Abstract
Oral valacyclovir for cytomegalovirus (CMV) prophylaxis in bone marrow transplantation (BMT) was investigated in a randomized, double-blind, acyclovir-controlled, multicenter clinical trial in recipients of allogeneic BMT who were CMV seropositive (or donor positive) before transplantation and were aged 13 years or older. Patients were randomized before BMT. All initially received intravenous acyclovir (500 mg/m2) 3 times daily until day 28 after transplantation or after discharge, then oral valacyclovir (2 g) or acyclovir (800 mg) 4 times daily until week 18 after transplantation. Evidence of CMV infection, CMV disease, and death were documented for 22 weeks. Primary end points were time to CMV infection (detection of CMV in blood, broncho-alveolar lavage) or CMV disease and survival. Preemptive CMV therapy was permitted. Seven hundred twenty-seven patients were evaluable for efficacy. After the administration of intravenous acyclovir, valacyclovir was significantly more effective than oral acyclovir in reducing the incidence of CMV infection. CMV infection or disease developed in 102 (28%) valacyclovir patients, compared with 143 (40%) acyclovir patients (HR, 0.59; 95% CI, 0.46-0.76; P < .0001). Survival did not differ between treatments (76% and 75% in the valacyclovir and acyclovir groups, respectively). The safety of oral valacyclovir was similar to that of high-dose oral acyclovir. Valacyclovir was more effective than acyclovir in preventing CMV reactivation in BMT recipients and showed a similar safety profile. CMV disease incidence was low, and no differences were observed between oral valacyclovir and acyclovir. Survival was similar in each group. Valacyclovir prophylaxis provides a clinically valuable intervention but must be part of an overall strategy for CMV prevention in BMT.
Introduction
Cytomegalovirus (CMV) infection and disease are major complications of allogeneic bone marrow transplantation (BMT). Different strategies have been developed to reduce the risk for CMV-associated sickness and death, including the blood transfusion policy,1 prophylaxis with high-dose intravenous (IV) immunoglobulin,2 high-dose acyclovir (2-amino-1,9-dihydro-9-[(2-hydroxy-ethoxy)methyl]-6H-purin-6-one) or IV ganciclovir (2-amino-1,9-[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]-6H-purin-6-one), and preemptive therapy based on the detection of CMV infection before disease develops.3,4 In spite of these advances significant morbidity and mortality levels are still associated with CMV, particularly in patients receiving grafts from HLA-mismatched or unrelated donors.5
Acyclovir was the first antiviral agent shown to be effective in preventing CMV reactivation in BMT recipients.6 A large, randomized study showed that high-dose acyclovir reduced the risk for CMV viremia and improved overall survival.7 Acyclovir has a favorable safety profile compared with other available CMV-suppressing agents,8,9 but its usefulness has been limited by low oral bioavailability.10 Valacyclovir (2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-ethyl L-valinate hydrochloride), the L-valyl ester oral prodrug of acyclovir, increases acyclovir bioavailability 3- to 5-fold compared with oral acyclovir. The resultant plasma exposures to acyclovir achieved with valacyclovir are similar to those of IV acyclovir administration.11 The study by Prentice et al7in BMT recipients suggested that the beneficial effects of acyclovir are dosage- and duration-dependent, leading us to hypothesize that increased exposure to acyclovir could further suppress CMV replication. We therefore performed a randomized, double-blind study comparing oral acyclovir and valacyclovir as prophylaxis against CMV infection after allogeneic BMT.
Patients and methods
Study design
This was a randomized, controlled, double-blind study conducted at 48 centers in 15 countries. The study followed European Community and US Good Clinical Practice guidelines and was approved by the appropriate ethics committees.
Patients aged 13 years or older who gave their informed consent and who received a matched or mismatched allogeneic bone marrow graft from a family member or an unrelated donor were eligible for enrollment if they or their donor was CMV seropositive before transplantation. Patients were assessed up to 7 days before BMT for inclusion in the study. Key exclusion criteria were impaired renal function, defined as creatinine clearance less than 50 mL/min (calculated using the Cockcroft and Gault formula), and use of other antiviral drugs at enrollment. Prophylaxis with other antiviral drugs was not allowed during the study period. The use of immunoglobulins was allowed.
All patients initially received prophylactic intravenous acyclovir (500 mg/m2) 3 times daily starting as early as 5 days before transplantation until 28 days after BMT or until discharge, whichever was earlier, followed by oral therapy. Each intravenous dose was administered over 1 hour, using a minimum volume of 50 mL/250 mg acyclovir. In overweight patients (more than 130% ideal body weight), dosing of intravenous acyclovir was based on ideal body weight rather than on total body weight. Equal randomization to the 2 oral study drug regimens, stratified by center, occurred on the first day of intravenous acyclovir administration, before transplantation. Patients received either oral valacyclovir (2 g) 4 times daily or oral acyclovir (800 mg) 4 times daily until the end of the 18th week after transplantation. Patients were followed up until the end of week 22 after transplantation. Drug dosages were adjusted for renal function while the double-blind design of the study was maintained. For creatinine clearance rates of 25 to 50 mL/min, 10 to 25 mL/min, and 0 to 10 mL/min, the intravenous acyclovir dosage was reduced to 500 mg/m2 every 12 hours, 500 mg/m2every 24 hours, and 250 mg/m2 every 24 hours, respectively. For the same creatinine clearance rates, oral valacyclovir and oral acyclovir were reduced to 2 tablets 4 times a day, 2 tablets 3 times a day, and 1 tablet twice a day, respectively.
Preemptive ganciclovir or foscarnet (trisodium phosphonoformate) (on the basis of laboratory evidence of CMV infection) was allowed in the study according to each center's usual practice. The study drug was withdrawn during the period of therapy for CMV infection.
Sample size
From the Kaplan-Meier estimates reported in a previous study,7 a 30% CMV rate in blood or disease and a 30% death rate at the end of the study (day 150, 21-22 weeks) were anticipated in the oral acyclovir group. The sample size of 300 per group provided 80% power to detect a minimum reduction of 10% in these rates at day 150 for the valacyclovir group. This was equivalent to hazard ratios (HRs) of 0.62 or smaller and 1.6 or larger.
Efficacy end points
The primary efficacy end points were, first, time to detection of CMV in blood or broncho-alveolar lavage (BAL) or evidence of definitive or presumptive CMV disease and, second, time to death. Secondary end points included time to development of CMV infection at any site, time to detection of CMV in blood, time to development of CMV disease, and time to ganciclovir or foscarnet use. Other parameters analyzed included time to development of other herpesvirus disease, time to occurrence of opportunistic infection, and time to onset of acute graft-versus-host disease (GVHD).
Definitions of CMV disease
Definitive CMV disease was defined as follows.
Pneumonitis.
The characteristic clinical picture included signs, symptoms, or both of pulmonary disease and detection of CMV in BAL or from lung tissue.
Retinitis.
Typical lesions of retinitis were confirmed by an ophthalmologist.
Gastrointestinal disease.
Clinical symptoms developed in the upper or lower gastrointestinal tract, including macroscopic mucosal lesions seen at endoscopy, and CMV was demonstrated in a biopsy specimen from the gastrointestinal tract.
Hepatitis.
Elevated liver function test results (greater than 3.5 times normal), no other documented cause of hepatitis, and CMV detected on liver biopsy pointed to this condition. If other medical conditions produced chronically raised liver enzyme activity, the baseline was reset and elevated was redefined as more than 2 times the reset baseline.
In the absence of BAL or biopsy evidence, presumptive CMV disease was defined as the presence of pneumonitis, gastrointestinal disease, or hepatitis or of CMV syndrome (including fever of 38°C on 3 or more consecutive days, associated with a decrease in white blood cell count or platelet levels to abnormal values and a 30% reduction in initial counts after these counts had shown initial signs of recovery after transplantation, in the absence of any other known causes) and detection of CMV in blood within 2 weeks of the onset of signs or symptoms. A diagnosis of presumptive CMV pneumonitis was only accepted if BAL was not performed. If BAL was performed and found to be CMV negative, the diagnosis of presumptive CMV was rejected.
In patients in whom presumptive CMV disease was possible, the diagnosis was rejected if other infectious causes were medically plausible and were proven positive by laboratory test. All CMV disease episodes were reviewed by an End points Committee (4 principal investigators under the chairmanship of an independent expert in the field of CMV); only those episodes verified by the Committee were included in the analysis.
Laboratory methods
CMV serostatus of donor and recipient was determined by enzyme immunoassay or radioimmunoassay before enrollment. Blood and urine samples were tested for the presence of CMV (by tissue culture, shell vial/early antigen detection, antigenemia or polymerase chain reaction [PCR]12 at screen) on first day of intravenous acyclovir administration, the day of BMT (day 0), and then weekly until week 12 and every 2 weeks until the end of 22 weeks. Diagnosis by PCR required 2 consecutive positive results. The antigenemia technique was performed using monoclonal antibodies against pp65.13 At each center, the same virology technique was used throughout the study for CMV surveillance in blood and in urine.
Safety
Safety was assessed from adverse event reports and from regular hematologic and clinical chemistry monitoring. In particular, monitoring for early signs of thrombotic microangiopathy (TMA) was performed. Data on reticulocytes, lactate dehydrogenase, and schistocytes on blood film were prospectively collected.
An interim analysis of the survival data was conducted by an independent Data and Safety Monitoring Board (DSMB) 15 months after the first patient started using the study drug. The following were collected for this analysis: demographics, details of the BMT (as in Table 1), start dates for intravenous acyclovir infusion and oral study drug, and study termination record (including all deaths). Only a database administrator, a statistician (independent to the study), and the DSMB members were unblinded to the treatment code so that the interim analysis was conducted without compromising the power of the study.
Demography and disease characteristics: safety and principal efficacy populations
| . | Safety population . | Principal efficacy population . | ||
|---|---|---|---|---|
| Acyclovir n = 372 . | Valacyclovir n = 376 . | Acyclovir n = 300 . | Valacyclovir n = 305 . | |
| Median age, y (range) | 37.0 (13.0-59.0) | 37.0 (13.0-60.0) | 37.0 (13.0-59.0) | 36.0 (13.0-58.0) |
| Male/female (%) | 66/34 | 60/40 | 65/35 | 61/39 |
| Median weight, kg (range) | 71.0 (34.5-141.2) | 70.0 (41.0-134.1) | 71.5 (43.0-141.2) | 70.4 (42.0-134.1) |
| Median height, cm (range) | 171.0 (117.0-194.0) | 170.0 (148.0-190.0) | 172.0 (117.0-192.0) | 170.0 (149.0-190.0) |
| CMV serostatus (%) | ||||
| D+R+ | 54 | 57 | 54 | 59 |
| D−R+ | 28 | 24 | 32 | 25 |
| D+R− | 16 | 17 | 14 | 16 |
| Donor (%) | ||||
| Related/unrelated | 82/17 | 81/18 | 68/32 | 75/25 |
| HLA match (%) | ||||
| Identical/mismatch | 91/8 | 92/6 | 92/6 | 92/7 |
| Marrow T cell (%) | ||||
| Depleted/nondepleted | 15/84 | 13/86 | 13/86 | 12/86 |
| Conditioning regimen (%) | ||||
| Chemotherapy | 32 | 35 | 32 | 35 |
| Single TBI + chemo | 17 | 15 | 17 | 14 |
| Fract TBI + chemo | 46 | 49 | 48 | 49 |
| Other | 3 | 0 | 2 | 0 |
| Unknown | 2 | 1 | 1 | 1 |
| Underlying disease (%) | ||||
| Acute myeloblastic leukemia | 32 | 27 | 30 | 26 |
| Acute lymphoblastic leukemia | 17 | 16 | 18 | 16 |
| Chronic myelogenous leukemia/chronic granulocytic leukemia | 30 | 30 | 32 | 31 |
| Aplastic anemia | 4 | 4 | 3 | 4 |
| Non-Hodgkin lymphoma | 3 | 4 | 4 | 4 |
| Hodgkin lymphoma | 2 | 2 | 1 | 1 |
| Other | 12 | 16 | 12 | 16 |
| Unknown | 1 | 1 | 1 | 1 |
| Risk category (%)* | ||||
| Low | 63 | 62 | 65 | 65 |
| Intermediate | 25 | 24 | 26 | 22 |
| High | 10 | 12 | 6 | 11 |
| . | Safety population . | Principal efficacy population . | ||
|---|---|---|---|---|
| Acyclovir n = 372 . | Valacyclovir n = 376 . | Acyclovir n = 300 . | Valacyclovir n = 305 . | |
| Median age, y (range) | 37.0 (13.0-59.0) | 37.0 (13.0-60.0) | 37.0 (13.0-59.0) | 36.0 (13.0-58.0) |
| Male/female (%) | 66/34 | 60/40 | 65/35 | 61/39 |
| Median weight, kg (range) | 71.0 (34.5-141.2) | 70.0 (41.0-134.1) | 71.5 (43.0-141.2) | 70.4 (42.0-134.1) |
| Median height, cm (range) | 171.0 (117.0-194.0) | 170.0 (148.0-190.0) | 172.0 (117.0-192.0) | 170.0 (149.0-190.0) |
| CMV serostatus (%) | ||||
| D+R+ | 54 | 57 | 54 | 59 |
| D−R+ | 28 | 24 | 32 | 25 |
| D+R− | 16 | 17 | 14 | 16 |
| Donor (%) | ||||
| Related/unrelated | 82/17 | 81/18 | 68/32 | 75/25 |
| HLA match (%) | ||||
| Identical/mismatch | 91/8 | 92/6 | 92/6 | 92/7 |
| Marrow T cell (%) | ||||
| Depleted/nondepleted | 15/84 | 13/86 | 13/86 | 12/86 |
| Conditioning regimen (%) | ||||
| Chemotherapy | 32 | 35 | 32 | 35 |
| Single TBI + chemo | 17 | 15 | 17 | 14 |
| Fract TBI + chemo | 46 | 49 | 48 | 49 |
| Other | 3 | 0 | 2 | 0 |
| Unknown | 2 | 1 | 1 | 1 |
| Underlying disease (%) | ||||
| Acute myeloblastic leukemia | 32 | 27 | 30 | 26 |
| Acute lymphoblastic leukemia | 17 | 16 | 18 | 16 |
| Chronic myelogenous leukemia/chronic granulocytic leukemia | 30 | 30 | 32 | 31 |
| Aplastic anemia | 4 | 4 | 3 | 4 |
| Non-Hodgkin lymphoma | 3 | 4 | 4 | 4 |
| Hodgkin lymphoma | 2 | 2 | 1 | 1 |
| Other | 12 | 16 | 12 | 16 |
| Unknown | 1 | 1 | 1 | 1 |
| Risk category (%)* | ||||
| Low | 63 | 62 | 65 | 65 |
| Intermediate | 25 | 24 | 26 | 22 |
| High | 10 | 12 | 6 | 11 |
Low indicates first remission/chronic phase; intermediate, first relapse/second or subsequent remission/accelerated phase; high, second relapse/blast crisis/primary refractory.
Oncology patients only. Safety population, n = 323 (acyclovir) and n = 333 (valacyclovir). Efficacy population, n = 263 (acyclovir) and n = 268 (valacyclovir).
Statistical methods: analysis populations
The following populations were defined for analysis (Figure1).
Patients recruited into the study.
*Patients excluded from these efficacy populations for reasons outlined in “Statistical methods: analysis populations.”
Patients recruited into the study.
*Patients excluded from these efficacy populations for reasons outlined in “Statistical methods: analysis populations.”
Safety population.
All randomized patients who received at least one dose of medication (intravenous acyclovir) were included in the safety analysis. This population was considered primary.
Principal efficacy population.
This population comprised all patients in the safety population except 6 CMV-seronegative patients with CMV seronegative donors (these were protocol violations at study entry), 2 patients for whom donor and recipient CMV serology were missing, and all 13 patients recruited at a center that failed to comply with Good Clinical Practice (GCP) and Food and Drug Administration regulations. This population was the primary population for efficacy analysis. Two additional efficacy populations were defined: intravenous/oral and oral.
Intravenous/oral efficacy population.
This population included all patients in the efficacy population who had evidence of taking the randomized (oral) study drug. This population was considered only for analysis of the primary end points.
Oral efficacy population.
This included patients reaching a primary efficacy end point during the oral phase (or not at all). It excluded those who had reached the primary end points or whose data were censored before they started taking the oral drug. Time to event was measured from oral drug start date.
Statistical methods: analysis methods
Efficacy.
All time-to-event parameters were compared between treatment groups using Cox proportional hazards regression, allowing for important prognostic influences. For the primary end points, the validity of the proportional hazards model (that the hazard ratio did not change with time) was checked by plotting the log of the negative log of the survival distribution against time. Hazard ratio (HR), 95% confidence interval, and probability (P) values were calculated as measures of treatment difference. The distributions of time-to-event parameters were estimated using cumulative incidence estimates to take account of competing risk events (ie, subjects who died before acquiring CMV infection.)14 Exploratory analyses of prognostic factors influencing the major efficacy end points were also carried out.
Safety.
Adverse experiences were categorized according to intensity, seriousness, causality, onset, and cessation. The proportion of patients in each treatment group reporting adverse experiences was summarized, but no formal hypothesis testing was carried out. Clinical laboratory values were assessed using quartile plots and confidence intervals for the changes from baseline and treatment differences in the changes from baseline.
Results
Randomized patients
Seven hundred forty-eight patients were enrolled in the study (Figure 1). Next, 376 patients were randomized to valacyclovir and 372 to acyclovir. Seven hundred twenty-seven (97%) patients were evaluable for efficacy, 366 patients in the valacyclovir arm and 361 in the acyclovir arm. Of the 727 patients included in the principal efficacy population, 605 received the oral study drug (305 in the valacyclovir arm and 300 in the acyclovir arm). One hundred thirty patients in the safety population did not proceed to the oral phase of the study. Eighty-four (65%, 45 and 39 patients randomized to valacyclovir and acyclovir, respectively) of these patients died before receiving the oral study drug. Sixty-four percent of patients in both treatment groups completed 22 weeks of clinical and laboratory assessments. The most common reason for premature discontinuation was one or more adverse experiences, which occurred in 94 (25%) patients in the acyclovir arm and 89 (24%) in the valacyclovir arm. One hundred sixty-eight (45%) patients in the valacyclovir arm and 180 (48%) in the acyclovir arm completed study drug administration.
Demography and disease characteristics
Patient demographics are shown in Table 1. Treatment groups were well matched. Eighty-nine percent of patients randomized to valacyclovir and 87% of patients randomized to acyclovir underwent transplantation for an underlying malignancy. Of these, most (62% and 63% for valacyclovir and acyclovir, respectively) were in first remission or chronic phase.
Detection of CMV in blood or BAL, or CMV disease
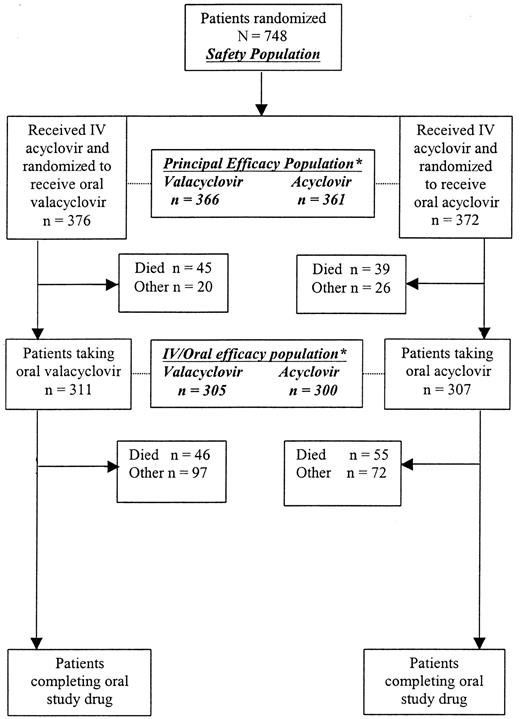
The time to CMV infection in BAL or blood or of CMV disease was significantly prolonged in the group receiving oral valacyclovir prophylaxis compared with those allocated to oral acyclovir (HR, 0.59; 95% CI, 0.46-0.76; P < .0001, Figure2). The cumulative incidence estimate of CMV infection was 33% on valacyclovir compared with 44% on oral acyclovir. (Table 2). Similar results were observed for the intravenous/oral efficacy population (HR, 0.59; 95% CI, 0.45-0.78; P = .0002) and for the oral efficacy population (HR, 0.47; 95% CI, 0.35-0.64; P < .0001).
Kaplan-Meier curves.
Cumulative incidence estimate of the time to CMV infection in BAL/blood or CMV disease.
Kaplan-Meier curves.
Cumulative incidence estimate of the time to CMV infection in BAL/blood or CMV disease.
Cumulative incidence estimates of CMV infection and disease: principal efficacy population
| CMV infection end point . | Oral acyclovir n = 361 . | Valacyclovir n = 366 . | ||
|---|---|---|---|---|
| Cumulative incidence estimates, % . | Incidence, % . | Cumulative incidence estimates, % . | Incidence, % . | |
| Blood, BAL, or disease | 44 | 40 | 33 | 28 |
| Disease or any positive culture | 53 | 50 | 40 | 34 |
| Blood | 41 | 39 | 29 | 26 |
| Urine | 34 | 28 | 20 | 14 |
| BAL | 3 | — | 2 | — |
| Definitive CMV disease | 4 | 3.6 | 4 | 2.5 |
| Presumed/definitive CMV disease | 6 | 5.5 | 5 | 3.5 |
| Presumed CMV disease | 1.9 | — | 1.0 | — |
| CMV infection end point . | Oral acyclovir n = 361 . | Valacyclovir n = 366 . | ||
|---|---|---|---|---|
| Cumulative incidence estimates, % . | Incidence, % . | Cumulative incidence estimates, % . | Incidence, % . | |
| Blood, BAL, or disease | 44 | 40 | 33 | 28 |
| Disease or any positive culture | 53 | 50 | 40 | 34 |
| Blood | 41 | 39 | 29 | 26 |
| Urine | 34 | 28 | 20 | 14 |
| BAL | 3 | — | 2 | — |
| Definitive CMV disease | 4 | 3.6 | 4 | 2.5 |
| Presumed/definitive CMV disease | 6 | 5.5 | 5 | 3.5 |
| Presumed CMV disease | 1.9 | — | 1.0 | — |
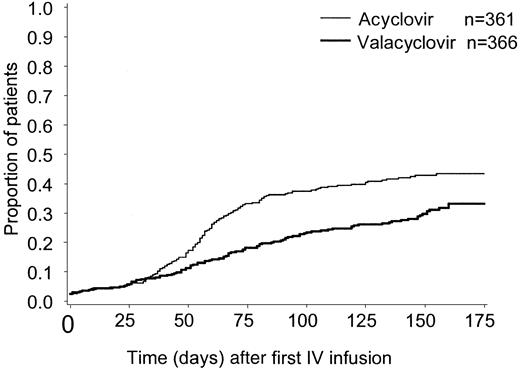
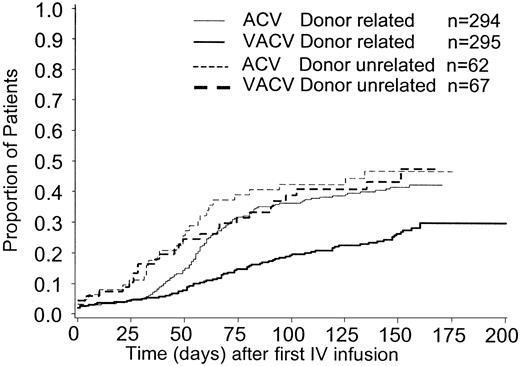
The cumulative incidence estimate of CMV infection in BAL/blood or CMV disease was lower in patients with related (vs unrelated) donors, (30% vs 48%, respectively for valacyclovir recipients; 43% vs 47% for acyclovir recipients), (Figure3). The cumulative incidence estimate of this end point, according to donor/recipient serostatus for CMV before transplantation, is presented in Figure4A-C. CMV-seronegative transplant recipients were at the lowest risk for CMV and in CMV-seronegative (vs -seropositive) transplant recipients (21% vs 36% respectively for valacyclovir recipients; 29% vs 46% for acyclovir recipients). There was no clear evidence of an effect of either of these prognostic factors—donor-relatedness or CMV serostatus—on the efficacy of valacyclovir compared with acyclovir.
Cumulative incidence estimate of the time to CMV infection in BAL/blood or CMV disease by donor relatedness.
Cumulative incidence estimate of the time to CMV infection in BAL/blood or CMV disease by donor relatedness.
Cumulative incidence estimate of the time to CMV infection in BAL/blood or CMV disease by donor-recipient CMV serostatus.
(A) Donor positive/recipient positive. (B) Donor negative/recipient positive. (C) Donor positive/recipient negative.
Cumulative incidence estimate of the time to CMV infection in BAL/blood or CMV disease by donor-recipient CMV serostatus.
(A) Donor positive/recipient positive. (B) Donor negative/recipient positive. (C) Donor positive/recipient negative.
Survival
Survival did not differ between the 2 treatment groups (valacyclovir vs acyclovir; HR, 0.98; 95% CI, 0.73-1.31;P = .89). Ninety-one patients (24%, 25.2%) randomized to valacyclovir compared with 94 (25%, 25.5%) randomized to acyclovir died during the study. When analysis was restricted to the oral efficacy subset, the mortality rate was also similar in the 2 treatment groups, with 46 of 305 (15%, 15.6%) deaths in the valacyclovir group, compared with 53 of 300 (18%, 17.2%) in the acyclovir group (HR, 0.89; 95% CI, 0.60-1.32; P = .55).
Time to cytomegalovirus infection
Valacyclovir was more effective than acyclovir in preventing or delaying CMV infection (disease or any positive culture), with a hazard ratio for valacyclovir versus acyclovir of 0.56 (95% CI, 0.45-0.71;P < .0001; Tables 2 and 3). Valacyclovir was also effective in preventing or delaying CMV viremia and CMV viruria (Table 3). The influence of donor/recipient CMV serostatus on this end point was similar to that presented above for the primary end point of CMV in blood, BAL, or CMV disease.
Prevention of CMV infection with valacyclovir vs acyclovir: principal efficacy population
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Time to CMV in blood, BAL, or disease | 0.59 (0.46, 0.76) | < .0001 |
| Time to CMV disease or any positive culture | 0.56 (0.45, 0.71) | < .0001 |
| Time to definitive CMV disease | 0.71 (0.30, 1.65) | .421 |
| Time to CMV in blood | 0.57 (0.44, 0.75) | < .0001 |
| Time to CMV in urine | 0.47 (0.34, 0.66) | < .0001 |
| . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Time to CMV in blood, BAL, or disease | 0.59 (0.46, 0.76) | < .0001 |
| Time to CMV disease or any positive culture | 0.56 (0.45, 0.71) | < .0001 |
| Time to definitive CMV disease | 0.71 (0.30, 1.65) | .421 |
| Time to CMV in blood | 0.57 (0.44, 0.75) | < .0001 |
| Time to CMV in urine | 0.47 (0.34, 0.66) | < .0001 |
CMV disease
A definitive CMV disease episode was documented for 22 patients; another 11 were accepted as having presumed CMV disease (Table 2). The times to definitive and presumed CMV disease did not differ between the treatment groups. For definitive CMV disease, the HR for valacyclovir versus acyclovir was 0.71 (95% CI, 0.30-1.65; P = .421). For presumed CMV disease, the HR for valacyclovir versus acyclovir was 0.67 (95% CI, 0.33-1.36; P = .269). Of the 13 patients in the valacyclovir group with definitive or presumed CMV disease, 2 cases occurred during therapy with intravenous acyclovir, 2 during oral valacyclovir therapy, 3 during ganciclovir/foscarnet therapy, and 6 when the patient was not receiving antiviral therapy. Of the 20 patients in the acyclovir group with definitive or presumed CMV disease, 2 cases occurred during intravenous acyclovir therapy, 9 during oral acyclovir therapy, 4 during ganciclovir/foscarnet therapy, and 5 when the patient was not receiving antiviral therapy.
Use of ganciclovir/foscarnet therapy
Ganciclovir or foscarnet was used by fewer valacyclovir patients (86 patients, 23%) than acyclovir patients (134 patients, 37%). There was a significant difference in time to use of ganciclovir/foscarnet, with an HR of 0.57 (95% CI, 0.43-0.75; P < .0001). These drugs were first used preemptively in 195 of 220 (88%) patients and as treatment for presumptive and confirmed CMV disease in 10 of 220 (5%) and 15 of 220 (7%) patients, respectively. Fourteen of 86 (16%) valacyclovir patients required 2 or more courses of ganciclovir/foscarnet (separated by 1 week) compared with 26 of 134 (19%) acyclovir patients.
Opportunistic infections: bacterial, fungal, and other herpesviruses
The incidence of microbiologically confirmed bacterial and fungal infections was similar between treatment groups. The incidence of HSV infections (including those virologically confirmed) was also similar between treatment groups (Table 4). Twenty-five patients in the acyclovir group had virologically confirmed HSV infection, compared with 19 in the valacyclovir group. At the time of virologically confirmed HSV infection, these patients were receiving therapy as follows: no therapy (20 acyclovir, 15 valacyclovir); intravenous acyclovir (1 acyclovir, 2 valacyclovir); oral study drug (2 acyclovir, 2 valacyclovir); ganciclovir/foscarnet (2 acyclovir, 0 valacyclovir). Diagnoses of varicella zoster virus infections (mostly localized zoster) and Epstein-Barr–virus infections were made rarely (Table 4).
Summary of opportunistic infections: bacterial, fungal, and other herpesvirus infections (1 or more episodes)
| Opportunistic infection . | % subjects (principal efficacy population) . | |
|---|---|---|
| Acyclovir . | Valacyclovir . | |
| Bacterial | 633-150 | 593-150 |
| Fungal | 303-150 | 313-150 |
| Herpes simplex virus | 103-151 (73-150) | 73-151 (53-150) |
| Varicella zoster virus | 23-151 | 23-151 |
| Epstein-Barr virus | 0 | 13-151 |
| Opportunistic infection . | % subjects (principal efficacy population) . | |
|---|---|---|
| Acyclovir . | Valacyclovir . | |
| Bacterial | 633-150 | 593-150 |
| Fungal | 303-150 | 313-150 |
| Herpes simplex virus | 103-151 (73-150) | 73-151 (53-150) |
| Varicella zoster virus | 23-151 | 23-151 |
| Epstein-Barr virus | 0 | 13-151 |
Laboratory confirmed.
Clinical diagnosis with or without laboratory confirmation.
Graft-versus-host disease
The incidence and severity of acute or chronic GVHD was similar in both treatment groups. Four percent of patients in both groups experienced acute GVHD of grades 3 to 4, and 3% of valacyclovir recipients and 5% of acyclovir recipients experienced extensive chronic GVHD. The times to acute and chronic GVHD did not differ between treatment groups. Acute GVHD HR for valacyclovir versus acyclovir was 1.03 (95% CI, 0.85-1.26; P = .74), and chronic GVHD HR for valacyclovir versus acyclovir was 0.80 (95% CI, 0.54-1.17; P = .24).
Safety
As expected, given the nature of the underlying disease, adverse events were frequently reported during the study (Table5). The most common adverse events were nausea, vomiting, abdominal pain, and diarrhea.
Most frequently reported adverse events: safety population
| . | Frequency (% subjects reporting adverse events) . | ||
|---|---|---|---|
| Intravenous acyclovir N = 748 . | Oral acyclovir n = 307 . | Oral valacyclovir n = 311 . | |
| Nausea | 53 | 21 | 24 |
| Vomiting | 59 | 22 | 22 |
| Abdominal pain | 27 | 16 | 19 |
| Diarrhea | 45 | 21 | 19 |
| Hypertension | 21 | 11 | 13 |
| Cough | 10 | 10 | 12 |
| Headache | 28 | 14 | 12 |
| Pain | 14 | 10 | 12 |
| Kidney function abnormality | 11 | 8 | 10 |
| Rash | 21 | 10 | 10 |
| Arthralgia | 7 | 7 | 10 |
| Constipation | 11 | 3 | 5 |
| Anxiety | 13 | 3 | 4 |
| Epistaxis | 14 | 4 | 3 |
| Stomatitis | 68 | 2 | 2 |
| TMA-like episode | 1 | 2 | 3 |
| . | Frequency (% subjects reporting adverse events) . | ||
|---|---|---|---|
| Intravenous acyclovir N = 748 . | Oral acyclovir n = 307 . | Oral valacyclovir n = 311 . | |
| Nausea | 53 | 21 | 24 |
| Vomiting | 59 | 22 | 22 |
| Abdominal pain | 27 | 16 | 19 |
| Diarrhea | 45 | 21 | 19 |
| Hypertension | 21 | 11 | 13 |
| Cough | 10 | 10 | 12 |
| Headache | 28 | 14 | 12 |
| Pain | 14 | 10 | 12 |
| Kidney function abnormality | 11 | 8 | 10 |
| Rash | 21 | 10 | 10 |
| Arthralgia | 7 | 7 | 10 |
| Constipation | 11 | 3 | 5 |
| Anxiety | 13 | 3 | 4 |
| Epistaxis | 14 | 4 | 3 |
| Stomatitis | 68 | 2 | 2 |
| TMA-like episode | 1 | 2 | 3 |
During the oral phase, events were reported with similar frequency in the valacyclovir and acyclovir treatment groups. Treatment-limiting adverse events, including nausea and vomiting, were of a similar nature and frequency in the 2 study arms during the oral phase (17% and 16% of the valacyclovir and acyclovir groups, respectively).
Twenty patients experienced a TMA-like episode; in 11 patients this was described as hemolytic–uremic syndrome, in 8 patients it was described as thrombotic thrombocytopenic purpura, and in 1 patient it was described as both. During oral treatment, cases occurred with similar frequency among the 2 groups (8 and 6 cases for valacyclovir and acyclovir, respectively). Two cases (1 in each oral treatment group) were not thought to be serious, and 3 cases (1 during the intravenous phase and 1 in each oral treatment regimen) were fatal.
One hundred eighty-five patients died during the study, 86 from events commencing during the intravenous acyclovir phase and 99 during the oral treatment phase. Forty-five recipients of valacyclovir died (14%) compared with 54 (18%) recipients of oral acyclovir. Reported causes of death were similar in the 2 groups. The most common causes of death were those anticipated for this patient population, and significant contributory factors included opportunistic infection (34%), GVHD (30%), relapse of underlying disease (11%) and CMV infection and disease (total, 9.2%-3.2% and 6.0% of those receiving valacyclovir or acyclovir, respectively).
Clinical chemistry and hematology data revealed no marked differences between treatment groups. In particular, serum creatinine levels were similar throughout the trial in each patient group.
Discussion
How to achieve optimal prevention of CMV infection and disease is still an important question in allogeneic bone marrow transplantation. When the first study of high-dose acyclovir prophylaxis showed a reduction in CMV disease and an improvement in survival a decade ago,6 it was received with some skepticism because acyclovir has poor activity against CMV in vitro. Since then it has been clarified that acyclovir can be phosphorylated by the UL97 gene product of CMV,15 and several studies in different patient populations have shown the efficacy of acyclovir in inhibiting CMV replication in vivo.7,16-18 The results of the current study, the largest randomized CMV prophylaxis study in allogeneic BMT patients published so far, show that valacyclovir was more effective in suppressing CMV viremia and CMV infection than the high-dose acyclovir regimen studied by Prentice et al.7 There was, similar to what was found previously,7 no difference in the frequency of CMV disease and no improvement in survival. This is not surprising given that preemptive therapy, based on the sensitive diagnostic tests for CMV infection available today, has been shown an effective strategy to reduce CMV disease, mortality from CMV infection, and overall transplant-related mortality.5 19
The lack of additional benefit of valacyclovir on CMV disease incidence may have several explanations. In our study, valacyclovir was compared with high-dose oral acyclovir, and both arms had the same initial intravenous acyclovir phase. In another recent study in renal transplant patients, valacyclovir significantly reduced CMV disease compared with placebo.20 Another explanation might be the permitted use of preemptive ganciclovir therapy.5,19 It should be noted that the documented CMV disease incidence was very low; only 2% of patients in the valacyclovir arm and 4% in the acyclovir arm had definitive CMV disease. Moreover, valacyclovir significantly reduced the requirement for preemptive therapy by approximately 40%. This reduction has practical implications; ganciclovir is associated with the development of neutropenia, and ganciclovir-associated neutropenia has been shown to be an independent risk factor for reduced survival.4 21
Our results in BMT patients indicate that the higher acyclovir exposure achieved with valacyclovir has additional benefits in preventing CMV infection but no additional benefit (over high-dose oral acyclovir) on survival. Other individual studies in solid organ transplantation have not, so far, demonstrated an impact of acyclovir prophylaxis on survival, though a meta-analysis of such studies did so.22Furthermore, an impact of acyclovir on survival was seen in patients with advanced HIV disease.23
The safety profile of valacyclovir was similar to that of high-dose acyclovir; adverse events were comparable between the groups. In particular the increased risk for TMA reported in a study of human immunodeficiency–infected patients23,24 was not seen in this study. The low incidence (17%) of acute GVHD grades 2 to 4 in this study of CMV-seropositive patients (or negative with a positive donor) is interesting because several studies have shown an association between pretransplantation CMV seropositivity and the probability for GVHD.25 26
One recent development is the concept of viral load as a risk for CMV disease in BMT patients.27 It is possible that valacyclovir prophylaxis might result in lower viral loads in BMT patients, as in HIV-infected patients.28 Therefore, an individualized preemptive therapy based on viral load measurements might mean that a smaller proportion of patients need potentially toxic antiviral therapy. This concept needs, however, to be tested in a controlled study.
The results of this study show that valacyclovir is effective in protecting patients from CMV reactivation. Valacyclovir prophylaxis could, therefore, play an important role in combination approaches to the prevention of CMV in allogeneic BMT patients. The strategy of using a well-tolerated prophylactic agent and of monitoring for CMV reactivation should ultimately reduce the requirement for preemptive therapy. Provided that the prophylactic agent is sufficiently efficacious, a well-tolerated oral medication is likely to be more acceptable to patients and to have economic advantages.29Whether valacyclovir prophylaxis followed by preemptive therapy would be better than preemptive therapy alone has not been tested in a controlled setting.
A complete list of the investigators who participated in the Valacyclovir International Bone Marrow Transplant Study Group appears in the at the end of this article.
The following investigators participated in the Valacyclovir International Bone Marrow Transplant Study Group: Australia, Dr R. Herrmann, Dr S. Durrant; Austria, Dr H. Greinix; Belgium, Prof A. Ferrant, Dr R. Schots, Dr D. Bron, Dr D. Seleslagh; Denmark, Dr L Vindelov; Finland, Dr L. Volin, Dr J. Nikoskelainen; France, Prof E. Gluckman, Prof N. Milpied, Dr D. Blaise, Prof J. Cahn, Prof J. Reiffers, Prof M. Attal; Germany, Prof G. Ehninger, Prof N. Schmitz, Dr H. Link, Prof R. A. Zander; Ireland, Prof McCann, Dr G. Crotty; Israel, Dr R. Or; Italy, Prof F. Martelli, Prof S. Tura, Prof P. di Bartolomeo, Prof F. Mandelli, Dr D. A. Bosi; Portugal, Dr M. Abecassis, Prof Lacerda; South Africa, Prof P. Jacobs; Spain, Dr M. Sanz, Dr R. de la Camara, Dr A. Domingo-Albos (deceased), Prof C. Rozman, Prof A. Tores-Gomez, Prof F. J. Lopez; Sweden, Prof P. Ljungman, Dr G. Öberg, Dr A. Fasth, Dr S. Rödjer, Dr P.-G. Nilsson; United Kingdom, Dr R. E. Clark, Dr N. Russell, Prof H. G. Prentice, Prof R. Powles, Prof S. Proctor, Dr E. J. Kanfer, Dr A. Foot.
Supported by research funding from Glaxo Wellcome Research and Development.
A.C. and A.W. are employed by Glaxo SmithKline Research and Development whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Per Ljungman, Department of Haematology, Huddinge University Hospital, SE-141 86 Huddinge, Stockholm, Sweden; e-mail:per.ljungman@medhs.ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal