Abstract
Collagen activates platelets by transducing signals through glycoprotein VI (GPVI). It is not clear whether collagen can directly activate fibrinogen receptors on the adherent platelets without a role for positive feedback agonists. We investigated the contribution of secondary G protein signaling to the mechanism of GPVI-stimulated platelet aggregation using the GPVI-selective agonists, convulxin and collagen-related peptide (CRP) as well as collagen. Adenosine diphosphate (ADP) scavengers or ADP receptor antagonists shifted the concentration-response curve slightly to the right at low concentrations of convulxin, whereas platelet aggregation at higher concentrations of convulxin was unaffected by these agents. ADP receptor antagonists shifted the concentration-response curve of collagen- or CRP-induced platelet aggregation to the right at all the concentrations. Protein kinase C inhibitor, Ro 31-8220, or a calcium chelator 5,5′-dimethyl-BAPTA shifted the concentration-response curve of convulxin-induced platelet aggregation to the right. In addition, pretreatment with both Ro 31-8220 and dimethyl-BAPTA resulted in total inhibition of convulxin-mediated aggregation. Blockade of either the calcium- or protein kinase C–regulated pathway leads to inhibition of fibrinogen receptor activation on platelets adherent to collagen, but inhibition of both pathways leads to abolished fibrinogen receptor activation. We conclude that collagen-induced activation of fibrinogen receptor on adherent platelets through GPVI signaling occurs without any significant role for secreted ADP or thromboxane A2. Furthermore, protein kinase C– and calcium-regulated pathways independently contribute to GPVI-mediated platelet aggregation.
Introduction
Contact of circulating platelets with exposed subendothelium results in the release, generation, or exposure of agonists, which in turn can activate platelets in a positive feedback loop. Among these agonists are collagen (exposed), thrombin and thromboxane A2 (generated), and adenosine diphosphate (ADP), epinephrine and serotonin (released).1Agonist-stimulated platelets change shape and subsequently aggregate, which requires activation of the platelet integrin adhesion receptor αIIBβ3 to bind fibrinogen and link adjacent platelets together in an aggregate.2
Even though platelets express a number of receptors for collagen, it is generally accepted that adhesion of platelets to collagen is mainly mediated by integrin α2β1, whereas activation is mediated by the signal-transducing glycoprotein VI (GPVI).3 GPVI has been recently cloned and shown to belong to the immunoglobulin receptor superfamily.4-7 Fc receptor-γ chain is coexpressed with GPVI in platelets and is phosphorylated on tyrosine residues of its ITAM motif on GPVI-collagen binding.8-12 This phosphorylation process, which is initiated presumably by a src family kinase, activates a signaling cascade resulting in tyrosine phosphorylation and activation of phospholipase Cγ2 (PLCγ2).3,13Therefore, unlike most other platelet agonist receptors that use G proteins for signaling and activation of PLCβ,14GPVI-mediated platelet signal transduction more closely mimics the signaling by immunoglobulin receptors in lymphocytes, which leads to intracellular calcium mobilization and protein kinase C activation independent of G protein activation. Identification of GPVI as the platelet receptor underlying collagen-stimulated signal transduction enabled the use of the GPVI selective agonists convulxin (a snake venom protein belonging to the heterodimeric C-type lectin family)15 and collagen-related peptide (CRP)16as tools to study collagen-induced platelet responses. Convulxin stimulation results in tyrosine phosphorylation of Fc receptor-γ chain, PLCγ2, and several other proteins,15 17 a profile that resembles collagen-induced tyrosine phosphorylation.
There has been a rapid accumulation of knowledge with respect to the signaling molecules involved prior to PLCγ2 activation in GPVI-mediated platelet signaling. However, the molecular mechanisms linking PLCγ2 activation to platelet physiologic responses and aggregation has not been extensively investigated. None of the collagen receptors identified to date have been linked to G proteins.1 However, collagen stimulation of platelets results in secretion of agonists such as ADP.5 In previous reports we showed that ADP-induced platelet aggregation required concomitant signaling from 2 P2 receptor subtypes, the Gq-coupled P2Y1 and the recently cloned Gi-coupled P2Y12 (formerly known as P2TAC).18,19 Similarly, thromboxane A2 was shown to independently mobilize intracellular calcium and induce shape change via Gq but depended exclusively on secretion of other agonists that stimulate Gi-coupled receptors for platelet aggregation.20 Thus, collagen stimulation of platelets may be able to activate G proteins indirectly through secretion of G protein–coupled receptor agonists. It has been known that collagen-induced platelet aggregation occurs indirectly through the generation of thromboxane A2 and release of ADP from dense granules of activated platelets. However, Nakamura et al21 demonstrated that collagen can cause fibrinogen receptor activation on platelets in the presence of ADP scavengers. It was previously reported that collagen-induced platelet aggregation in mouse platelets lacking Gq is dramatically inhibited.22,23This diminished aggregation response to collagen in Gq-deficient mouse platelets could be rescued by supplementing with agonists that activate Gi pathways,22 suggesting that secreted ADP plays an important role in collagen-induced platelet aggregation. Furthermore, cross-talk between GPVI signaling and Gi signaling during collagen-induced platelet aggregation was proposed using a GPVI-specific antibody, JAQ1, wherein Gi signaling blockade using P2Y12 receptor antagonists abolished GPVI-mediated fibrinogen receptor activation.24 In addition, FC receptor γII cross-linking, activating similar pathways as GPVI, has been shown to cause platelet aggregation also dependent on Gi signaling through secreted ADP.25 Hence, it is not clear whether collagen can directly activate fibrinogen receptors on the adherent platelets without a role for positive feedback agonists. Here, we investigated the contribution of secondary G protein signaling to the mechanism of GPVI-stimulated platelet aggregation using the selective agonists convulxin and CRP. In this report, we conclude that collagen activates fibrinogen receptor on adherent platelets directly through GPVI signaling without any significant role for secreted ADP or thromboxane A2. Furthermore, protein kinase C- and calcium-regulated pathways independently contribute to GPVI-mediated platelet aggregation.
Materials and methods
Materials
Adenosine-3′-phosphate-5′-phosphate (A3P5P), apyrase (type V specific activity 4.2 U/mg protein; ATPase/ADPase = 1.4), fibrinogen (type I), MRS2179, and bovine serum albumin (BSA) were obtained from Sigma Chemical (St Louis, MO). Convulxin was purified according to the method of Polgar et al.15[3H]5-Hydroxytryptamine (serotonin; 25.5 Ci/mmol; 940 GBq/mmol) and 9,10-3H(N)oleic acid, (9.2 Ci/mmol; 340 GBq/mmol) were purchased from NEN (Boston, MA). The acetoxymethyl ester of Fura PE-3 was purchased from Teflabs (Austin, TX). The acetoxymethyl ester of the calcium chelator 5,5′-dimethyl-bis-(o-aminophenoxy)ethane-N, N, N′, N′-tetraacetic acid (dimethyl-BAPTA) was purchased from Molecular Probes (Eugene, OR). PAC-1 monoclonal antibody was purchased from Becton Dickinson (San Jose, CA). The stable thromboxane analogue 9,11-dideoxy-9,11-epoxymethanoprostaglandin F2 (U46619) and Ro 31-8220 (bisindolylmaleimide IX) were purchased from Biomol (Plymouth Meeting, PA). AR-C66096 and AR-C69931MX were gifts from Astra Research Laboratories-Charnwood, Loughborough, United Kingdom. SC57101 was a gift from Searle Research and Development (Skokie, IL). Collagen-related peptide (CRP) was prepared as described previously.26 All other chemicals were purchased from Sigma.
Isolation of platelets
Human blood was collected from informed healthy volunteers all of whom are students or staff at Temple University School of Medicine. The donated blood was collected into a one-sixth volume of acid-citrate-dextrose (2.5 g sodium citrate, 1.5 g citric acid, and 2.0 g glucose in 100 mL deionized H2O). Platelet-rich plasma (PRP) was isolated by centrifugation of citrated blood at 180g for 15 minutes at room temperature. PRP was incubated with 1 mM acetylsalicylic acid (aspirin treated) for 30 minutes at 37°C followed by centrifugation at 1000g for 10 minutes at room temperature. The platelet pellet was resuspended in Hepes-buffered Tyrode solution (138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3.0 mM NaH2PO4, 5 mM glucose, 10 mM Hepes, adjusted to pH 7.4) supplemented with 0.2% BSA, and 0.05 U/mL apyrase. The platelet count was adjusted to 3.5 × 108 cells/mL. All experiments were repeated at least 3 times using platelets from different donors.
Analysis of platelet aggregation
Agonist-induced platelet aggregation was determined by measuring the transmission of light through a 0.5-mL sample of washed platelets with constant stirring (900 rpm) in a lumiaggregometer at 37°C (Chrono-Log, Havertown, PA). The recorder output speed was set to 0.2 mm/s. The baseline was set using Tyrode solution as a blank. All treatments were performed just prior to the addition of agonist. Platelets were loaded with 20 μM dimethyl-BAPTA by incubating the washed platelets with the acetoxylmethyl ester form or dimethylsulfoxide (control) for 10 minutes. Platelet aggregation in response to different concentrations of agonist was measured in the presence of exogenously added fibrinogen (1 mg/mL) whenever Ro 31-8220 (10 μM) or 5-5′-dimethyl-BAPTA (20 μM) was used. Extent of aggregation was measured 210 seconds after the addition of the agonist. The maximum aggregation extent in the absence of any agent was taken as 100% and the remaining values were normalized to this value. In the case of collagen, extent of aggregation in the presence of SC57101, a fibrinogen receptor antagonist, was taken as 0%. All experiments were repeated at least 3 times using platelets from different donors.
Measurement of platelet secretion
[3H]5-HT was loaded into platelets by incubating PRP with 1 μCi/mL (37 KBq/mL) for 30 minutes, followed by 15 minutes at room temperature. Platelets were then processed as described above. Imipramine was added to the Tyrode solution at a final concentration of 1 μM during resuspension of the washed platelets to prevent reuptake of secreted [3H]5-HT. The activation of labeled [3H]5-HT platelets was performed in the aggregometer at 37°C with stirring (900 rpm) and was stopped after 2 minutes with the addition of formaldehyde/EDTA. Samples were collected and centrifuged at 5000g for 1 minute, and the radioactivity of the supernatant was measured using Wallac 1409 liquid scintillation counter (Perkin Elmer Life Sciences, Gaithersburg, MD). Platelet secretion was expressed as the percentage of the total [3H]5-HT content.
Measurement of cytoplasmic concentrations of ionized Ca++
The PRP was incubated at 37°C with 3 μM Fura PE-3 acetoxymethyl ester for 30 minutes followed by 15 minutes at room temperature. Platelets were then isolated as described above. Aliquots (0.5 mL) of the platelet suspension were stirred (900 rpm) in a water-jacketed cuvette maintained at 37°C during activation. Fluorescence was constantly measured using an Aminco-Bowman Series 2 luminescence spectrometer with settings of 340 nm (excitation) and 510 nm (emission). All experiments were repeated at least 3 times using platelets from different donors.
Platelet adhesion to collagen
The method of Smith and Dangelmaier27 was used to determine platelet adhesion to collagen. Briefly, PRP was incubated with [3H]-oleic acid (1 μCi/mL; 37 KBq/mL) and 1 mM aspirin at 37°C for 1 hour. Platelets were then isolated as described above. One milliliter samples were stirred at 800 rpm in an aggregometer at 37°C with addition of SC57101 (1 μM), SQ29.548 (100 μM), A3P5P (1 mM), and AR-C66096 (1 μM). Collagen (50 μg/mL) was added and the incubation continued for 2 minutes. Some samples were treated with Ro 31-8220 (10 μM, 5 minutes at 37°C) or dimethyl-BAPTA (20 μM, 10 minutes at 37°C) before collagen treatment. Following incubation, the platelet suspensions were decanted into a manifold containing 10-μm nylon disks under vacuum. The disks were rinsed with 2 mL Tyrode solution and either transferred directly to liquid scintillation cocktail for measurement of adhesion or placed in a 6-well plate for PAC-1 binding measurements.
PAC-1 binding to platelets adhered to collagen
The 10-μm nylon disks were placed in 6-well plates, each well containing 2 mL Hanks balanced salt solution (HBSS). The disks were blocked with HBSS containing 1% BSA for 45 minutes at room temperature with gentle agitation. The blocking solution was replaced with a PAC-1 antibody (1:1000 dilution in blocking buffer) and agitated for 30 minutes at room temperature. The disks were then washed 3 times in HBSS, blocked for an additional 15 minutes at room temperature, and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000 dilution in blocking buffer) for 30 minutes at room temperature. The disks were washed 3 times and exposed to O-phenylenediamine (OPD) and H2O2. When adequate color change was reached (10 minutes), an equal volume of sample was mixed with 2.5M H2SO4 and the optical density was read at 490 nm. Data are expressed as relative PAC-1 binding (a ratio of the absorbance at 490 nm to the percent of platelets adhered to collagen times 100%).
Results
Effect of ADP scavengers on GPVI-mediated platelet aggregation
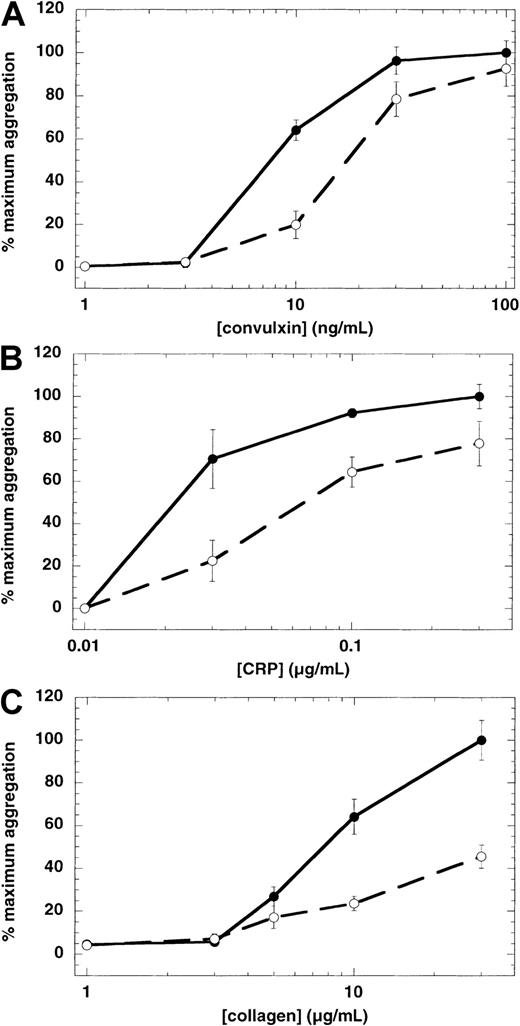
In the cases of ADP and thromboxane A2, it is known that concomitant signaling through both Gi-coupled and Gq-coupled receptors is required for platelet aggregation.19,28 29However, the GPVI receptor does not couple directly to either Gi or Gq. Therefore, to determine whether G protein–coupled ADP receptors play any significant role in GPVI-mediated platelet aggregation through secreted agonists, we used ADP-scavenging enzyme systems. Because we used aspirin-treated platelets throughout the study, we have eliminated the generation of thromboxane A2 and its contribution to platelet activation in this system. A concentration-response analysis of convulxin-mediated aggregation revealed that platelet shape change occurred at lower concentrations, whereas higher concentrations caused platelet aggregation (Figure 1). When creatine phosphate (10 mM) and creatine phosphokinase (40 U/mL) were used as ADP scavengers, a significant inhibitory effect was achieved on convulxin-induced platelet aggregation at lower convulxin concentrations (Figure 2A). The concentration-response curve shifted slightly to the right at lower concentrations of convulxin, but the extent of platelet aggregation was unaffected with higher concentrations of convulxin. However, in the case of collagen or CRP, the ADP scavenger system shifted the concentration response curves to the right with all concentrations (Figure 2B,C). This implied that at low concentrations of convulxin and all concentrations of collagen and CRP, platelet aggregation is potentiated by secreted ADP.
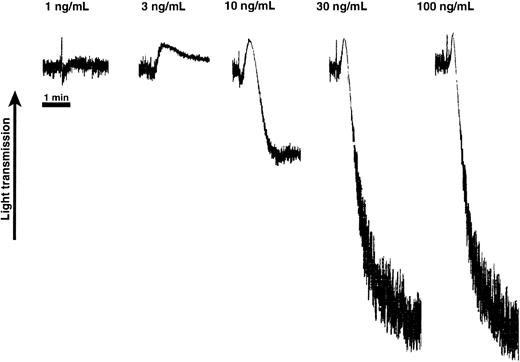
Effect of varying concentrations of convulxin on platelet shape change and aggregation.
Aspirin-treated human platelets were washed and resuspended in Hepes-buffered Tyrode solution. Platelet response was measured in the presence of extracellular fibrinogen (1 mg/mL) and was performed in a cuvette maintained at 37°C with stirring. The ordinate represents the observed changes in light absorbance (optical density) due to light scattering by the platelets. These tracings are representative of results observed on 3 separate occasions from 3 different donors.
Effect of varying concentrations of convulxin on platelet shape change and aggregation.
Aspirin-treated human platelets were washed and resuspended in Hepes-buffered Tyrode solution. Platelet response was measured in the presence of extracellular fibrinogen (1 mg/mL) and was performed in a cuvette maintained at 37°C with stirring. The ordinate represents the observed changes in light absorbance (optical density) due to light scattering by the platelets. These tracings are representative of results observed on 3 separate occasions from 3 different donors.
Effect of ADP scavengers on GPVI-mediated platelet aggregation.
Washed, aspirin-treated human platelets were treated with an ADP scavenger system, creatine phosphate (10 mM), and creatine phosphokinase (40 U/mL), then challenged with various concentrations of convulxin (A), CRP (B), and collagen (C). Extent of aggregation was measured 210 seconds after the addition of the agonist in the absence (●) or presence (○) of the ADP scavenger system. The maximum aggregation extent in the absence of the ADP scavenger system was taken as 100% and the remaining values were normalized to this value. In the case of collagen, extent of aggregation in the presence of SC57101, a fibrinogen receptor antagonist, was taken as zero. Each data point is the mean ± SE of 3 measurements with different donors.
Effect of ADP scavengers on GPVI-mediated platelet aggregation.
Washed, aspirin-treated human platelets were treated with an ADP scavenger system, creatine phosphate (10 mM), and creatine phosphokinase (40 U/mL), then challenged with various concentrations of convulxin (A), CRP (B), and collagen (C). Extent of aggregation was measured 210 seconds after the addition of the agonist in the absence (●) or presence (○) of the ADP scavenger system. The maximum aggregation extent in the absence of the ADP scavenger system was taken as 100% and the remaining values were normalized to this value. In the case of collagen, extent of aggregation in the presence of SC57101, a fibrinogen receptor antagonist, was taken as zero. Each data point is the mean ± SE of 3 measurements with different donors.
Effect of receptor-selective antagonists on GPVI-mediated platelet aggregation
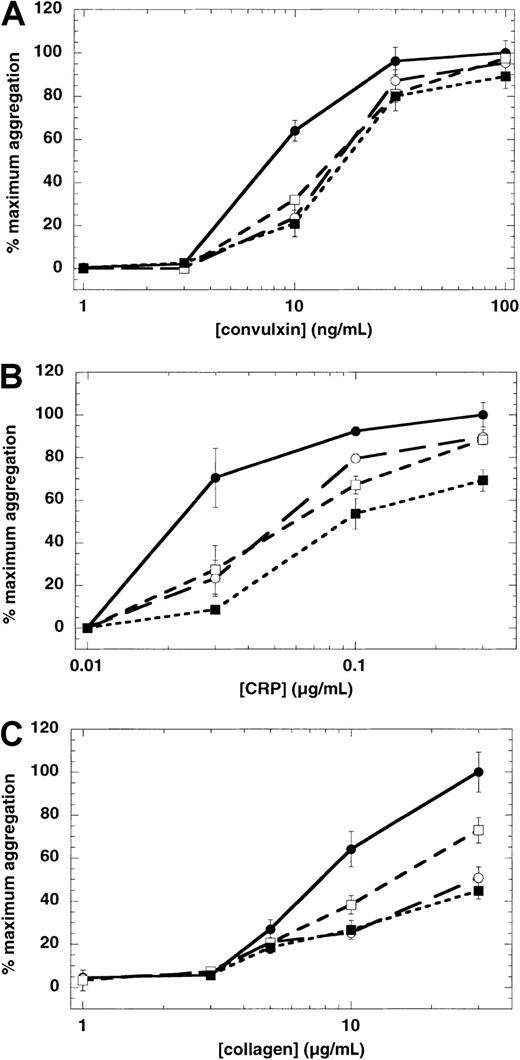
To elucidate the role of the specific ADP receptor involved in GPVI-mediated platelet aggregation, we used ADP receptor selective antagonists. The Gq-coupled P2Y1 receptor antagonist A3P5P and the Gi-coupled P2Y12 receptor antagonist AR-C66096 marginally inhibited convulxin-induced platelet aggregation at the low convulxin concentrations (Figure 3A). The Gi-coupled α2A receptor antagonist yohimbine was without any significant effect (not shown). When both P2 receptor antagonists were combined, no cumulative effect on convulxin-mediated aggregation was observed (Figure 3A). When collagen or CRP was used as a GPVI agonist, either the P2Y1 receptor antagonist (MRS2179) or the P2Y12 receptor antagonist (AR-C69931MX) shifted the concentration-response curve to the right (Figure 3B,C). In case of collagen, MRS2179 appeared to be more potent than AR-C69931MX in causing the rightward shift, whereas both antagonists were equally effective in CRP-induced platelet aggregation. When both P2 receptor antagonists were combined, the concentration-response curve for CRP-induced platelet aggregation shifted further to the right.
Effect of receptor-specific antagonists on GPVI-mediated platelet aggregation.
Washed, aspirin-treated human platelets were treated with various antagonists (as indicated), then challenged with various concentrations of convulxin (A), CRP (B), and collagen (C). Extent of aggregations were measured 210 seconds after the addition of the agonist, in the absence of any antagonist (●), in the presence of a P2Y1 antagonist (○), a P2Y12 antagonist (■), or both P2Y1 and P2Y12 antagonists (▪). The maximum aggregation extent in the absence of any antagonist was taken as 100% and the remaining values were normalized to this value. In the case of collagen, extent of aggregation in the presence of SC57101, a fibrinogen receptor antagonist, was taken as zero. Concentrations of agents used were: P2Y1 antagonists A3P5P, 1 mM (A) or MRS2179, 100 μM (B,C); P2Y12 antagonists AR-C66096, 1 μM (A) or AR-C69931MX, 100 nM (B,C). Each data point is the mean ± SE of 3 measurements with different donors.
Effect of receptor-specific antagonists on GPVI-mediated platelet aggregation.
Washed, aspirin-treated human platelets were treated with various antagonists (as indicated), then challenged with various concentrations of convulxin (A), CRP (B), and collagen (C). Extent of aggregations were measured 210 seconds after the addition of the agonist, in the absence of any antagonist (●), in the presence of a P2Y1 antagonist (○), a P2Y12 antagonist (■), or both P2Y1 and P2Y12 antagonists (▪). The maximum aggregation extent in the absence of any antagonist was taken as 100% and the remaining values were normalized to this value. In the case of collagen, extent of aggregation in the presence of SC57101, a fibrinogen receptor antagonist, was taken as zero. Concentrations of agents used were: P2Y1 antagonists A3P5P, 1 mM (A) or MRS2179, 100 μM (B,C); P2Y12 antagonists AR-C66096, 1 μM (A) or AR-C69931MX, 100 nM (B,C). Each data point is the mean ± SE of 3 measurements with different donors.
Effect of protein kinase C inhibition on GPVI-mediated platelet responses
We have previously shown that U46619-induced aggregation is dependent on secretion because the aggregation was completely blocked by the cell-permeable PKC inhibitor Ro 31-8220, a known inhibitor of platelet secretion.30 Therefore, we wanted to determine whether convulxin-induced aggregation was also sensitive to inhibition of secretion by Ro 31-8220. Platelet secretion of dense granule contents is totally abolished by 10 μM Ro 31-8220 in response to different concentrations of convulxin (Figure4). In the presence of Ro 31-8220, lower concentrations of convulxin only caused shape change and did not induce significant aggregation (Figure 5A). However, when higher concentrations of convulxin were used, the concentration response curve of convulxin-induced platelet aggregation shifted to the right in the presence of Ro 31-8220. Similarly the concentration-response curves for platelet aggregation were shifted to the right with collagen (Figure 5B) in the presence of Ro 31-8220.
Effect of Ro 31-8220 on convulxin-induced platelet dense granule release.
Aspirin-treated and [3H]-serotonin loaded washed platelets were stimulated with varying concentrations of convulxin in the presence (shaded bars) or absence (open bars) of Ro 31-8220 (10 μM). The released [3H]-serotonin was measured as described in “Materials and methods.” Each data point is the mean ± SE of 3 measurements using different donors.
Effect of Ro 31-8220 on convulxin-induced platelet dense granule release.
Aspirin-treated and [3H]-serotonin loaded washed platelets were stimulated with varying concentrations of convulxin in the presence (shaded bars) or absence (open bars) of Ro 31-8220 (10 μM). The released [3H]-serotonin was measured as described in “Materials and methods.” Each data point is the mean ± SE of 3 measurements using different donors.
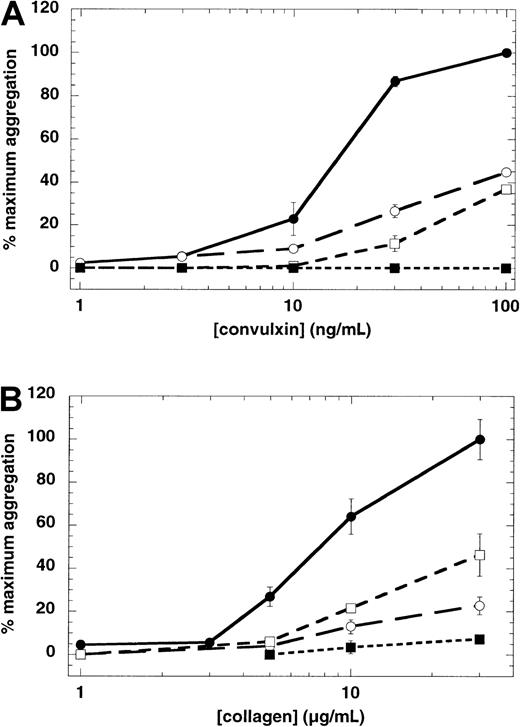
Effect of Ro 31-8220 and 5-5′-dimethyl-BAPTA on GPVI-mediated platelet aggregation.
Aspirin-treated human platelets were washed and resuspended in Hepes-buffered Tyrode solution. Platelet aggregation in response to different concentrations of convulxin (A) or collagen (B) was measured in the presence of exogenously added fibrinogen (1 mg/mL). Extent of aggregation was measured 210 seconds after the addition of the agonist, without pretreatment of platelets with any agent (●), pretreatment with Ro 31-8220 (10 μM) (○), dimethyl-BAPTA (20 μM) (■), or both Ro 31-8220 and dimethyl-BAPTA (▪). The maximum aggregation extent in the absence of any agent was taken as 100% and the remaining values were normalized to this value. In the case of collagen, extent of aggregation in the presence of SC57101, a fibrinogen receptor antagonist, was taken as zero. Each data point is the mean ± SE of 3 measurements using platelets from different donors.
Effect of Ro 31-8220 and 5-5′-dimethyl-BAPTA on GPVI-mediated platelet aggregation.
Aspirin-treated human platelets were washed and resuspended in Hepes-buffered Tyrode solution. Platelet aggregation in response to different concentrations of convulxin (A) or collagen (B) was measured in the presence of exogenously added fibrinogen (1 mg/mL). Extent of aggregation was measured 210 seconds after the addition of the agonist, without pretreatment of platelets with any agent (●), pretreatment with Ro 31-8220 (10 μM) (○), dimethyl-BAPTA (20 μM) (■), or both Ro 31-8220 and dimethyl-BAPTA (▪). The maximum aggregation extent in the absence of any agent was taken as 100% and the remaining values were normalized to this value. In the case of collagen, extent of aggregation in the presence of SC57101, a fibrinogen receptor antagonist, was taken as zero. Each data point is the mean ± SE of 3 measurements using platelets from different donors.
Effects of 5,5′-dimethyl-BAPTA on convulxin-induced platelet aggregation
We investigated the role of increased cytosolic Ca++as a possible means by which platelets aggregate in response to convulxin. The normal increase in cytosolic Ca++, which occurs in response to convulxin, does not occur in platelets loaded with the calcium chelator 5,5′-dimethyl-BAPTA (not shown). In the absence of increased cytosolic Ca++, platelet aggregation in response to convulxin or collagen was inhibited significantly (Figure 5). As in the case of Ro 31-8220 treatment, the concentration-dependence of convulxin- or collagen-induced platelet aggregation shifted to the right in the presence of dimethyl-BAPTA.
Because blockade of either the PKC-regulated pathway (with Ro 31-8220) or calcium-regulated pathway (with dimethyl-BAPTA treatment) did not abolish GPVI-mediated platelet aggregation, we investigated the effect of inhibiting both the pathways on GPVI-mediated platelet aggregation. In the presence of both Ro 31-8220 and dimethyl-BAPTA, convulxin- or collagen-induced platelet aggregation is abolished (Figure 5).
Although platelet aggregation reflects the activation of fibrinogen receptor, in the case of collagen, platelet aggregation is a reflection of fibrinogen receptor activation on platelets not adherent to collagen fibers. To evaluate the role of PKC-regulated and calcium-regulated pathways in GPVI-induced fibrinogen receptor activation on platelets adherent to collagen, we established a PAC-1 binding assay. In this assay, bound PAC-1 was estimated by a colorimetric assay using an enzyme-linked secondary antibody. The platelets adherent to collagen were estimated by [3H]-oleic acid. Thus, the ratio of PAC-1 binding to adherent platelets reflects relative fibrinogen receptor activation. As seen in Figure 6, fibrinogen receptor is activated on aspirin-treated platelets adherent to collagen fibers, even in the presence of ADP receptor antagonists. This indicated that collagen activates fibrinogen receptor directly on the adherent platelet without a role for ADP and thromboxane A2. Furthermore, pretreatment of platelets with Ro 31-8220 or dimethyl-BAPTA resulted in a decrease in fibrinogen receptor activation on platelets adherent to collagen. The blockade of both PKC-regulated and calcium-regulated pathways resulted in dramatic inhibition of fibrinogen receptor activation. These results indicate that collagen, through GPVI-mediated stimulation of PLCγ2, causes fibrinogen receptor activation on adherent platelets independently through both PKC-regulated and calcium-regulated pathways.
Effect of Ro 31-8220 or dimethyl-BAPTA on collagen-induced fibrinogen receptor activation.
Collagen was added to aspirin-treated, washed human platelets that were pretreated with Ro 31-8220 (10 μM) or dimethyl-BAPTA (20 μM) or both in the presence of feedback inhibitors, and filtered through 10-μm filters. The filters were then probed with PAC-1 followed by HRP-conjugated secondary antibody, and the color was measured using OPD as the substrate. The adhesion was measured by counting of [3H]-oleic acid in platelets. Adhesion ranged from 23% to 32%. The ratio of PAC-1 binding (OPD reading) to fraction of adhesion was then expressed as relative PAC-1 binding. Each data point is the mean ± SE of 3 measurements using platelets from different donors.
Effect of Ro 31-8220 or dimethyl-BAPTA on collagen-induced fibrinogen receptor activation.
Collagen was added to aspirin-treated, washed human platelets that were pretreated with Ro 31-8220 (10 μM) or dimethyl-BAPTA (20 μM) or both in the presence of feedback inhibitors, and filtered through 10-μm filters. The filters were then probed with PAC-1 followed by HRP-conjugated secondary antibody, and the color was measured using OPD as the substrate. The adhesion was measured by counting of [3H]-oleic acid in platelets. Adhesion ranged from 23% to 32%. The ratio of PAC-1 binding (OPD reading) to fraction of adhesion was then expressed as relative PAC-1 binding. Each data point is the mean ± SE of 3 measurements using platelets from different donors.
Discussion
Upon vascular injury, adhesion of platelets to collagen in the subendothelium is the first step in platelet activation. Because collagen forms insoluble fibers at physiologic pH and represents a solid surface, the nature of the collagen-platelet interaction is more complicated than with other agonists. Activation of collagen-adhered platelets results in generation and secretion of soluble agonists, namely thromboxane A2 and ADP, that further activate nonadherent platelets.31,32 Therefore, the platelet aggregation response to low concentrations of collagen that is observed in an aggregometer is the reflection of aggregation of nonadherent platelets rather than the collagen-adherent platelets. Thus, the defective platelet aggregation response to collagen in Gαq–deficient mice23 and P2Y1 receptor–deficient mice,33,34instead of providing information on the response in collagen-adherent cells, rather reflects the effect of secreted granule contents on the cells that have not yet adhered to collagen. When higher concentrations of collagen are used, many platelets adhere to collagen fibers and this phenomenon is in some ways similar to limited platelet aggregation in an aggregometer. Interestingly, selective activation of GPVI using JAQ1, a monoclonal antibody against GPVI, indicated that GPVI-mediated fibrinogen receptor activation depends on Gi signaling,24whereas collagen fibrils have been shown to activate fibrinogen receptor independently of secreted ADP.21 Thus, whether collagen can activate fibrinogen receptors on the platelets that adhere to it independently of thromboxane A2 and ADP was not clearly addressed. In this study we address this question and delineate the signaling pathways downstream of collagen activation in platelets. Because we have used aspirin-treated and washed platelets throughout the study, we have eliminated the generation of thromboxane A2 and its feedback contribution to the platelet activation.
Among the many proposed platelet surface receptors for collagen, GPVI has gained credence as the receptor responsible for collagen-induced platelet signal transduction.8,11,35-40 GPVI signaling is rather distinct from that of G protein–coupled receptor agonists in that it goes through a series of tyrosine phosphorylation steps that are not yet fully understood.10,35 Because collagen is insoluble at physiologic pH, it is imperative to use a soluble collagen receptor agonist to delineate the signal transduction events through GPVI on platelets. By virtue of its specific interaction with collagen receptor GPVI, the snake venom protein convulxin15,41,42 or CRP16 is well suited for this task.
Because convulxin induces platelet shape change at lower concentrations and aggregation at higher concentrations (Figure 1), we speculated that convulxin-induced aggregation requires secretion of granule contents as is the case for U46619-induced platelet aggregation.20 The role of secreted ADP on aggregation mediated by convulxin, CRP, or collagen was examined using receptor-selective antagonists as well as ADP scavengers. Significant inhibition of aggregation was seen with the ADP receptor antagonists and ADP scavenger enzymes at low concentrations of convulxin, whereas little if any effect of the inhibitors was seen at high convulxin concentrations (Figures 2A and3A). The Gi-coupled α2A receptor antagonist yohimbine had no effect on convulxin-mediated aggregation (not shown). This suggests that stimulation of G protein–coupled receptors by secreted components (ie, ADP and epinephrine) is not essential for convulxin-induced platelet aggregation, but instead can potentiate the response initiated by activation of GPVI signaling. However, in the case of CRP or collagen, both ADP scavengers and ADP receptor antagonists inhibited platelet aggregation at all concentrations. Thus, the defective platelet aggregation observed in response to collagen in Gαq-deficient mice23 and P2Y1 receptor–deficient mice33,34 may reflect the need for secreted granule contents in potentiation of initial GPVI signaling. We have observed that the P2Y12 receptor antagonist (AR-C69931MX) had less inhibitory effect than the P2Y1 receptor antagonist (MRS2179) on collagen-induced platelet aggregation. Aggregation mediated by high convulxin concentrations is not inhibited by the antagonism of ADP receptors because the PKC- and calcium-regulated pathways are still activated by the PLCγ2 pathway via GPVI signaling. The potentiation of convulxin-mediated aggregation by G protein–coupled receptor signaling is seen when lower PLCγ2 activity from low convulxin concentrations synergizes with the activation of PLCβ2from stimulation by ADP. However, this synergism may become less important at higher convulxin concentrations in that greater PLCγ2 activity would be stimulated43 and less dependence on G protein–coupled PLC activity is required. Because convulxin is a soluble agonist and a multimeric protein, it is conceivable that, due to much higher affinity for GPVI, convulxin can cause much better clustering of possibly more GPVI molecules than conformationally constrained collagen fibers. Similarly, although CRP is a soluble agonist, its small size might become limiting in efficient clustering of GPVI and subsequent signaling. Thus, convulxin at higher concentrations can cause platelet aggregation independently of secreted ADP, whereas CRP depends on potentiating effects of secreted ADP.
Consistent with the role for PKC activation in platelet secretion,20,30 44 we observed that Ro 31-8220 inhibited convulxin-induced secretion in platelets (Figure 4). The presence of Ro 31-8220 significantly inhibited aggregation even at higher concentrations of convulxin. However, the studies with ADP scavengers and receptor antagonists revealed that convulxin can cause platelet aggregation in the absence of secreted ADP. These results suggested that convulxin-induced platelet aggregation may be dependent on PKC activation. In addition, the inability of Ro 31-8220 to abolish platelet aggregation at high concentrations of convulxin raises the possibility that multiple signaling pathways contribute to convulxin-induced platelet aggregation. Thus, we investigated the contribution of GPVI-induced calcium mobilization on platelet aggregation through the use of the high affinity Ca++chelator 5,5′-dimethyl-BAPTA. In a profile similar to that seen with Ro 31-8220, the aggregation induced by lower concentrations of convulxin was abolished in the presence of dimethyl-BAPTA, whereas up to 60% inhibition occurred at higher concentrations (Figure 5). Under conditions in which PKC activity and calcium mobilization are simultaneously inhibited by Ro 31-8220 and dimethyl-BAPTA, respectively, GPVI-stimulated platelet aggregation was completely inhibited even at high convulxin concentrations (Figure 5). Similar results were obtained with collagen-induced platelet aggregation (Figure 5B).
Fibrinogen receptor activation occurred on platelets adherent to collagen fibers even in the presence of ADP receptor antagonists (Figure 6). These data confirm the previous studies of Nakamura and coworkers21,39 through the use of receptor antagonists. When the PKC-regulated pathway or calcium-regulated pathway is blocked, fibrinogen receptor is still activated on collagen-adherent platelets. Thus on the adherent platelet, collagen can cause fibrinogen receptor activation through either the PKC-regulated pathway or the calcium-regulated pathway. When the PKC-regulate pathway is inhibited by Ro 31-8220, both phosphorylation events downstream of PKC leading to αIIbβ3 activation and platelet secretion are blocked. Under these conditions, the calcium-sensitive pathway alone is sufficient to cause fibrinogen receptor activation. Conversely, with dimethyl-BAPTA treatment, calcium-regulated pathways leading to fibrinogen receptor activation and platelet secretion are inhibited, leaving only PKC-regulated pathways to mediate αIIbβ3 activation. In the presence of both dimethyl-BAPTA and Ro 31-8220, both calcium-regulated and PKC-regulated pathways are blocked leading to abolished αIIbβ3 activation. Thus, either of the molecular events downstream of PLC activation (namely, activation of PKC and mobilization of calcium ions from intracellular stores through diacylglycerol and inositol triphosphate, respectively) is independently capable of fibrinogen receptor activation. Conversely, when collagen-induced PLCγ2 activity is absent, platelet secretion and aggregation are abolished, as is established with mice deficient in PLCγ2.45
In conclusion, our results show that GPVI-stimulated aggregation is mediated independently by 2 different mechanisms, namely the PKC- and calcium-regulated pathways. The role of secreted agonists in response to GPVI-mediated signaling appears to be stimulation of G protein–coupled ADP receptors and the resultant potentiation of the primary aggregation response.
Supported by research grants HL64943 and HL60683 from the National Institutes of Health. T.M.Q. is the recipient of a Postdoctoral Fellowship from the Pennsylvania-Delaware affiliate of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
Subsequent to the original submission of this manuscript, Atkinson et al46 showed that Ca++ and protein kinase C, but not release of the secondary agonists ADP and thromboxane A2, are required for full aggregation induced by convulxin, whereas the response induced by collagen shows a much greater dependence on secretion of secondary agonists.
Author notes
Satya P. Kunapuli, Temple University Medical School, Department of Physiology, Rm 224 OMS, 3420 N Broad St, Philadelphia, PA 19140; e-mail: kunapuli@nimbus.temple.edu.

![Fig. 4. Effect of Ro 31-8220 on convulxin-induced platelet dense granule release. / Aspirin-treated and [3H]-serotonin loaded washed platelets were stimulated with varying concentrations of convulxin in the presence (shaded bars) or absence (open bars) of Ro 31-8220 (10 μM). The released [3H]-serotonin was measured as described in “Materials and methods.” Each data point is the mean ± SE of 3 measurements using different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3228/6/m_h80922502004.jpeg?Expires=1765885944&Signature=aWzi7LTbsT7yvdNTHKfIO3Ft0OKbVVuTlCosqwzgO4~yd~U5Dd2Xqx92YnLbK5U-mpIdB-oJiNH4T3E-qMkODdORVXSjCR32PEo2hFLrjelmPbiiXVElnPc9FMqDJZNvPS0JHzFkUqd5fgQ8MsADeKhi6KEeAZ2GnXJWgWiKHuGbke4nHUx9i-dc4p3kHA3G9-3fwx9ylNSX-4IzwNijS2Ix6hK8wTz5w1RPa4zvQ0~VPPHOyldIFosc-FaosNzRhl5L81s8K6feirGypBVVO1XEOodUfaGRoa9RgQa6cTY48MiKvvXq5OGtLL8wD3252i02KBNyRYIPxrrYnN~RUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Effect of Ro 31-8220 or dimethyl-BAPTA on collagen-induced fibrinogen receptor activation. / Collagen was added to aspirin-treated, washed human platelets that were pretreated with Ro 31-8220 (10 μM) or dimethyl-BAPTA (20 μM) or both in the presence of feedback inhibitors, and filtered through 10-μm filters. The filters were then probed with PAC-1 followed by HRP-conjugated secondary antibody, and the color was measured using OPD as the substrate. The adhesion was measured by counting of [3H]-oleic acid in platelets. Adhesion ranged from 23% to 32%. The ratio of PAC-1 binding (OPD reading) to fraction of adhesion was then expressed as relative PAC-1 binding. Each data point is the mean ± SE of 3 measurements using platelets from different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/9/10.1182_blood.v99.9.3228/6/m_h80922502006.jpeg?Expires=1765885945&Signature=a92s~b~TtHqXWFCPwHbfmfMbmdG0CBvJ6sD8Hx7oEnQuYniSHcf47YeI1DSppngTvbMeX9hfiXFbDOtOjV807XNQMxi8bp6tFl4UQqSyHmBC1~T28xVqpx4QGLK1DQb8lueGYTsrTN8ax0DMUv1Mwn5UnaIUUP3MIJO2UUm0FAsYPbmER1Blwa32XRkvrxD1GOFViTzmyceYcV4mujrd99WVxBNFVgDB2iisAyGlNq8ke2i~9YVkfuBKd13YqEwtcRcUxmKP9ZSgZw1Au7vD2VgyUpFprClZmETLLofDlYN0HYvp1Q2nQ8h22AqDmsieqBCQAev~GNNRrQpwEvuSpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal