Abstract
Vitamin C is present in the cytosol as ascorbic acid, functioning primarily as a cofactor for enzymatic reactions and as an antioxidant to scavenge free radicals. Human granulocyte macrophage–colony-stimulating factor (GM-CSF) induces an increase in reactive oxygen species (ROS) and uses ROS for some signaling functions. We therefore investigated the effect of vitamin C on GM-CSF–mediated responses. Loading U937 cells with vitamin C decreased intracellular levels of ROS and inhibited the production of ROS induced by GM-CSF. Vitamin C suppressed GM-CSF–dependent phosphorylation of the signal transducer and activator of transcription 5 (Stat-5) and mitogen-activated protein (MAP) kinase (Erk1 and Erk2) in a dose-dependent manner as was phosphorylation of MAP kinase induced by both interleukin 3 (IL-3) and GM-CSF in HL-60 cells. In 293T cells transfected with alpha and beta GM-CSF receptor subunits (αGMR and βGMR), GM-CSF–induced phosphorylation of βGMR and Jak-2 activation was suppressed by vitamin C loading. GM-CSF–mediated transcriptional activation of a luciferase reporter construct containing STAT-binding sites was also inhibited by vitamin C. These results substantiate the importance of ROS in GM-CSF signaling and indicate a role for vitamin C in downmodulating GM-CSF signaling responses. Our findings point to vitamin C as a regulator of cytokine redox-signal transduction in host defense cells and a possible role in controlling inflammatory responses.
Introduction
Human granulocyte macrophage–colony-stimulating factor (GM-CSF) is a glycoprotein cytokine involved in the growth, differentiation, and maturation of myeloid precursor cells and in the function of mature neutrophils, eosinophils, and monocytes.1 GM-CSF is secreted by a variety of cells and tissue types in response to mediators of inflammation such as tumor necrosis factor (TNF) and interleukin 1 (IL-1), and GM-CSF is thought to be involved in pathologic inflammatory conditions such as rheumatoid arthritis.2 3
GM-CSF elicits its diverse responses by interacting with a receptor complex that consists of 2 transmembrane glycoprotein subunits known as GM-CSF receptor alpha (αGMR) and beta (βGMR) of 85 kd and 120 kd, respectively.4-6 Whereas αGMR binds to GM-CSF with low affinity, βGMR itself is unable to bind GM-CSF, but forms a high-affinity receptor complex with αGMR.7,8 GM-CSF receptors are expressed widely in hematopoietic tissue, however, they are also present in nonhematopoietic cells and tissues including placenta, endothelium, melanocytes, oligodendrocytes, and prostate tissue as well as various tumors.9-14 Membrane signaling events initiated by GM-CSF include the activation of Jak-2 and tyrosine phosphorylation of the βGMR, leading to transcriptional activation of the signal transducers and activators of transcription (STATs).15-17 Other responses to GM-CSF include the activation of the ras/raf signaling pathway, inducing mitogen-activated protein (MAP) kinase phosphorylation and downstream transcription factors.18 19
Recent evidence indicates that growth factors induce the generation of reactive oxygen species (ROS) as chemical second messenger molecules.20,21 GM-CSF induces a rapid increase in ROS required for signaling.22 Since vitamin C is a critical biologic antioxidant in the cell whose role in redox-signaling responses is poorly understood,23 we investigated its effect on GM-CSF–dependent signaling pathways. Vitamin C is transported into most cells in the oxidized form, dehydroascorbic acid (DHA), via facilitative glucose transporters (Gluts)24,25and as ascorbic acid (AA) in specialized cells, by sodium-dependent AA transporters.26 When transported as DHA, vitamin C is rapidly reduced inside the cell and accumulates as AA.27Recently, Bowie and O'Neill reported that vitamin C inhibits TNF-dependent activation of NF-kappa beta.28
We loaded the human monocytic cell-line U937, HL-60, and 293T kidney cells with vitamin C by incubation with DHA and found that vitamin C inhibits GM-CSF–induced signaling responses at a proximal point in the pathway. These results indicate a role for vitamin C in downregulating GM-CSF signaling. Since GM-CSF itself increases cellular uptake of vitamin C,29 the signal-modulating effects of the vitamin may be part of a homeostatic mechanism for controlling immune responses.
Materials and methods
Cell culture
Human monocytic U937 cells were obtained from American Type Culture Collection (ATCC), and grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine, and 1% penicillin/streptomycin. 293T30 kidney cells were grown in Dulbecco modified Eagle medium-high glucose (DME-HG) medium supplemented with 10% FBS, 1% penicillin/streptomycin, 1% L-glutamine, and 1% modified Eagle medium (MEM) sodium pyruvate. Human myeloid HL-60 cells were obtained from ATCC and grown in Iscoves modified Dulbecco medium (IMDM) supplemented with 10% heat-inactivated FBS, 1% L-glutamine, and 1% penicillin/streptomycin. Human megakaryocytic MO7e cells were grown in IMDM with 10% FBS, 1% L-glutamine, 1% penicillin/streptomycin, and supplemented with 400 pM GM-CSF.
AA, DHA, GM-CSF, and IL-3 treatments
Cells were incubated for 16 hours in serum-free medium containing 0.2% bovine serum albumin (BSA), and then washed with phosphate buffered saline (PBS) before incubation with 15 mM HEPES, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 0.8 mM at pH 7.4 (incubation buffer, pH 7.4), for 1 hour at 37°C. Next, cells were incubated with different concentrations of DHA or AA for 30 minutes at 37°C, washed, resuspended (1 × 106 cells/mL) in the same buffer and incubated with 1 nM GM-CSF (R&D Systems, Minneapolis, MN) or with 0.6 nM IL-3 (R&D Systems) for the indicated period of time. Cells were then washed with cold PBS, resuspended in 20 mM Tris, 1 mM ethyleneglycotetraacetic acid (EGTA), 1 mM NaV, 50 mM NaF, and protease inhibitors cocktail at pH 7.4, and lysed by sonication on ice (3 times, 15 seconds each). The protein concentration was measured using the Bradford Method (Pierce, Rockford, IL). All reagents were from Sigma (St Louis, MO).
Cellular proliferation assay
MO7e cells were incubated with 500 μM DHA in incubation buffer for 30 minutes at 37°C and then washed with PBS. Tissue culture plates (96-well) were seeded with 17 × 103 cells per well in the presence or absence of 400 pM GM-CSF and allowed to grow for 24 hours or 48 hours. Cell proliferation was measured by colorimetric assay using WST-1 reagent (Abs 450-690 nm) (Roche Molecular Biochemicals, Indianapolis, IN).
Analysis of phosphorylation of MAP kinase and Stat5
Cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane (Bio-rad Laboratories, Hercules, CA) in a buffer containing 25 mM Tris pH 8.3, 192 mM glycine, and 20% methanol in a semidry system (Bio-rad Laboratories) for 15 minutes, 15 V, at 25°C. Residual binding sites on the membrane were blocked by incubating the membrane in 20 mM Tris-HCl (pH 7.4), 0.15 M NaCl (TBS), containing 5% nonfat dry milk and 0.1% Tween-20, for 2 hours at 25°C (blocking buffer). Membranes were incubated with either polyclonal antiphospho p44/42 MAP kinase antibody (New England Biolabs, Beverly, MA), or polyclonal antiphospho Stat5 antibody (Cell Signaling Technology, Beverly, MA), for 16 hours at 4°C. The primary antibody was removed, and the blots were washed 3 times in TBS. To detect antibody reaction, the blots were incubated for 1 hour with anti–rabbit IgG horseradish peroxidase (HRP)–conjugated antibody (Bio-rad) diluted 1:3000 in blocking buffer, washed with TBS, and the protein bands were revealed using the enhanced chemiluminescence (ECL) Western blotting detection system (Amersham Pharmacia Biotech, Piscataway, NJ). To ensure the presence of equal amounts of proteins, the membranes were routinely reprobed with anti–MAP kinase 1/2 (ErK 1/2-CT) (Upstate Biotechnology, Lake Placid, NY) or anti-Stat5b (C-17) (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. Detection was conducted as described above.
Casein kinase-2 assay
U937 cells treated with or without 500 μM DHA for 30 minutes at 37°C were washed with PBS and lysed. The assay to measure the activity of casein kinase-2 (CK2) was conducted as described by the manufacturer (Upstate Biotechnology). Briefly, extracts containing the CK2 were mixed with 200 μM CK2 substrate peptide (Upstate Biotechnology) and 10 μCi (0.37 MBq) of γ-[32P]-ATP in assay dilution buffer (20 mM MOPS, pH 7.2, 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiotreitol). The reactions were allowed to proceed for 10 minutes at 30°C and then stopped with 40% trichloroacetic acid (TCA). An aliquot of the sample was transferred to P81 paper and washed 3 times with 0.75% phosphoric acid and once with acetone. The paper was dried and the radioactivity was measured in a scintillation counter.
Analysis of reactive oxygen species
Cells were treated with DHA and/or GM-CSF as described above. After treatment with 1 nM GM-CSF, cells were washed 3 times in buffer containing 20 mM HEPES (pH 7.4), 10 mM dextrose, 127 mM NaCl, 5.5 mM KCl, 1 mM CaCl2, 2 mM MgSO4, and resuspended (1 × 106 cells/mL) in the same buffer. The DCF-DA (2′, 7′-dichlorodihydroflurescein diacetate; Molecular Probes, Eugene, OR) was added to a final concentration of 20 μM and cells were incubated for 20 minutes at 37°C.31 The level of ROS was measured in a Flow Cytometer FACScalibur Immunocytometry System (Becton and Dickinson, San Jose, CA).
Transfection of 293T cells and luciferase assays
293T cells were transiently transfected by calcium phosphate method in 100 mm plates at 80% of confluency using pMX plasmids containing the GM-CSF receptor cDNA, αGMR and βGMR,30and the p8xGAS-luc plasmid.32 Cells treated with DHA and/or GM-CSF were washed with PBS and lysed with Reporter Lysis Buffer (Promega, San Luis Obispo, CA). Luciferase activity was determined with the Luciferase Reporter Assay System (Promega) using a Berthold Luminometer Lumet (Model B9501).
Vitamin C uptake
Uptake assays were performed as described.24,27Briefly, 2 × 106 cells were incubated with 500 μM AA in the presence of 2 units of ascorbate oxidase (Sigma) and 0.5 μCi (18.5 KBq) of L-[14C]-AA (specific activity, 8.0 mCi/mmol (DuPont NeN) at room temperature for the periods of time indicated in the figures. Cells were washed twice with ice-cold PBS before lysis with 10 mM Tris-HCl pH 8.0 and 0.2% SDS. Cell-associated radioactivity was determined by scintillation spectrometry.24
Analysis of phosphorylation of βGMR and Jak-2
293T cells were cotransfected with plasmids containing the GM-CSF receptor subunits, αGMR and βGMR, as described previously. Cells were incubated 12 hours after transfection with 500 μM DHA for 30 minutes and then treated with 1 nM GM-CSF. Cell extracts were prepared as described before, incubated with 1 μg of anti-βGMR antibody (S-16; Santa Cruz Biotechnology) for 16 hours at 4°C on an oscillating platform. A quantity of 20 μg of Protein A–Sepharose (Sigma) was then added and left for 1 hour at 4°C. The beads were collected by centrifugation, washed 3 times with lysis buffer, resuspended with SDS sample loading buffer, and heated for 5 minutes at 95°C before separation by SDS-PAGE. Western blotting to analyze the phosphorylation of βGMR was performed in the following manner: nonspecific sites were blocked using 5% milk in TBS buffer before incubating the membrane with antiphosphotyrosine antibody (clone: 4G10; Upstate Biotechnology) for 16 hours at 4°C at final dilution of 1:1000 in blocking buffer. The membrane was washed 3 times in TBS, incubated for 1 hour at room temperature with anti–mouse IgG-HRP linked whole antibody (Amersham Pharmacia Biotech) diluted to 1:4000 in blocking buffer, and washed with TBS. The protein bands were revealed using the ECL detection system. The membrane was reprobed with the immunoprecipitating antibody to confirm the presence of equal amounts of proteins. Western blotting to analyze the phosphorylation of Jak-2 was performed as described for the analysis of the βGMR using an antiphospho–Jak-2 antibody (Y1007, Y1008; Upstate Biotechnology).
Results
Vitamin C suppresses GM-CSF–induced formation of reactive oxygen species
GM-CSF induces an increase in the formation of ROS in hematopoietic cells that is repressed in vitro by antioxidants such as N-acetyl cysteine.22 This result led us to investigate the effect of vitamin C in the production of ROS induced by GM-CSF in U937, a human monocyticlike cell line. Cells were loaded with vitamin C by exposing them to the oxidized form of vitamin C, DHA, and the intracellular formation of ROS was quantified. The generation of ROS was measured using 2′, 7′-dichlorofluorescein diacetate (DFC) and fluorescence flow cytometry.31 Cells loaded with vitamin C showed a 30% decrease in the constitutive intracellular levels of ROS, compared with untreated cells (Figure1A). U937 cells incubated with 1 nM GM-CSF for 10 minutes showed a 2-fold increase in formation of ROS (Figure 1C). A time-course analysis demonstrated that GM-CSF induced a linear increase of ROS up to a period of at least 10 minutes. The generation of ROS induced by GM-CSF at these early time points was completely inhibited in cells loaded with vitamin C (Figure 1C). The inhibitory effect of vitamin C on the production of ROS was not due to cellular toxicity, as assessed by viability assays (data not shown).
Vitamin C inhibits GM-CSF–induced production of ROS.
(A) U937 cells incubated for 30 minutes with (black histogram) or without (white histogram) 500 μM dehydroascorbic (DHA) were analyzed for the production of ROS. (B) U937 cells treated (black histogram) or untreated (white histogram) for 30 minutes with 500 μM DHA were incubated for 10 minutes with 1 nM GM-CSF and the production of ROS analyzed. (C) Analysis of the production of ROS in U937 cells pretreated (closed circle) or untreated (open circle) with 500 μM DHA for 30 minutes before incubation with 1 nM GM-CSF. The relative production of ROS was measured by fluorescence using DFC in a flow cytometer.
Vitamin C inhibits GM-CSF–induced production of ROS.
(A) U937 cells incubated for 30 minutes with (black histogram) or without (white histogram) 500 μM dehydroascorbic (DHA) were analyzed for the production of ROS. (B) U937 cells treated (black histogram) or untreated (white histogram) for 30 minutes with 500 μM DHA were incubated for 10 minutes with 1 nM GM-CSF and the production of ROS analyzed. (C) Analysis of the production of ROS in U937 cells pretreated (closed circle) or untreated (open circle) with 500 μM DHA for 30 minutes before incubation with 1 nM GM-CSF. The relative production of ROS was measured by fluorescence using DFC in a flow cytometer.
Vitamin C inhibits GM-CSF–induced phosphorylation of MAP kinase
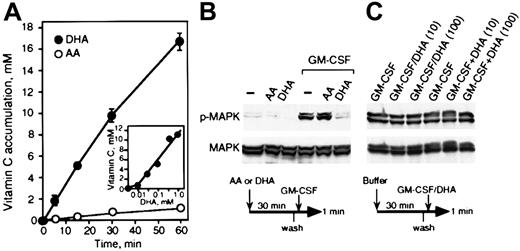
Vitamin C is transported into U937 cells preferentially as DHA via the glucose transporters24 (Figure2A). We calculated the accumulated vitamin C using an estimated intracellular volume of 1.0 μL per 106 cells. Uptake of 500 μM DHA was linear for up to 1 hour (Figure 2A). A time-course analysis of DHA uptake revealed that at the end of 30 minutes incubation with 500 μM DHA, U937 cells accumulated nearly 10 mM vitamin C. Cells incubated with AA under the same conditions, however, accumulated only 0.7 mM vitamin C. To assess the ability of vitamin C to regulate intracellular responses mediated by GM-CSF, U937 cells were treated with either vehicle buffer, DHA, or AA, and GM-CSF–mediated phosphorylation of p44 and p42 MAP kinases (Erk1 and Erk2) was then analyzed. U937 cells incubated with 1 nM GM-CSF for 1 minute showed a significant increase in phosphorylation of MAP kinase (p-MAPK), as analyzed by immunoblotting with a phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody (Figure 2B). Dose-response experiments indicated that 1 nM GM-CSF was needed to obtain maximum induction of p-MAPK (data not shown). Cells treated with DHA, but not AA, showed inhibition of GM-CSF–dependent p-MAPK (Figure2B). To be certain that the inhibitory effect of vitamin C was not extracellular, perhaps due to inactivation of the receptor or the ligand, DHA and GM-CSF were preincubated together for 5 minutes before addition (GM-CSF/DHA), or added simultaneously (GM-CSF+DHA) to the cells (Figure 2C). GM-CSF preincubated for 5 minutes with 10 μM or 100 μM DHA was able to activate p-MAPK at levels comparable to the nontreated GM-CSF. Likewise, when DHA and GM-CSF were both added simultaneously to the cells and incubated for 1 minute, the activity of GM-CSF was normal (Figure 2C). The immunoblots were stripped and reprobed with anti–MAP kinase antibody, demonstrating that equal amounts of MAP kinase were loaded on the gel (lower panels in Figure 2B-C). The results indicated that DHA does not alter the activity of the ligand during the treatment with DHA. Also, GM-CSF binding analysis showed that in cells incubated with DHA the GM-CSF receptor had unaltered binding properties (data not shown). DHA and AA were prepared under controlled pH buffered conditions to avoid changes in pH during incubation with cells. To further assess the signaling inhibitory properties of vitamin C, we investigated if cells loaded with vitamin C suppress p-MAPK induced by 1 nM GM-CSF for increasing periods of time. We found that vitamin C considerably inhibited p-MAPK induced by 1 nM GM-CSF (Figure 3A). This inhibitory effect of vitamin C was dose-dependent, as shown in Figure 3B-C. Exposing the cells to 100 μM DHA for 30 minutes was sufficient to inhibit p-MAPK induced by 1 nM GM-CSF for 3 minutes (Figure 3B). Under these conditions, U937 cells accumulated 6 mM vitamin C (Figure 2A, insert). Time-dependent analysis of the inhibitory effect of vitamin C indicated that cells incubated with 500 μM DHA for 5 minutes accumulated approximately 9 mM, abolishing p-MAPK induced by 1 nM GM-CSF. These results indicated that U937 cells required a critical intracellular concentration of vitamin C of approximately 6 mM to abolish p-MAPK induced by 1 nM GM-CSF. Similar intracellular concentrations of vitamin C have been reported in mononuclear leukocytes.33
Vitamin C inhibits GM-CSF–induced MAP kinase phosphorylation.
(A) Accumulation of vitamin C by U937 cells. Cells were incubated for the time indicated with 500 μM DHA (closed circle) or 500 μM AA (open circle). The insert shows the accumulation of vitamin C incubation with increasing concentrations of DHA for 30 minutes. The uptake of DHA and AA was determined as described in “Materials and methods.” (B) U937 cells treated with either buffer (–), 500 μM AA, or 500 μM DHA for 30 minutes were incubated with 1 nM GM-CSF for 1 minute. Phosphorylated (p-MAPK) and nonphosphorylated MAP kinase (MAPK) were detected by immunoblotting as shown in the upper and lower panels, respectively. (C) DHA did not affect ligand or GM-CSF receptor functions. DHA and GM-CSF were incubated together for 5 minutes before addition (GM-CSF/DHA) or added simultaneously (GM-CSF+DHA). Concentrations of DHA are indicated by 10 μM and 100 μM. The phosphorylated and nonphosphorylated MAP kinase are shown in the upper and lower panels, respectively. A schematic representation of the experiments is shown below the figure.
Vitamin C inhibits GM-CSF–induced MAP kinase phosphorylation.
(A) Accumulation of vitamin C by U937 cells. Cells were incubated for the time indicated with 500 μM DHA (closed circle) or 500 μM AA (open circle). The insert shows the accumulation of vitamin C incubation with increasing concentrations of DHA for 30 minutes. The uptake of DHA and AA was determined as described in “Materials and methods.” (B) U937 cells treated with either buffer (–), 500 μM AA, or 500 μM DHA for 30 minutes were incubated with 1 nM GM-CSF for 1 minute. Phosphorylated (p-MAPK) and nonphosphorylated MAP kinase (MAPK) were detected by immunoblotting as shown in the upper and lower panels, respectively. (C) DHA did not affect ligand or GM-CSF receptor functions. DHA and GM-CSF were incubated together for 5 minutes before addition (GM-CSF/DHA) or added simultaneously (GM-CSF+DHA). Concentrations of DHA are indicated by 10 μM and 100 μM. The phosphorylated and nonphosphorylated MAP kinase are shown in the upper and lower panels, respectively. A schematic representation of the experiments is shown below the figure.
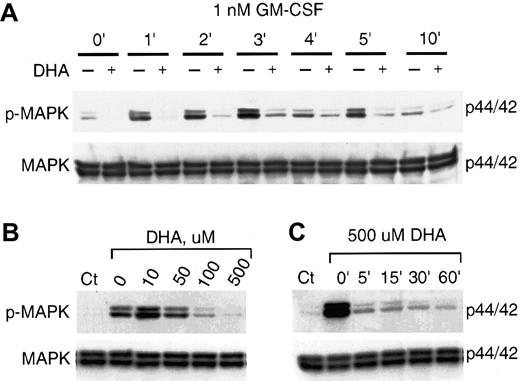
Time-course and concentration dependence of vitamin C suppressing of GM-CSF–induced phosphorylation of MAP kinase.
(A) U937 cells incubated for 30 minutes with 500 μM DHA (+) or without DHA (–) were treated with 1 nM GM-CSF for the time periods shown. Phosphorylated MAP kinase (p-MAPK) was visualized by immunoblotting with an antiphospho-MAP kinase antibody (upper panel). Equal protein loading was demonstrated by immunoblotting the membrane with anti–MAP kinase antibody as shown in the lower panel (MAPK). (B) Concentration dependence of the effect of vitamin C on the GM-CSF–induced phosphorylation of MAP kinase. U937 cells preincubated for 30 minutes with different concentrations of DHA were treated with 1 nM GM-CSF for 3 minutes. (C) Time dependence of the effect of vitamin C on GM-CSF–induced phosphorylation of MAP kinase. Cells preincubated with 500 μM DHA for the time indicated were incubated with 1 nM GM-CSF for 3 minutes. Control (Ct) represents cells left untreated with either GM-CSF or DHA.
Time-course and concentration dependence of vitamin C suppressing of GM-CSF–induced phosphorylation of MAP kinase.
(A) U937 cells incubated for 30 minutes with 500 μM DHA (+) or without DHA (–) were treated with 1 nM GM-CSF for the time periods shown. Phosphorylated MAP kinase (p-MAPK) was visualized by immunoblotting with an antiphospho-MAP kinase antibody (upper panel). Equal protein loading was demonstrated by immunoblotting the membrane with anti–MAP kinase antibody as shown in the lower panel (MAPK). (B) Concentration dependence of the effect of vitamin C on the GM-CSF–induced phosphorylation of MAP kinase. U937 cells preincubated for 30 minutes with different concentrations of DHA were treated with 1 nM GM-CSF for 3 minutes. (C) Time dependence of the effect of vitamin C on GM-CSF–induced phosphorylation of MAP kinase. Cells preincubated with 500 μM DHA for the time indicated were incubated with 1 nM GM-CSF for 3 minutes. Control (Ct) represents cells left untreated with either GM-CSF or DHA.
Vitamin C suppresses GM-CSF–induced phosphorylation of Stat5
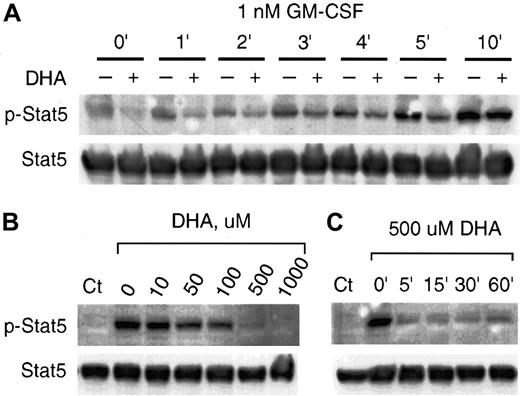
Independent of the ras/raf pathway, GM-CSF activates the phosphorylation of Stat5, leading to its dimerization and translocation to the nucleus.15-17 We investigated the effect of vitamin C on phosphorylation of Stat5 mediated by GM-CSF. U937 cells incubated with 1 nM GM-CSF showed a prominent increase of Stat5 phosphorylation (Figure 4). Cells incubated with 500 μM DHA and treated with 1 nM GM-CSF for up to 5 minutes showed markedly decreased GM-CSF–mediated Stat5 phosphorylation (Figure 4A). Control cells incubated under the same conditions, however, did not evidence inhibition of phosphorylation of Stat5 at 10 minutes incubation with 1 nM GM-CSF (Figure 4A). The dose-dependent inhibitory effect of vitamin C is shown in Figure 4B. Cells incubated for 30 minutes with 500 μM DHA had no phosphorylation of Stat5 induced by treating the cells with 1 nM GM-CSF for 1 minute (Figure 4B). Similar to the effect of vitamin C on p-MAPK (Figure 3), incubation for 5 minutes with 500 μM DHA efficiently blocked the phosphorylation of Stat5 induced by GM-CSF (Figure 4C).
Vitamin C suppresses GM-CSF–induced phosphorylation of Stat5.
(A) U937 cells incubated for 30 minutes with (+) or without (–) 500 μM DHA were treated with 1 nM GM-CSF for the periods shown. The phosphorylated Stat5 (p-Stat5) was visualized by immunoblotting with an antiphosphorylated Stat5 antibody (upper panel). Equal protein loading was demonstrated by immunoblotting the same membrane with anti-Stat5 (Stat5) antibody as shown in the lower panel. (B) Concentration dependence of the effect of vitamin C on the GM-CSF–induced phosphorylation of Stat5. U937 cells incubated for 30 minutes with different concentrations of DHA were treated with 1 nM GM-CSF for 3 minutes. The phosphorylated (p-Stat5) and nonphosphorylated Stat5 (Stat5) are shown at the upper and lower panels, respectively. (C) Cells were incubated with 500 μM DHA for the indicated time periods and treated with 1 nM GM-CSF for 3 minutes. The phosphorylated and nonphosphorylated Stat5 are shown at the upper and lower panels, respectively. Control (Ct) represents cells unexposed to GM-CSF or DHA.
Vitamin C suppresses GM-CSF–induced phosphorylation of Stat5.
(A) U937 cells incubated for 30 minutes with (+) or without (–) 500 μM DHA were treated with 1 nM GM-CSF for the periods shown. The phosphorylated Stat5 (p-Stat5) was visualized by immunoblotting with an antiphosphorylated Stat5 antibody (upper panel). Equal protein loading was demonstrated by immunoblotting the same membrane with anti-Stat5 (Stat5) antibody as shown in the lower panel. (B) Concentration dependence of the effect of vitamin C on the GM-CSF–induced phosphorylation of Stat5. U937 cells incubated for 30 minutes with different concentrations of DHA were treated with 1 nM GM-CSF for 3 minutes. The phosphorylated (p-Stat5) and nonphosphorylated Stat5 (Stat5) are shown at the upper and lower panels, respectively. (C) Cells were incubated with 500 μM DHA for the indicated time periods and treated with 1 nM GM-CSF for 3 minutes. The phosphorylated and nonphosphorylated Stat5 are shown at the upper and lower panels, respectively. Control (Ct) represents cells unexposed to GM-CSF or DHA.
Vitamin C suppresses GM-CSF–induced Stat5-dependent transcription
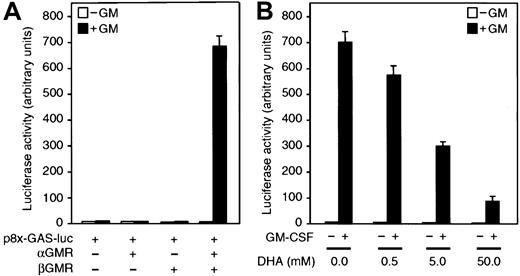
The previous data indicated that vitamin C inhibits GM-CSF–dependent phosphorylation of Stat5. We then investigated whether vitamin C would inhibit Stat5-dependent transcription. An efficient method of analyzing the transcriptional Stat5 responsiveness to GM-CSF involves the transfection of cells with the construct p8xGAS-luciferase (p8xGAS-luc).32 This construct contains 8 interferon γ–activated site elements cloned upstream of a luciferase reporter gene.32 We use 293T cells as an assay system because they are highly transfectable as compared with U937 cells.30 The 293T cells preferentially take up DHA and after 30 minutes incubation with 500 μM DHA, accumulate approximately 3 mM vitamin C intracellularly. No significant induction of luciferase activity was detected when 293T cells transiently transfected with p8xGAS-luc alone or cotransfected with plasmids encoding the cDNA for αGMR or βGMR were incubated with 1 nM GM-CSF for 5 hours (Figure5A). However, cotransfection of p8xGAS-luc with a combination of plasmids encoding the cDNA for αGMR and βGMR into 293T cells resulted in a strong induction of luciferase activity by GM-CSF. Induction was 80-fold over the basal level after 5 hours treatment (Figure 5A-B). These results suggested that the lack of responsiveness in 293T cells was due to a limited expression of functional GM-CSF receptors. We therefore examined the effect of vitamin C in the triple transfectants by incubating them with increasing concentrations of DHA prior to the GM-CSF treatment. Transfectants incubated with 5 mM DHA showed a 50% decrease in luciferase activity induced by 1 nM GM-CSF, as compared with control cells (Figure 5B). At 500 μM DHA treatment, there was an 18% inhibition of luciferase activity and at 5 mM DHA, luciferase activity was reduced by more than 50% (Figure 5B).
Vitamin C inhibits Stat5 transcriptional activation mediated by GM-CSF.
(A) 293T cells transfected with either the reporter construct p8xGAS-luc or cotransfected with plasmids containing the cDNAs for αGMR and/or βGMR were incubated for 5 hours with 1 nM GM-CSF (closed bars) or buffer (open bars), and luciferase activity was measured in cell lysates. (B) 293T cells cotransfected with plasmids encoding the cDNAs for αGMR, βGMR, and the reporter construct p8xGAS-luc were incubated for 30 minutes with increasing concentrations of DHA. Transfectants were incubated for 5 hours with 1 nM GM-CSF (closed bars) or no additions (open bars), and luciferase activity was measured in cell lysates. Bars represent the average values of triplicate determinations ± SD.
Vitamin C inhibits Stat5 transcriptional activation mediated by GM-CSF.
(A) 293T cells transfected with either the reporter construct p8xGAS-luc or cotransfected with plasmids containing the cDNAs for αGMR and/or βGMR were incubated for 5 hours with 1 nM GM-CSF (closed bars) or buffer (open bars), and luciferase activity was measured in cell lysates. (B) 293T cells cotransfected with plasmids encoding the cDNAs for αGMR, βGMR, and the reporter construct p8xGAS-luc were incubated for 30 minutes with increasing concentrations of DHA. Transfectants were incubated for 5 hours with 1 nM GM-CSF (closed bars) or no additions (open bars), and luciferase activity was measured in cell lysates. Bars represent the average values of triplicate determinations ± SD.
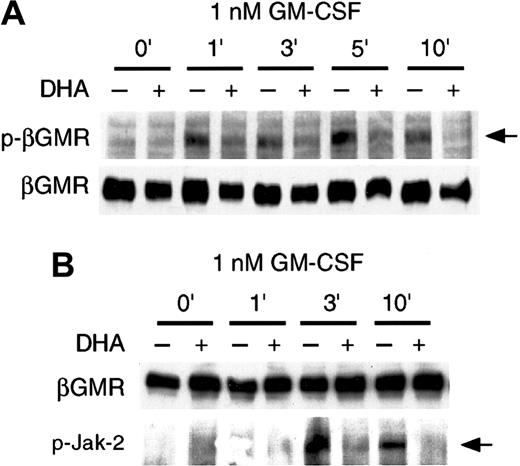
Vitamin C inhibits GM-CSF–induced phosphorylation of βGMR
Although vitamin C inhibits cytoplasmic phosphorylation events mediated by GM-CSF, it was important to also examine the phosphorylation of βGMR, which is an early signaling event initiated at the membrane level.34 Ligand-binding to the high-affinity GMR complex triggers the phosphorylation of Jak-2, and Jak-2 phosphorylates tyrosine residues within the cytoplasmic domain of βGMR.34 Thus, we investigated whether vitamin C could inhibit the phosphorylation of the βGMR induced by GM-CSF. Cells cotransfected with plasmids encoding αGMR and βGMR cDNAs were loaded with vitamin C and incubated with 1 nM GM-CSF for up to 10 minutes, and cell lysates were immunoprecipitated with an anti-βGMR antibody. βGMR was phosphorylated at tyrosine residues after incubation with GM-CSF, as shown by immunoblotting with antiphosphotyrosine antibody (Figure 6); βGMR was not phosphorylated in the absence of the ligand. When the tranfectants were incubated for 30 minutes with 500 μM DHA prior to GM-CSF treatment, the GM-CSF–dependent phosphorylation of βGMR was strongly inhibited (Figure 6A).
Vitamin C suppresses GM-CSF–induced phosphorylation of βGMR.
293T cells cotransfected with plasmids containing the cDNAs for αGMR and βGMR were incubated for 30 minutes in the presence (+) or absence (–) of 500 μM DHA. 1 nM GM-CSF was added for the periods of time shown. βGMR was immunoprecipitated, subjected to SDS-PAGE, and the phosphorylated βGMR (p-βGMR) was visualized by immunoblotting with an antiphosphotyrosine antibody (arrow, upper panel). (B) Jak-2 phosphorylation is suppressed by vitamin C. βGMR was immunoprecipitated, subjected to SDS-PAGE, and the phosphorylated Jak-2 (p-Jak-2) was visualized by immunoblotting with antiphospho Jak-2 antibody (arrow, lower panel). Equal protein loading was demonstrated by immunoblotting the same membrane with an anti-βGMR antibody, as shown in the upper panel.
Vitamin C suppresses GM-CSF–induced phosphorylation of βGMR.
293T cells cotransfected with plasmids containing the cDNAs for αGMR and βGMR were incubated for 30 minutes in the presence (+) or absence (–) of 500 μM DHA. 1 nM GM-CSF was added for the periods of time shown. βGMR was immunoprecipitated, subjected to SDS-PAGE, and the phosphorylated βGMR (p-βGMR) was visualized by immunoblotting with an antiphosphotyrosine antibody (arrow, upper panel). (B) Jak-2 phosphorylation is suppressed by vitamin C. βGMR was immunoprecipitated, subjected to SDS-PAGE, and the phosphorylated Jak-2 (p-Jak-2) was visualized by immunoblotting with antiphospho Jak-2 antibody (arrow, lower panel). Equal protein loading was demonstrated by immunoblotting the same membrane with an anti-βGMR antibody, as shown in the upper panel.
Vitamin C inhibits the GM-CSF–mediated phosphorylation of Jak-2
The foregoing results suggested that vitamin C could be inhibiting the phosphorylation of downstream signaling molecules by impairing the kinase activity of Jak-2. We analyzed the effect of vitamin C on phosphorylation of Jak-2 with immunoprecipitation experiments. Extracts of cells cotransfected with αGMR and βGMR cDNAs, and immunoprecipitated with anti-βGMR, showed that the phosphorylation of Jak-2 is dependent on GM-CSF treatment (Figure 6B). We found that Jak-2 phosphorylation induced by GM-CSF was strongly inhibited by vitamin C. These results imply that vitamin C may be downmodulating GM-CSF signaling by inhibiting phosphorylation of Jak-2 and therefore preventing it from phosphorylating βGMR and initiating downstream phosphorylation events.
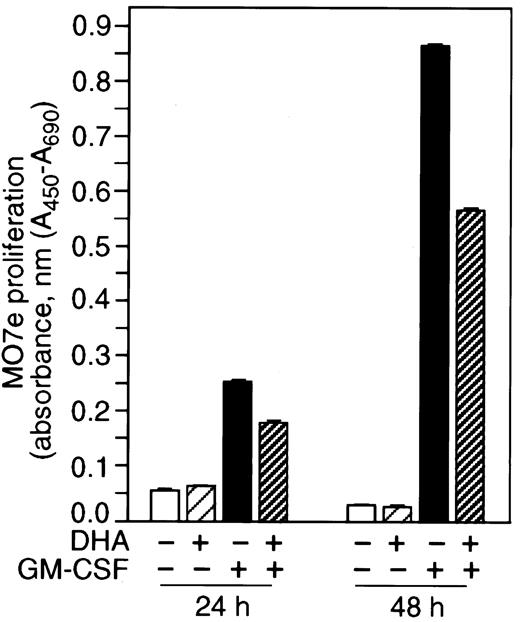
Vitamin C inhibits GM-CSF–mediated cell proliferation
We found that vitamin C inhibited GM-CSF–induced signaling responses at a proximal point in the pathway, inactivating the phosphorylation of βGMR. These results suggest that vitamin C would have an effect on cell proliferation induced by GM-CSF. We therefore analyzed the effect of vitamin C loading on proliferation of cultures of GM-CSF–dependent cell line MO7e. Cells loaded with vitamin C showed a 30% to 40% reduced growth in response to GM-CSF as compared with control (Figure 7). These data indicate that vitamin C affects the proliferation signaling pathway mediated by GM-CSF.
Vitamin C inhibits GM-CSF–dependent cell proliferation.
MO7e cells incubated for 30 minutes with or without 500 μM DHA were grown in a 96-well tissue culture plate in the presence or absence of 400 pM GM-CSF for 24 hours or 48 hours. Proliferation was measured by colorimetric assay. WST-1 was added and 3 hours later the absorbance was measured (450 nm-690 nm) by ELISA reader.
Vitamin C inhibits GM-CSF–dependent cell proliferation.
MO7e cells incubated for 30 minutes with or without 500 μM DHA were grown in a 96-well tissue culture plate in the presence or absence of 400 pM GM-CSF for 24 hours or 48 hours. Proliferation was measured by colorimetric assay. WST-1 was added and 3 hours later the absorbance was measured (450 nm-690 nm) by ELISA reader.
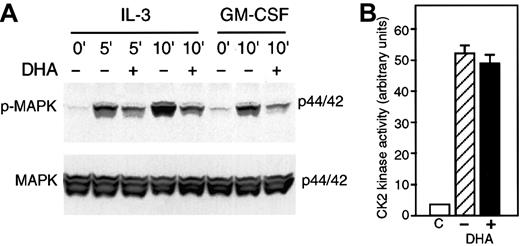
Vitamin C prevents IL-3–induced p-MAPK
The previous results demonstrated that GM-CSF–induced phosphorylation of βGMR was suppressed by vitamin C (Figure 6A). Since the human high-affinity receptors for IL-3, IL-5, and GM-CSF share a common β subunit (βGMR),34 we reasoned that vitamin C may modulate IL-3– and/or IL-5–mediated cytokine responses. As previously shown for U937 cells, treatment with 1 nM GM-CSF for 10 minutes induced p-MAPK in HL-60 cells (Figure8A). We also found that incubation with 0.6 nM IL-3 for 5 or 10 minutes induced p-MAPK (Figure 8A). However, IL-5 under similar conditions was unable to induce p-MAPK in HL-60 or U937 cells (data not shown). Loading HL-60 cells with vitamin C by incubation with 500 μM DHA for 30 minutes suppressed the induction of p-MAPK by either IL-3 or GM-CSF (Figure 8A). To determine if the inhibitory activity of vitamin C is specific to the redox-mediated signaling of GM-CSF and IL-3 by inhibiting key kinases involved in their pathway, we investigated if cells loaded with vitamin C showed changes in CK2 activity. Protein kinase CK2 is ubiquitously expressed and is a constitutively active serine/threonine kinase.35U937 cells loaded with vitamin C showed identical protein kinase CK2 activity as compared with controls (Figure 8B). This result indicated that vitamin C loading does not generally inhibit constitutive intracellular kinases.
Vitamin C suppresses IL-3–induced phosphorylation of MAP kinase.
(A) HL-60 cells incubated for 30 minutes with 500 μM DHA (+) or without DHA (–) were treated with 1 nM GM-CSF for 10 minutes or incubated with 0.6 nM IL-3 for the period indicated. Phosphorylated MAP kinase (p-MAPK) was visualized by immunoblotting with an antiphospho–MAP kinase antibody (upper panel). Equal protein loading was demonstrated by immunoblotting the membrane with anti–MAP kinase antibody as shown in the lower panel (MAPK). (B) U937 cells were incubated with DHA (+) or without DHA (–) and CK2 kinase activity was measured in cell lysates. One representative experiment is shown. C represents background activity in absence of lisate. Bars represent the average values of triplicate determinations ± SD.
Vitamin C suppresses IL-3–induced phosphorylation of MAP kinase.
(A) HL-60 cells incubated for 30 minutes with 500 μM DHA (+) or without DHA (–) were treated with 1 nM GM-CSF for 10 minutes or incubated with 0.6 nM IL-3 for the period indicated. Phosphorylated MAP kinase (p-MAPK) was visualized by immunoblotting with an antiphospho–MAP kinase antibody (upper panel). Equal protein loading was demonstrated by immunoblotting the membrane with anti–MAP kinase antibody as shown in the lower panel (MAPK). (B) U937 cells were incubated with DHA (+) or without DHA (–) and CK2 kinase activity was measured in cell lysates. One representative experiment is shown. C represents background activity in absence of lisate. Bars represent the average values of triplicate determinations ± SD.
Discussion
AA is a potent antioxidant that quenches ROS and serves as a cofactor for enzymes involved in the synthesis of collagen, neurotransmitters, and carnitine.36-38 In this study we demonstrate that vitamin C can also function as a modulator of cytokine signal transduction pathways. GM-CSF signaling responses were suppressed by vitamin C in the human monocytic U937, HL-60, and kidney 293T cell lines. U937 cells loaded with vitamin C showed a decrease in GM-CSF–induced p-MAPK p42/44 and Stat5. GM-CSF–dependent p-MAPK is activated via the ras/raf signaling pathway. This MAP kinase cascade is involved in host defense cellular responses and regulation of gene expression, suggesting that the vitamin could downregulate host defense responses and expression of specific cytokine-inducible genes.39 The vitamin also suppressed the phosphorylation of the transcription factor Stat5, and since the phosphorylation of Stat5 is required for its nuclear translocation the vitamin suppressed GM-CSF–induced transcription of a Stat5 reporter assay system. These data suggested that the inhibitory effect of vitamin C on GM-CSF–induced Stat5-dependent transcription could have important effects seen at the level of gene expression regulated by GM-CSF. Other responses to GM-CSF may be also modulated by the intracellular level of the vitamin; for instance, GM-CSF is a proinflammatory cytokine that may be involved in rheumatoid arthritis, therefore our finding that vitamin C inhibits GM-CSF implies a role of the vitamin in anti-inflammatory responses.
We found that vitamin C suppressed early membrane signaling events initiated by GM-CSF. Ligand-induced phosphorylation of βGMR and Jak-2 was inhibited by vitamin C loading, implying that the inhibition of these early events could explain the GM-CSF downstream responses affected by vitamin C. This conclusion is based on the notion that the phosphorylation of Jak-2 and βGMR are required for most of GM-CSF signaling pathways.40
Our studies demonstrated that IL-3–induced phosphorylation of MAP kinase was also suppressed by vitamin C. This result is consistent with the knowledge that the high-affinity receptors for IL-3, IL-5, and GM-CSF share a common β subunit (βGMR) that is considered to be the primary signaling subunit. Since IL-3– and GM-CSF–induced signaling was suppressed by vitamin C, it is possible that vitamin C may regulate IL-5 cytokine responses. Therefore, evidence leads to the conclusion that vitamin C may be an important player in controlling cellular signaling pathways mediated by cytokines, including IL-3 and IL-5.
The molecular mechanism underlying vitamin C–mediated suppression of GM-CSF–induced phosphorylation is unclear; however, a possible explanation is that vitamin C may directly inhibit the kinases involved in GM-CSF responses, or alternatively, it activates protein phosphatases. We discarded both possibilities, since AA failed to inhibit phosphotyrosine and serine/threonine kinases in vitro (data not shown). Also, in vitro phosphatase assays indicated that AA does not activate phosphatases (data not shown). The likely mechanism of GM-CSF signaling inhibition by vitamin C is at the level of controlling the quantity of ROS production induced by GM-CSF. The role of ROS as signaling mediators has been suggested for GM-CSF and other growth factors, but the molecular mechanisms of their generation and precise function are not well known. ROS as messenger molecules appear to regulate the activity of redox-sensitive enzymes, including protein kinases and protein phosphatases. The activation of Jak-2 by ROS has been demonstrated41 42 and the finding that GM-CSF–dependent phosphorylation of Jak-2 was suppressed by vitamin C supports our notion that vitamin C may be blocking the activation of Jak-2 by quenching the ROS generated in response to GM-CSF, which is needed to induce this kinase. Therefore, we propose that the mechanism by which vitamin C inhibits GM-CSF responses is due to a strong inhibition of ROS, which is required to activate the kinase(s) involved in signaling, induced by GM-CSF.
Under physiologic conditions, vitamin C circulates in the blood in its reduced form, AA, at approximately 50 μM; however, cells accumulate a wide range of intracellular concentrations of vitamin C up to 6 mM in mononuclear leukocytes.33 Our data showed that the inhibition of GM-CSF signaling was evident at intracellular concentrations of 5 mM vitamin C. These results suggest a physiologic role of vitamin C as modulator of GM-CSF signaling. Furthermore, GM-CSF induces an increase in glucose and vitamin C uptake in target cells, presumably by modifying the affinity of the facilitative hexose transporters for the transport of glucose and DHA in human host defense cells, spermatozoa, and U937 cells,29,43,44 which allows them to accumulate higher intracellular concentrations of vitamin C. It is possible that GM-CSF could induce an increase in the levels of vitamin C to inhibitory levels functioning as a feedback system to control its biologic activities. Since the oxidized form of vitamin C is transported by the glucose transporters, it is at least plausible to consider a homeostatic and feedback system whereby GM-CSF can stimulate oxidative events and transport functions that lead to increased accumulation of vitamin C and subsequent downmodulation of GM-CSF action (Figure 9). This theory is based on the notion that GM-CSF primes host defense cells for oxidation metabolism and the oxidative burst.45 It is known that the activation of neutrophils induces accumulation of vitamin C by inducing the oxidation of the vitamin.46
Schematic representation of GM-CSF signaling and vitamin C transport and functions.
GM-CSF binds to αGMR, and in concert with βGMR forms the high-affinity receptor-signaling complex (M: membrane). Vitamin C enters the cells through the glucose transporters as DHA, and is rapidly reduced to AA intracellularly. The GM-CSF–dependent signaling events modulated by vitamin C are indicated.
Schematic representation of GM-CSF signaling and vitamin C transport and functions.
GM-CSF binds to αGMR, and in concert with βGMR forms the high-affinity receptor-signaling complex (M: membrane). Vitamin C enters the cells through the glucose transporters as DHA, and is rapidly reduced to AA intracellularly. The GM-CSF–dependent signaling events modulated by vitamin C are indicated.
We concluded that vitamin C modulates GM-CSF signaling responses, and we postulate that cells may, by changing their intracellular content of antioxidants such as vitamin C, alter their responses to cytokines.
Stat5-responsive p8xGAS-luciferase reporter plasmid was obtained from S. J. Ackerman (originally obtained from C. K. Glass).
Supported by Public Health Service grant CA30388 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David W. Golde, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail:d-golde@ski.mskcc.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal