surface markers (except CD11c) was evaluated after gating on CD11c+cells. These data are from 1 representative experiment of 3. MFI indicates mean fluorescence intensity.
Expression of IFN-α and IFN-β mRNA in sDCs and BM-DCs.
(A) BM-DCs. DCs were generated by culture of BM cells from B6 mice for 6 days in the presence of GM-CSF. CD11c+ cells were purified by means of MACS beads (greater than 98% CD11c+), and mRNA was extracted and assayed for IFN-α and IFN-β mRNAs by RT-PCR. As a negative control, 2 μL sterile water was used instead of cDNA template. (B) sDCs. The sDCs were purified from the spleens of B6 mice as described in “Materials and methods” (greater than 98% CD11c+) and immediately placed in lysis buffer. After cell lysis, the sample was treated with DNAse. Total RNA was then extracted and assayed for IFN-α and IFN-β mRNA by RT-PCR (left lane). The negative control (right lane) corresponds to 2 μL total RNA subjected to direct PCR (ie, without reverse transcription) using the same IFN-α and IFN-β primers. Amplified products were separated by electrophoresis on a 1.2% agarose gel in the presence of molecular markers (not shown).
Expression of IFN-α and IFN-β mRNA in sDCs and BM-DCs.
(A) BM-DCs. DCs were generated by culture of BM cells from B6 mice for 6 days in the presence of GM-CSF. CD11c+ cells were purified by means of MACS beads (greater than 98% CD11c+), and mRNA was extracted and assayed for IFN-α and IFN-β mRNAs by RT-PCR. As a negative control, 2 μL sterile water was used instead of cDNA template. (B) sDCs. The sDCs were purified from the spleens of B6 mice as described in “Materials and methods” (greater than 98% CD11c+) and immediately placed in lysis buffer. After cell lysis, the sample was treated with DNAse. Total RNA was then extracted and assayed for IFN-α and IFN-β mRNA by RT-PCR (left lane). The negative control (right lane) corresponds to 2 μL total RNA subjected to direct PCR (ie, without reverse transcription) using the same IFN-α and IFN-β primers. Amplified products were separated by electrophoresis on a 1.2% agarose gel in the presence of molecular markers (not shown).
Secretion of interferon-α/β by splenic and bone marrow–derived dendritic cells
| Sample and treatment . | Experiment no. . | IFN-α/β, U/106 cells . |
|---|---|---|
| BM-DCs | ||
| None | 1 | 8 |
| None | 2 | 8 |
| sDCs | ||
| None | 1 | 16 |
| Anti–IFN-α/β | 1 | < 4 |
| None | 2 | 8 |
| Anti–IFN-α/β | 2 | < 4 |
| None | 3 | 16 |
| Anti–IFN-α/β | 3 | < 4 |
| Sample and treatment . | Experiment no. . | IFN-α/β, U/106 cells . |
|---|---|---|
| BM-DCs | ||
| None | 1 | 8 |
| None | 2 | 8 |
| sDCs | ||
| None | 1 | 16 |
| Anti–IFN-α/β | 1 | < 4 |
| None | 2 | 8 |
| Anti–IFN-α/β | 2 | < 4 |
| None | 3 | 16 |
| Anti–IFN-α/β | 3 | < 4 |
CD11c+ populations were isolated from the spleens of B6 mice (sDCs) and from 10- to 14-day cultures of bone marrow cells from B6 mice (BM-DCs). One million DCs were plated in each well of a 96-well culture plate in a volume of 200 μL, in the presence or absence of 1000 neutralizing units of antimouse IFN-α/β sheep immunoglobulin. Cultures were incubated at 37°C in 5% CO2 for 18 hours, after which supernatant was harvested. An aliquot of 100 μL or 50 μL of each sample was assayed for IFN-α/β activity. Data represent the IFN-α/β titer in each individual experiment, expressed in experimental units of IFN-α/β per 106 cells. One of these units is the equivalent of 4 international IFN units. IFN indicates interferon; BM-DC, bone marrow–derived dendritic cell; sDC, splenic DC.
To determine whether the IFN-α/β in the culture was influencing the maturation of BM-DCs, a comparison was made between BM-DCs generated from mice lacking a functional receptor for type I IFN (type I IFN-R KO mice, which were on the 129 background) and control mice. A similar percentage of CD11c+ cells were generated in the cultures derived from the 2 strains of mice, showing that BM-DCs can develop in the absence of signaling via type I IFN. However, when the phenotype of the BM-DCs was compared, it was apparent that those derived from type I IFN-R KO mice were less mature than those generated from control bone marrow (Figure 1). Thus, type I IFN-R KO BM-DCs had lower surface expression of the costimulatory markers CD80 and CD86, as well as MHC class II, and the percentage of CD40+ cells was reduced compared with control cultures. The differences between control and type I IFN-R KO BM-DCs became more pronounced with increasing time of culture.
The less activated phenotype of type I IFN-R KO BM-DCs suggested that these cells may also have a reduced ability to act as APCs. To assess this possibility, type I IFN-R KO and control BM-DCs were tested for their ability to present peptides to naive antigen-specific T cells derived from TCR transgenic mice. Responder CD8+ T cells were purified from 2C TCR transgenic mice; these cells recognize an 8-aa peptide (Ser-Ile-Tyr-Arg-Tyr-Tyr-Gly-Leu) in association with Kb.48 As shown in Figure3, type I IFN-R KO BM-DCs were much less potent APCs than control BM-DCs for the induction of CD8+T-cell proliferation. Approximately 4 times as many type I IFN-R KO BM-DCs were required to induce a response similar to that elicited by control BM-DCs (Figure 3A). Furthermore, an equivalent number of control BM-DCs were able to induce significant T-cell proliferation at about a 15-fold lower peptide concentration (Figure 3B). Type I IFN-R KO BM-DCs were also less efficient than control BM-DCs in presenting peptides to CD4+ T cells, although the differences were less dramatic than for presentation to CD8+ T cells (Figure3C). Here, using ovalbumin peptide–specific (aa 323 through 339) DO11.10 T cells41 as responders, it was found that control BM-DCs did stimulate greater proliferation than type I IFN-R KO BM-DCs, but only when DCs were present in low numbers (6250 BM-DCs per well). These results therefore show that although BM-DCs can be generated in the absence of signaling via type I IFN, these DCs are phenotypically and functionally less mature than those produced from precursors responsive to type I IFN.
Ability of BM-DCs from type I IFN-R KO mice to stimulate CD8+ and CD4+ T-cell proliferation.
BM-DCs from type I IFN-R KO mice exhibit a reduced ability to stimulate CD8+ and CD4+ T-cell proliferation. BM-DCs from type I IFN-R KO mice (dotted line [panels A-B] and open bars [panel C] or control mice (solid line [panels A-B]) and solid bars [panel C]) were harvested after 10 to 14 days of culture, irradiated, and used as APCs for TCR transgenic CD8+ (panels A-B) or CD4+ (panel C) T cells. (A) Different numbers of BM-DCs were added to culture wells together with 105CD8+ responder T cells purified from 2C TCR transgenic mice. Cells were cultured with (open symbols) or without (solid symbols) specific peptide (Ser-Ile-Tyr-Arg-Tyr-Tyr-Gly-Leu at 0.05 nM). (B) Aliquots of 5000 BM-DCs were added per well together with 105 purified CD8+ 2C T cells and the indicated concentration of specific peptide. (C) Different numbers of BM-DCs were added to culture wells together with 105 CD4+responder T cells purified from DO11.10 TCR transgenic mice in the presence or absence of specific peptide (ovalbumin [OVA] aa 323 through 339 at 144 nM). Each of the panels shown here is representative of results obtained in at least 3 independent experiments.
Ability of BM-DCs from type I IFN-R KO mice to stimulate CD8+ and CD4+ T-cell proliferation.
BM-DCs from type I IFN-R KO mice exhibit a reduced ability to stimulate CD8+ and CD4+ T-cell proliferation. BM-DCs from type I IFN-R KO mice (dotted line [panels A-B] and open bars [panel C] or control mice (solid line [panels A-B]) and solid bars [panel C]) were harvested after 10 to 14 days of culture, irradiated, and used as APCs for TCR transgenic CD8+ (panels A-B) or CD4+ (panel C) T cells. (A) Different numbers of BM-DCs were added to culture wells together with 105CD8+ responder T cells purified from 2C TCR transgenic mice. Cells were cultured with (open symbols) or without (solid symbols) specific peptide (Ser-Ile-Tyr-Arg-Tyr-Tyr-Gly-Leu at 0.05 nM). (B) Aliquots of 5000 BM-DCs were added per well together with 105 purified CD8+ 2C T cells and the indicated concentration of specific peptide. (C) Different numbers of BM-DCs were added to culture wells together with 105 CD4+responder T cells purified from DO11.10 TCR transgenic mice in the presence or absence of specific peptide (ovalbumin [OVA] aa 323 through 339 at 144 nM). Each of the panels shown here is representative of results obtained in at least 3 independent experiments.
Role of type I IFNs in sDC activation
The observation that BM-DCs both secrete and respond to type I IFN raised the question of whether DCs that arise in vivo behave in a similar way. To determine whether in vivo–generated DCs respond to type I IFN, we examined the phenotype of sDCs isolated from mice that had been injected with IFN-α/β 4 hours previously (Figure4A). Compared with control sDCs, sDCs from IFN-α/β–injected mice exhibited increased cell surface expression of CD40 and CD86, indicating that they had been activated in vivo. Similar phenotypic changes occurred when IFN-α/β was added to purified sDCs during overnight culture (Figure 4B). In this case, assessment of the effect of IFN-α/β was complicated by the fact that “spontaneous” phenotypic activation of sDCs occurred upon placing these cells in culture in the absence of added IFN-α/β (see below). Nevertheless, the in vitro results showed that type I IFN could act directly on sDCs to stimulate activation.
Activation of sDCs upon exposure to type I IFN in vivo or in vitro.
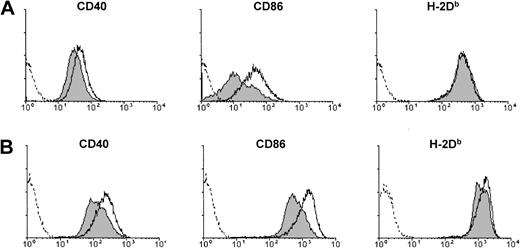
(A) Expression of CD40, CD86, or H-2Db on sDCs purified from B6 mice 4 hours after injection of PBS (filled histograms) or IFN-α/β (open histograms, solid line). (B) Expression of CD40, CD86, or H-2Db on CD11c+ sDCs purified from untreated B6 mice and placed in culture for 18 hours in medium alone (filled histograms) or in medium containing 5000 U/mL IFN-α/β (open histograms, solid line). Dashed lines show staining with isotype control antibodies.
Activation of sDCs upon exposure to type I IFN in vivo or in vitro.
(A) Expression of CD40, CD86, or H-2Db on sDCs purified from B6 mice 4 hours after injection of PBS (filled histograms) or IFN-α/β (open histograms, solid line). (B) Expression of CD40, CD86, or H-2Db on CD11c+ sDCs purified from untreated B6 mice and placed in culture for 18 hours in medium alone (filled histograms) or in medium containing 5000 U/mL IFN-α/β (open histograms, solid line). Dashed lines show staining with isotype control antibodies.
Having shown that sDCs respond to type I IFN, we then determined whether DCs isolated from the spleens of normal mice expressed type I IFN. As shown in Figure 2B, mRNA for both IFN-α and IFN-β were detectable in freshly isolated CD11c+ sDCs, demonstrating that these cells were expressing type I IFN in situ. Supernatants harvested from sDCs that had been cultured for 18 hours contained bioactive IFN-α/β (Table 1). Cultured sDCs also secreted IFN-γ and IL-18, but not IL-4 (Figure 7 and data not shown). The presence of IFN-α/β in the supernatant was confirmed by the ability of anti–IFN-α/β immunoglobulin to neutralize the IFN activity (Table 1). Furthermore, direct evidence that sDCs were in fact the source of type I IFN in the supernatant was provided by confocal microscopy. Here, purified sDCs were cultured briefly (1 hour) in the presence of an inhibitor of intracellular protein transport (GolgiPlug, containing brefeldin A) to cause accumulation of recently synthesized proteins within the cells, then stained with antibodies to IFN-α and CD11c. As shown in Figure 5, most of the cells stained positively for both CD11c and IFN-α. Therefore, like BM-DCs, sDCs expressed type I IFNs, which were secreted into the culture medium.
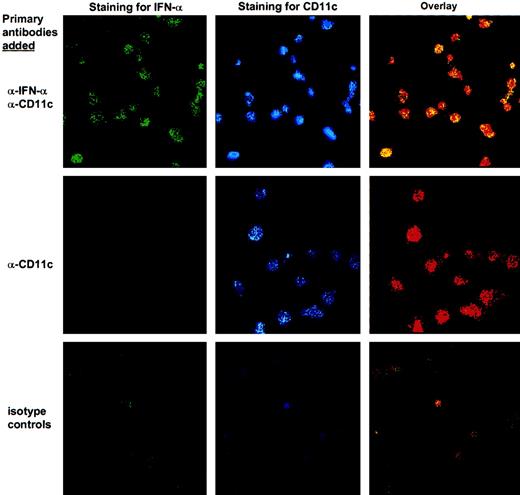
Expression of IFN-α by sDCs.
The sDCs were purified from B6 mice, incubated for 1 hour in medium containing GolgiPlug, placed on microscope slides, and fixed. In the upper row, cells were stained with rat antimouse IFN-α and biotinylated hamster antimouse CD11c followed by FITC-conjugated anti–rat immunoglobulin and Cy5-conjugated streptavin. Staining of cells in the middle row was done in the same manner, except that rat antimouse IFN-α was omitted, while in the lower row primary antibodies were replaced by isotype-matched controls. Cells were analyzed by confocal microscopy, where staining with FITC appears green. Cy5 staining was given a “false” blue color in the middle column and appears as a “true” red color in the right column. Colocalization of FITC and Cy5 staining appears yellow in the overlay. Magnification × 63 for all images.
Expression of IFN-α by sDCs.
The sDCs were purified from B6 mice, incubated for 1 hour in medium containing GolgiPlug, placed on microscope slides, and fixed. In the upper row, cells were stained with rat antimouse IFN-α and biotinylated hamster antimouse CD11c followed by FITC-conjugated anti–rat immunoglobulin and Cy5-conjugated streptavin. Staining of cells in the middle row was done in the same manner, except that rat antimouse IFN-α was omitted, while in the lower row primary antibodies were replaced by isotype-matched controls. Cells were analyzed by confocal microscopy, where staining with FITC appears green. Cy5 staining was given a “false” blue color in the middle column and appears as a “true” red color in the right column. Colocalization of FITC and Cy5 staining appears yellow in the overlay. Magnification × 63 for all images.
Overnight culture of sDCs was associated with phenotypic activation. Thus, sDCs cultured for 18 hours in medium alone had markedly higher surface expression of CD40, CD80, CD86, MHC class I, and MHC class II compared with sDCs examined directly ex vivo (Figure6A, and data not shown). These differences were seen on both the CD8+ and the CD8− subsets of sDCs (data not shown). Since the sDCs were actively secreting type I IFN into the supernatant during this time, the question arose as to whether the phenotypic changes were due to autocrine/paracrine activation by type I IFN. To address this question, the activity of type I IFN was blocked by adding neutralizing anti–IFN-α/β sheep immunoglobulin45 to the sDC culture. As shown in Figure 6B, anti–IFN-α/β treatment inhibited the up-regulation of CD40, CD86, and MHC class I, implicating type I IFN in the sDC activation; addition of control sheep immunoglobulin to cultures did not affect sDC phenotype (data not shown). Inhibition by anti–IFN-α/β was only partial, however, as sDCs cultured in the presence of anti–IFN-α/β still expressed higher levels of these molecules than ex vivo sDCs (compare empty histograms in Figure 6A with light gray–filled histograms in Figure 6B), and anti–IFN-α/β did not inhibit up-regulation of CD80 and MHC class II (data not shown). The failure of anti–IFN-α/β to completely inhibit sDC activation probably signifies that mechanisms acting independently of type I IFN were also involved, although an alternative possibility is that the antibody was unable to completely block interaction between IFN-α/β and its receptor.
Effect of overnight culture of sDCs.
Overnight culture of sDCs induces phenotypic activation that is partially dependent on type I IFN. (A) Phenotype of CD11c+sDCs from B6 mice directly ex vivo (solid lines) and after overnight culture (dark-gray filled histograms). (B) Phenotype of CD11c+ B6 sDCs after culture for 18 hours in the presence (light-gray filled histograms) or absence (dark-gray filled histograms) of anti–IFN-α/β immunoglobulin. Dashed histograms show background staining with isotype control antibodies. Data are representative results from 1 of 3 experiments.
Effect of overnight culture of sDCs.
Overnight culture of sDCs induces phenotypic activation that is partially dependent on type I IFN. (A) Phenotype of CD11c+sDCs from B6 mice directly ex vivo (solid lines) and after overnight culture (dark-gray filled histograms). (B) Phenotype of CD11c+ B6 sDCs after culture for 18 hours in the presence (light-gray filled histograms) or absence (dark-gray filled histograms) of anti–IFN-α/β immunoglobulin. Dashed histograms show background staining with isotype control antibodies. Data are representative results from 1 of 3 experiments.
Anti–IFN-α/β treatment of sDCs also markedly reduced the amount of IFN-γ secreted by these cells, but did not affect the production of IL-18 (Figure 7). The implication was that IFN-γ secretion, like phenotypic activation, was at least partially triggered by the autocrine action of type I IFN on sDCs. IFN-γ secreted by sDCs may also have been playing a role in the activation of these cells. Evidence in favor of this possibility came from the observation that addition of IFN-γ to anti–IFN-α/β–treated sDCs resulted in the up-regulation of costimulatory and MHC class I molecules to an extent similar to or greater than that in control cultures (Figure8).
Role of type I IFN in IFN-γ secretion by sDCs.
Secretion of IFN-γ by sDCs is partially dependent on type I IFN. Purified sDCs (greater than 97% CD11c+) were cultured in either medium alone, medium plus 1000 neutralizing units of anti–IFN-α/β sheep immunoglobulin, or medium plus an equivalent volume of control sheep immunoglobulin for 18 hours. Supernatants were collected and analyzed for IFN-γ and IL-18 by ELISA. Data shown are for 1 representative experiment of 3.
Role of type I IFN in IFN-γ secretion by sDCs.
Secretion of IFN-γ by sDCs is partially dependent on type I IFN. Purified sDCs (greater than 97% CD11c+) were cultured in either medium alone, medium plus 1000 neutralizing units of anti–IFN-α/β sheep immunoglobulin, or medium plus an equivalent volume of control sheep immunoglobulin for 18 hours. Supernatants were collected and analyzed for IFN-γ and IL-18 by ELISA. Data shown are for 1 representative experiment of 3.
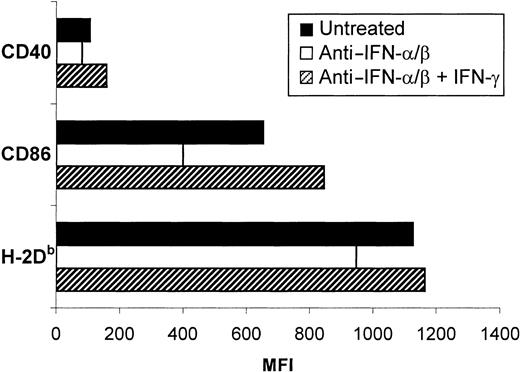
Effect of IFN-γ treatment on the inhibitory effects of anti–IFN-α/β.
Treatment with IFN-γ reverses the inhibitory effects of anti–IFN-α/β on the phenotypic activation of sDCs. The sDCs were isolated from B6 mice and placed in culture for 18 hours alone (solid bars) or in the presence of anti–IFN-α/β immunoglobulin without (open bar) or with (hatched bars) 12 ng/mL IFN-γ. Data shown are from 1 representative experiment of 3.
Effect of IFN-γ treatment on the inhibitory effects of anti–IFN-α/β.
Treatment with IFN-γ reverses the inhibitory effects of anti–IFN-α/β on the phenotypic activation of sDCs. The sDCs were isolated from B6 mice and placed in culture for 18 hours alone (solid bars) or in the presence of anti–IFN-α/β immunoglobulin without (open bar) or with (hatched bars) 12 ng/mL IFN-γ. Data shown are from 1 representative experiment of 3.
To determine whether the DC-secreted type I IFN affected the functional status of sDCs, cells cultured for 18 hours in the presence or absence of anti–IFN-α/β or control sheep immunoglobulin were washed, irradiated, and assessed for their ability to present peptides to antigen-specific CD4+ and CD8+ T cells. The stimulatory capacity of DCs treated with control sheep immunoglobulin did not differ from that of untreated DCs (data not shown). In contrast, sDCs that had been treated with anti–IFN-α/β were clearly less effective than control sDCs in stimulating proliferation of both CD8+ (Figure 9A,C) and CD4+ (Figure 9B,D) T cells. Induction of similar levels of proliferation required approximately 2 × and 5 × the number of anti–IFN-α/β–treated sDCs for CD8+ and CD4+ T-cell responses, respectively. Anti–IFN-α/β–treated sDCs were also less potent APCs on a per cell basis, requiring the addition of higher concentrations of peptide to induce equivalent responses to control sDCs; this was particularly marked with regard to the stimulation of CD4+ T cells (Figure 9D). Therefore, these results show that type I IFNs secreted by sDCs act in an autocrine manner to enhance the antigen-presenting ability of these cells.
Reduced ability of sDCs to stimulate T-cell proliferation when cultured with anti–IFN-α/β.
The sDCs (96% CD11c+) isolated from B6 mice were cultured for 18 hours in the presence (circles, dotted lines) or absence (squares, solid lines) of anti–IFN-α/β immunoglobulin, washed, irradiated, and used as APCs for TCR transgenic T cells. (A) Different numbers of sDCs and 105 CD8+ responder T cells purified from 2C TCR transgenic mice were added to wells with (open symbols) or without (solid symbols) antigenic peptide (Ser-Ile-Tyr-Arg-Tyr-Tyr-Gly-Leu at 0.05 nM). (B) Different numbers of sDCs and 105 CD4+ responder T cells purified from DO11.10 TCR transgenic mice were added to wells with (open symbols) or without (solid symbols) antigenic peptide (OVA aa 323 through 336 at 144 nM). (C) Five thousand sDCs were added to wells with 105 CD8+responder T cells purified from 2C TCR transgenic mice and differing amounts of specific peptide. (D) Five thousand sDCs were added to wells with 105 CD4+ responder T cells purified from DO11.10 TCR transgenic mice and differing amounts of specific peptide. Each of the panels shown here is representative of results obtained in at least 3 independent experiments.
Reduced ability of sDCs to stimulate T-cell proliferation when cultured with anti–IFN-α/β.
The sDCs (96% CD11c+) isolated from B6 mice were cultured for 18 hours in the presence (circles, dotted lines) or absence (squares, solid lines) of anti–IFN-α/β immunoglobulin, washed, irradiated, and used as APCs for TCR transgenic T cells. (A) Different numbers of sDCs and 105 CD8+ responder T cells purified from 2C TCR transgenic mice were added to wells with (open symbols) or without (solid symbols) antigenic peptide (Ser-Ile-Tyr-Arg-Tyr-Tyr-Gly-Leu at 0.05 nM). (B) Different numbers of sDCs and 105 CD4+ responder T cells purified from DO11.10 TCR transgenic mice were added to wells with (open symbols) or without (solid symbols) antigenic peptide (OVA aa 323 through 336 at 144 nM). (C) Five thousand sDCs were added to wells with 105 CD8+responder T cells purified from 2C TCR transgenic mice and differing amounts of specific peptide. (D) Five thousand sDCs were added to wells with 105 CD4+ responder T cells purified from DO11.10 TCR transgenic mice and differing amounts of specific peptide. Each of the panels shown here is representative of results obtained in at least 3 independent experiments.
Discussion
Up-regulation of expression of type I IFN is one of the earliest cellular responses upon contact with infectious agents. The rapid induction of type I IFN reflects the crucial role that these cytokines play in the inhibition of viral spread before the generation of a specific immune response. Indeed, type I IFNs were identified and named on the basis of their ability to confer an antiviral state on target cells, and much is now known about the mechanisms by which they achieve this.49 However, the timing of their expression also makes type I IFNs ideal signaling molecules for alerting the immune system to the presence of infection. This aspect of the activity of type I IFNs has also been recognized for many years, particularly with regard to their stimulatory effects on natural killer (NK) cells and macrophages.50-52 More recently, the development of techniques for generating DCs in vitro has allowed for an assessment of the effects of type I IFNs on these key regulators of the immune response. Consistent with a role in linking innate and adaptive immunity, treatment with type I IFNs has been shown to activate in vitro–derived DCs, enhancing and modulating their ability to initiate T-cell responses.8,30-34 More recently, it has been shown that type I IFNs promote antibody responses in vivo and that stimulation of DCs by type I IFNs plays a role in this adjuvant activity.53 In the present study, we have demonstrated that type I IFNs are in fact autocrine activation factors for in vitro– and in vivo–derived DCs, both of which secrete and respond to type I IFNs.
Type I IFNs were expressed not only by BM-DCs but also by DCs immediately after their isolation from spleen. The latter expression implies that type I IFNs are expressed by sDCs in situ, although one cannot formally exclude the possibility that expression was induced during the DC-isolation procedure. In situ expression of type I IFNs by sDCs could reflect in vivo contact with environmental microbes, or components thereof, which may have occurred either in the spleen or in peripheral tissues prior to migration of the DCs to the spleen. Conversely, DC expression of type I IFNs may be independent of stimuli derived from infectious agents. This possibility is worth considering, since it has been shown that type I IFNs are expressed constitutively by resting macrophages.25,54,55 Analogous to the role of type I IFNs in macrophages,55 56 a function of the background expression of these IFNs might be to confer a baseline level of resistance to virus infection on DCs. This baseline resistance could, for example, be crucial in preventing depletion of APCs by rapidly acting lytic viruses. However, the question of whether background expression of type I IFNs by DCs is independent of exposure to micro-organisms will require an analysis of DCs from axenic mice.
Regardless of the factors driving DC secretion of type I IFNs, we have shown here that these cytokines can act in an autocrine manner to activate DCs. Thus, a reduced state of activation was observed for DCs generated in vitro from the bone marrow of type I IFN-R KO mice and for sDCs isolated from normal mice and cultured in the presence of a neutralizing anti–IFN-α/β antiserum. These DCs had lower surface expression of costimulatory molecules and were much less efficient in presenting antigenic peptides and stimulating the proliferation of CD4+ and CD8+ T cells.
The precise reason for the reduced ability of DCs matured in the absence of signaling by type I IFN to present peptides to T cells is unknown. Although there were lower levels of surface MHC class II on type I IFN-R KO BM-DCs and class I on sDCs cultured with anti–IFN-α/β, this probably did not account for the reduced stimulatory ability of these cells, since both CD4+ and CD8+ responses were affected in each case. A more likely explanation is that the reduced APC function is due to lower surface expression of CD80 (type I IFN-R KO BM-DCs only), CD86, and CD40. However, the possibility that other differences between the DCs account for the disparity in stimulatory ability should be considered, especially in view of the relatively small differences in expression of CD40 and CD86 on sDCs cultured with and without anti–IFN-α/β (Figure 5B). In this regard, it is clear that activation (or maturation) of DCs cannot be thought of as an all-or-none phenomenon, since the sDCs cultured in the presence of anti–IFN-α/β would be considered fully mature by many criteria, yet can be driven to become more potent APCs through autocrine stimulation with type I IFN. Instead, DC activation may proceed through several different stages.
In recent years, it has become apparent that IFN-γ is secreted not only by activated T cells and NK cells, but also by APCs, including DCs.57 In the current study, we found that treatment of sDCs with anti–IFN-αβ immunoglobulin in vitro reduced their secretion of IFN-γ. This result suggests that basal expression of type I IFN by DCs leads to secretion of IFN-γ, which may in turn act to enhance DC activation (see Figure 6). On the other hand, IFN-γ may act in an autocrine manner to enhance the resistance of DCs to virus replication, as has been shown for macrophages.26 In this regard, a synergism between type I and type II interferons in inducing an antiviral state in DCs is in keeping with the complementary role that these cytokines play in antiviral defense in vivo.58Finally, regulation of secretion of IFN-γ by type I IFN may have implications relating to the early events that polarize T-cell responses. Thus, IFN-γ secreted by DCs could bias T-cell responses in favor of a TH1-type profile.59 It would therefore be of interest to determine whether stimuli that increase expression of type I IFN, such as virus infection, result in increased secretion of IFN-γ by DCs.
The authors thank Nicola Colburn and Andrew Worth for cell sorting and technical assistance with flow cytometric and confocal analysis.
Supported by the Edward Jenner Institute for Vaccine Research, the European Community (contract no. QLK2-CT-2001-02103 and Associazione Italiana per la Ricerca sul Cancro, Fasc n D5A), and a grant from the Italian Ministry of Health on “Cytokines as vaccine adjuvants.” This is publication no. 27 from the Edward Jenner Institute for Vaccine Research.
M.M. and G.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David F. Tough, The Edward Jenner Institute for Vaccine Research, Compton, Newbury, Berkshire RG20 7NN, United Kingdom; e-mail: david.tough@jenner.ac.uk.

![Fig. 3. Ability of BM-DCs from type I IFN-R KO mice to stimulate CD8+ and CD4+ T-cell proliferation. / BM-DCs from type I IFN-R KO mice exhibit a reduced ability to stimulate CD8+ and CD4+ T-cell proliferation. BM-DCs from type I IFN-R KO mice (dotted line [panels A-B] and open bars [panel C] or control mice (solid line [panels A-B]) and solid bars [panel C]) were harvested after 10 to 14 days of culture, irradiated, and used as APCs for TCR transgenic CD8+ (panels A-B) or CD4+ (panel C) T cells. (A) Different numbers of BM-DCs were added to culture wells together with 105CD8+ responder T cells purified from 2C TCR transgenic mice. Cells were cultured with (open symbols) or without (solid symbols) specific peptide (Ser-Ile-Tyr-Arg-Tyr-Tyr-Gly-Leu at 0.05 nM). (B) Aliquots of 5000 BM-DCs were added per well together with 105 purified CD8+ 2C T cells and the indicated concentration of specific peptide. (C) Different numbers of BM-DCs were added to culture wells together with 105 CD4+responder T cells purified from DO11.10 TCR transgenic mice in the presence or absence of specific peptide (ovalbumin [OVA] aa 323 through 339 at 144 nM). Each of the panels shown here is representative of results obtained in at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3263/6/m_h80922480003.jpeg?Expires=1767708068&Signature=O1ocBYNf-oKsCWQ4VVX3eCj5Ams5xy2sxz9uUetIrb~RzVBXqQRr2CHBjdnQ9gABCeW8tDnD8Amet~j3SuBPb9eOvlta43EZnbu-NuvJxTiUc0lHmq2L9~CdNbbwCfJCcgzvT3FUoRqcmZZTM-cOVOd9oTZlGOmjHOPy7T~~QcwL2vGV-YOamgPgblwdP5nAJcn9wakXZOVRAz7UjsJ~y0hAWlAgKnDflq3z5z8UARC~DygIefgIZi6Eimi6vDK4YFWdFcSrMDKd19t9LlC-OhrPpQ9pKzdf7wkRkqfF8qyZuVka2CKCp7kZOIPrT1qBkn-ss48YS5Y5i7zOwPjGSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal