Abstract
In the initial stage of cutaneous T-cell lymphoma (CTCL), proliferating CTCL cells are concentrated in the epidermis in close association with an immature dendritic cell (DC), the Langerhans cell. Because long-term in vitro culture of CTCL cells has proven difficult, the in vivo association with the major antigen-presenting cell (APC) of the epidermis has been postulated to play a role in directly stimulating the clonal T-cell proliferation. We report that CTCL cells can be reproducibly grown in culture for 3 months when cocultured with immature DCs. CTCL cells retain the phenotype and genotype of the initial malignant clone, whereas the APCs are a mixture of immature and mature DCs. CTCL cell and DC survival was dependent on direct membrane contact. Growth was inhibited by antibodies that bound to the T-cell receptor (TCR) or interfered with the interaction of CD40 with its ligand on the CTCL cell. Addition of antibody to CD3 or the clonotypic TCR caused rapid CTCL cell apoptosis followed by engulfment by avidly phagocytic immature DCs and subsequent DC maturation. The opportunity to study CTCL cells and immature DCs for prolonged periods will facilitate studies of tumor cell biology and will allow investigation of the intriguing hypothesis that CTCL cell growth is driven through TCR recognition of class II–presented self-peptides. In addition, the culture of CTCL cells will permit evaluation of therapies in vitro before clinical intervention, thereby improving safety and efficacy.
Introduction
Cutaneous T-cell lymphoma (CTCL) is a clonal,1 epidermotropic malignancy of memory2,3 inducer T cells,4,5 which has been difficult to study because of the inability to propagate the neoplastic cells in vitro and the absence of suitable animal models. The malignant cells initially proliferate in the epidermis and evolve more poorly differentiated subclones that later escape the confines of the skin and disseminate into the peripheral blood and internal organs.6 The close apposition of the CTCL cells and the Langerhans cell, an immature member of the dendritic cell (DC) series,7 has suggested that the malignancy may be driven by Langerhans cell triggering of CTCL cell growth through engagement of the CTCL cell T-cell receptor (TCR).8
Previous studies have shown that mitogen, antigen, and mixed leukocyte cultures (MLCs) with or without cytokines only minimally stimulate proliferation of isolated CTCL cells.9-11 CTCL cells cultured with interleukin-2 (IL-2) and IL-7 and DCs cultured with granulocyte-monocyte colony-stimulating factor (GM-CSF) and IL-412 do not survive and proliferate beyond a few weeks of culture. We have recently demonstrated that monocytes activated by passage through leukapheresis centrifugation and subsequent extracorporeal photochemotherapy (ECP) treatment develop the phenotypic and functional characteristics of immature DCs, which can engulf apoptotic CTCL cells.13 When cells from the leukapheresis harvest were cultured in the presence of supportive cytokines, both CTCL cells and DCs continued to proliferate in vitro for 3 months.
We report that CTCL cells appear to retard DC maturation and this immaturity enables avid phagocytosis of apoptotic tumor cells. Uptake of apoptotic CTCL cells leads to the up-regulation of some DC maturation markers and the potential for continued CTCL cell stimulation through presentation of peptide derived from the CTCL cells themselves in the DC class II molecules to the TCR of the CD4+ T cells or release of DC-derived growth factors. This codependency may explain the factors that influence the growth of CTCL cells and elucidate the role of immature DCs in the disease process.
Materials and methods
Cell cultures
Leukapheresis cells were obtained from patients with CTCL undergoing ECP, under the guidelines of the Yale Human Investigational Review Board. Informed consent was provided according to the Declaration of Helsinki. Leukocytes isolated by Ficoll-Hypaque flotation were cultured with the cytokines IL-7 (10 ng/mL) and IL-2 (10 U/mL) to support the CTCL cells12 and GM-CSF (800 U/mL), IL-4 (1000 U/mL; all from R & D Systems, Minneapolis, MN) to promote DC differentiation from monocytes,13in RPMI 1640 (Gibco, Gaithersburg, MD)/20% AB serum, 20 to 30 × 106 cells/well. Cultures were established from 10 patients with CTCL on multiple occasions and maintained for 3 months. The patients all had leukemic-phase Sézary syndrome with erythrodermic skin involvement and were negative for infection with human T-cell leukemia virus 1 (HTLV-1).
Outgrowth of normal CD4 and CD8 T cells or Epstein-Barr virus–transformed B cells may occur if normal cells predominate in the peripheral blood of the patient. In our studies, the phenotype and genotype of the cultured CTCL cells and DCs were evaluated to confirm their purity. The majority of cultures established for study were derived from the peripheral blood of patients with CTCL that contained less than 10% contaminating normal cells and only the initial CTCL cell malignant clone was propagated under those circumstances. The cultures were negative for Mycoplasma contamination as determined by a Mycotek Kit (Gibco Invitrogen, Grand Island, NY), performed according to the manufacturer's directions.
Immunophenotyping
Cultured cells were phenotyped with a panel of monoclonal antibodies specific for lymphocytes, monocytes, and DCs. Antibodies were either directly conjugated to fluorescein-isothiocyanate (FITC) or phycoerythrin (PE) or stained with a secondary fluorochrome-conjugated antimouse reagent. The antibody panel included CD3 (pan T cell); CD4 (inducer T cell); CD8 (cytotoxic T cell); Vβ5c, Vβ18 (variable region of family-specific β chain of the TCR); CD19 (pan B cell); CD14 (monocytes); CD56 (natural killer cells); class II major histocompatibility complex (MHC) molecule; W632 (class I MHC molecule, gift from Peter Cresswell, Yale University); CD83 (DC, activated B lymphocytes); DC-LAMP (mature DC); CD1a (immature DC); annexin V and APO-2 (early apoptotic cells); all antibodies (except W632) were obtained from Coulter-Immunotech (Hialeah, FL) and used at the predetermined optimum dilution. Background staining was assessed with the appropriate isotype control that was either directly conjugated or stained with a fluorochrome-conjugated secondary antimouse antibody. Reactivity was determined by analysis with a Coulter XL flow cytometer, direct observation with a fluorescent microscope equipped with phase contrast optics, or by confocal microscopy.
Two-color detection of membrane and cytoplasmic antigens
Two-color membrane staining was performed (representative results from 1 of 6 cultures) by adding the predetermined optimum concentrations of both monoclonals directly conjugated to FITC or PE (30 minutes at 4°C) followed by washing. Two-color isotype-matched controls were used to detect background staining. To examine membrane and cytoplasmic antigen expression, a fixation and permeabilization kit was used essentially as described in the manufacturer's directions (Coulter-Immunotech). Membrane staining was assessed with monoclonal antibodies reactive with either CD83 or class II directly conjugated to FITC. Cytoplasmic staining was determined with CD3-PE. DC-LAMP cytoplasmic staining was assessed after permeabilization with a secondary antimouse reagent conjugated to FITC. As a control for DC-LAMP, an isotype control and the secondary antibody were added after permeabilization.
Confocal microscopy
The DCs were freed of CTCL cells by CD2-bead depletion and adhered to Alcian blue–coated coverslips. DCs were stained with rabbit anti–human class II and mouse anti–human LAMP (gift from Ira Mellman, Yale University). Alexa-Fluor antibodies (Molecular Probes, Eugene, OR) were used to detect binding of the primary antibodies and FITC-conjugated goat antirabbit and Texas red–conjugated goat antimouse were used to detect irrelevant isotype control antibodies. Fluorescent staining was detected with a Zeiss confocal microscope.
Polymerase chain reaction assay
Polymerase chain reactions (PCRs) were performed at the Yale University, Department of Laboratory Medicine. A probe to detect rearrangement of consensus sequences of the β chain (D2-J2) and γ chain of the TCR were used.
Proliferation assays
Cell proliferation (representative results from 2 of 6 cultures) was evaluated by cultivation of magnetic bead–purified (as previously described,14 Dynal, Lake Success, NY) CD2+CTCL cells (2 × 105 tumor cells/well) and tumor cell–depleted DCs (1 × 105 DCs/well) for 3 days. The wells were pulsed during the final 16 hours with [3H]-thymidine, harvested, and counted in a liquid scintillation counter. Results are the mean of 5 replicates ± SD.
Because purified CTCL cells or DCs did not survive without direct contact, proliferation was assessed in the cocultures with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes). The cultures were stained with the predetermined optimal concentration of CFSE (2 μM, RPMI 1640) for 20 minutes at 37°C), washed with RPMI 1640 containing 20% fetal calf serum to bind up free CFSE, and cultivated under standard conditions with cytokines for 5 days. Samples were analyzed for staining by gating on the lymphoblast or large cell population with a flow cytometer. Cell division was quantified by measuring equidistant peaks that represented a halving of the fluorescent intensity with the initial peak determined at day 3, a time point taken after the initial nonspecific loss of CFSE had occurred.
Transwell cultures
CD2-magnetic bead-enriched CTCL cells (1 × 106/mL, results presented from 2 representative cultures) and bead-depleted DCs (1 × 105/mL) were cultured on either side of a 0.40-μm porous membrane (Corning Costar, Cambridge MA) in media containing the standard cytokines. To exclude deleterious effects of contact with the membrane, the positions of the DCs and the CTCL cells were reversed in parallel cultures. Cultures were maintained for 3 weeks and viability monitored by trypan blue exclusion.
Antibody-mediated inhibition of CTCL cell–antigen-presenting cell contact
Cocultivated cells (1 of 6 cultures) were incubated with monoclonal antibodies (CD3, family-specific Vβ, CD40), used at predetermined optimal dilutions, approximately 33 μg/mL, 10 × 106cells/100 μL/well. After overnight incubation with the antibodies, proliferation was measured by uptake of [3H]-thymidine. Results are the mean of 5 replicates ± SD.
Cytokine enzyme-linked immunosorbent assay
Supernatants were harvested from 2-month cultures (3 cultures of 6 tested, 4 days after media replenishment) and the presence of cytokines evaluated with commercial enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems) to detect tumor necrosis factor–α (TNF-α), interferon-γ (IFN-γ), IL-10, and IL-15 according to the manufacturer's directions.
To inhibit IL-10, a neutralizing antibody was added to 10 × 106 CTCL cells and 1 × 106 DCs from 2-month cultures (10 ng/mL, R & D Systems) with and without the addition of 2 × 106 CTCL cells that were rendered apoptotic by γ irradiation. The cocultures were incubated overnight and then analyzed by immunofluorescent staining and flow cytometry.
Apoptosis assays
Programmed cell death was examined (2 of 6 cultures) at intervals (0, 20, 30, 50 minutes and 24 hours) after the addition of 0.5 mL CD3 (33 μg/mL) or an antibody reactive with the variable region of the CTCL cell's clonotypic TCR. Two-color immunofluorescence was performed, after fixation and permeabilization, using antimouse FITC/APO-2–PE to detect internalized apoptotic T lymphocytes. Annexin V staining was performed to detect early apoptotic cells with a commercially available kit, following the manufacturer's directions (Coulter-Immunotech).
Videomicroscopy
Cells (1 × 105) from 1- and 2-month cultures were plated within 30 minutes after receiving CD3 antibody (3.3 μg/100 μL) on the uncoated glass surface of a chamber slide (Mattek, Ashland, MA). The cells were maintained at 37°C under a 7% CO2 atmosphere during the 1 hour of filming and observed with a Zeiss Axiovert microscope. Images were filmed with a CCD camera (Hamamatsu, Michigan City, IN) using Open Lab software to produce a Quicktime movie.
MLC
Normal control responder T cells were purified with CD2-magnetic beads. DCs were enriched from 1- and 2-month cultures by bead-depletion of CD2+ CTCL cells. Control T cells were added to DCs at concentrations of 100, 50, and 25 × 103 responder T cells added to 1 × 103 DCs. Control T cells were added at the same concentrations to allogeneic control B cells and monocytes that had been depleted of T cells. Stimulators were prevented from proliferating by γ irradiation (2000 rads, cesium irradiator) prior to the addition of responding T cells. Cultures were incubated for 6 days and received a 16-hour pulse of [3H]-thymidine, prior to harvesting. Results were calculated as the mean ± SD of 5 replicates (representative results from 1 of 2 normal controls). Statistics were analyzed with the Student t test.
Results
Characterization of CTCL and antigen-presenting cell cocultures
The CTCL cells and DCs were cultured, with supportive cytokines, from leukapheresis specimens obtained from 10 patients on multiple occasions and were routinely maintained in vitro for 3 months. The ability to grow both cell types was entirely reproducible and uniformly successful in all patients.
The CTCL cells were unable to proliferate when they were removed from contact with the antigen-presenting cells (APCs) (Figure1). Purified CTCL cells obtained from cultures that had been established for 1 and 3 months from 2 representative CTCL patients did not proliferate during the 72-hour incubation period, despite the presence of cytokines, unless they were cultivated in the presence of their autologous APCs.
CTCL cells proliferate only in the presence of APCs.
CTCL cells were obtained from 1- (▪) and 3-month (▨) cultures of 2 representative patients and were freed of APCs by CD2–magnetic bead enrichment. The purified CTCL cells were readded after overnight culture to γ-irradiated autologous APCs that had been CTCL cell bead-depleted. After 3 additional days of culture, only CTCL cells cocultivated with APCs proliferated, as shown by uptake of [3H]-thymidine. The results presented are the means of 5 replicate samples ± SD.
CTCL cells proliferate only in the presence of APCs.
CTCL cells were obtained from 1- (▪) and 3-month (▨) cultures of 2 representative patients and were freed of APCs by CD2–magnetic bead enrichment. The purified CTCL cells were readded after overnight culture to γ-irradiated autologous APCs that had been CTCL cell bead-depleted. After 3 additional days of culture, only CTCL cells cocultivated with APCs proliferated, as shown by uptake of [3H]-thymidine. The results presented are the means of 5 replicate samples ± SD.
In addition, when proliferating CTCL cells and DCs were purified from 2-month cultures and separated by a transwell membrane for 2 weeks, their viability declined from 91% ± 0.7% in the cocultures to 57% ± 10% in isolated CTCL cells and 0% in DCs. The viability of the isolated CTCL cells continued to decline until at 3 weeks only a few intact cells were visible. The addition of supernatants from long-term proliferating cocultures did not prolong the growth of the isolated cell populations, supporting the contention that direct membrane contact is required for CTCL cell and DC proliferation.
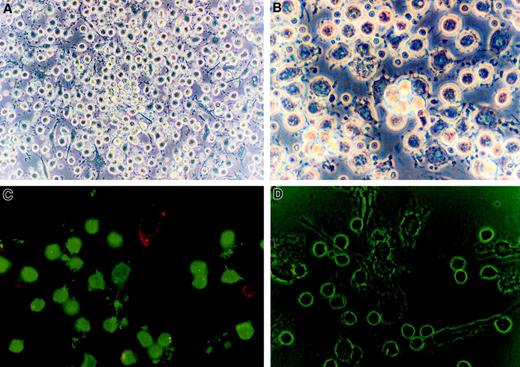
The CTCL cells were morphologically medium-sized lymphocytes clustered around the APCs (Figure 2A), in a fashion reminiscent of the Pautrier microabscess. APCs (Figure 2B) formed groups of nonadherent floating cells as well as adherent cells that were of large size and had a granular cytoplasm and multiple dendritic extensions. Immunofluorescent staining (Figure 2C) demonstrated many CD3+ T cells in direct contact with class II+APCs. The dendritic extensions of the APCs and the close approximation of the T cells to the APCs were clearly visible under phase contrast microscopy (Figure 2D).
CTCL cells are morphologically small CD3 + lymphocytes clustered around large granular class II + cells with dendritic appendages.
(A) Phase contrast microscopy (× 400) of cultured CTCL cells demonstrates that they have the morphology of small round lymphocytes. (B) The APCs were morphologically large granular cells with multiple dendritic extensions. (C) Immunofluorescent microscopy reveals that the CTCL cells are CD3+ (FITC, green) lymphocytes and the APCs express class II MHC molecules (phycoerythrin, red). (D) Phase contrast microscopy of the same field demonstrates the small round lymphocytes in direct contact with the membrane of large cells with multiple dendritic appendages. Original magnification × 400.
CTCL cells are morphologically small CD3 + lymphocytes clustered around large granular class II + cells with dendritic appendages.
(A) Phase contrast microscopy (× 400) of cultured CTCL cells demonstrates that they have the morphology of small round lymphocytes. (B) The APCs were morphologically large granular cells with multiple dendritic extensions. (C) Immunofluorescent microscopy reveals that the CTCL cells are CD3+ (FITC, green) lymphocytes and the APCs express class II MHC molecules (phycoerythrin, red). (D) Phase contrast microscopy of the same field demonstrates the small round lymphocytes in direct contact with the membrane of large cells with multiple dendritic appendages. Original magnification × 400.
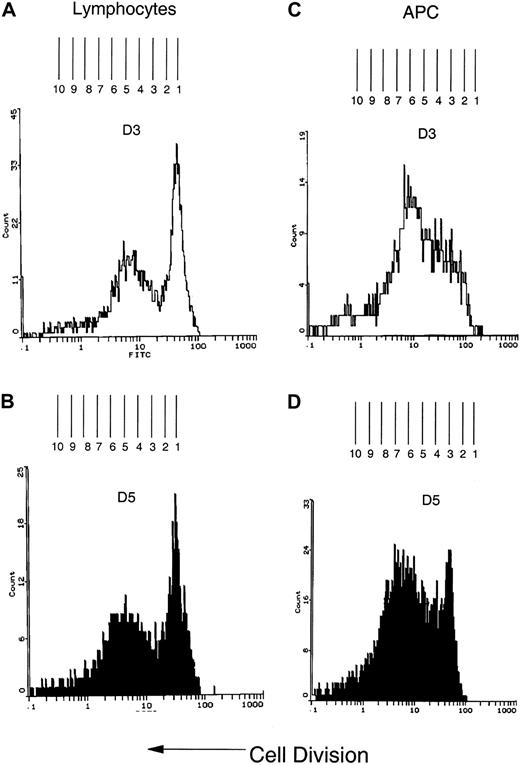
CFSE staining demonstrates proliferation of CTCL cells and APCs
Because separation of either cell type resulted in rapid cell death, proliferation was evaluated directly in the cocultures by CFSE staining. CFSE staining intensity is halved when the cells replicate, enabling direct quantification of cell division.15 The results are presented as the shift in fluorescence observed at day 5 measured from the initial staining monitored at day 3, a time point chosen because the initial nonspecific loss of CFSE has dissipated.15 Figure 3, panels A and B, demonstrate lymphocyte proliferation in a 1-month culture, followed over 5 days. The shift in CFSE intensity indicates that at least 8 rounds of lymphocyte cell division have occurred. The parallel cocultivated APC population (Figure 3C,D) also demonstrated at least 8 divisions over 5 days, indicating a doubling time of 15 hours for both the tumor cells and the APCs.
CFSE staining demonstrates multiple rounds of proliferation in both the lymphocyte and APC populations of the cocultivated cells.
(A) Three days after CFSE staining, the lymphoblast population was identified by flow cytometry scatter parameters. The lymphoblast population was biphasic with the majority of cells undergoing slower replication and a subset of cells that demonstrated a higher rate of proliferation. To determine the number of divisions, the fluorescence histogram was overlaid on a grid so that the center of each peak was equidistant from the next peak and represented one cycle of cell division. The number of divisions was determined by counting the number of peaks. (B) The change in fluorescence intensity was measured by determining the distance between the initial peak on day 3 and the shift in fluorescent intensity of equidistant peaks observed on day 5. The fluorescence shift indicates that 8 rounds of cell division have occurred by day 5. (C) The APC population was identified by flow cytometry light scatter parameters and the replication rate determined as described in panel A. (D) By 5 days, the CFSE staining curve has shifted to the left and at least 8 peaks of cell division are present, demonstrating a doubling time, which is equivalent to that found in the cocultured lymphocytes, of approximately 15 hours.
CFSE staining demonstrates multiple rounds of proliferation in both the lymphocyte and APC populations of the cocultivated cells.
(A) Three days after CFSE staining, the lymphoblast population was identified by flow cytometry scatter parameters. The lymphoblast population was biphasic with the majority of cells undergoing slower replication and a subset of cells that demonstrated a higher rate of proliferation. To determine the number of divisions, the fluorescence histogram was overlaid on a grid so that the center of each peak was equidistant from the next peak and represented one cycle of cell division. The number of divisions was determined by counting the number of peaks. (B) The change in fluorescence intensity was measured by determining the distance between the initial peak on day 3 and the shift in fluorescent intensity of equidistant peaks observed on day 5. The fluorescence shift indicates that 8 rounds of cell division have occurred by day 5. (C) The APC population was identified by flow cytometry light scatter parameters and the replication rate determined as described in panel A. (D) By 5 days, the CFSE staining curve has shifted to the left and at least 8 peaks of cell division are present, demonstrating a doubling time, which is equivalent to that found in the cocultured lymphocytes, of approximately 15 hours.
Immunophenotype and genotype
At 2 months, the CTCL cells retain the phenotype of the neoplastic, clonal, peripheral blood T cells from which they were derived, with the majority of the cells expressing CD3 (100%) and CD4 (99%), lacking CD8 (0%) and, if known, expressing a Vβ-reactive family-specific TCR Vβ8a (93%). No CD56+ natural killer cells or CD19+ B cells were identified. PCR studies demonstrated that cultured CTCL cells, tested at 2 months, retained the identical TCR-β and TCR-γ chain rearrangements found in the original peripheral blood isolate (results not shown).
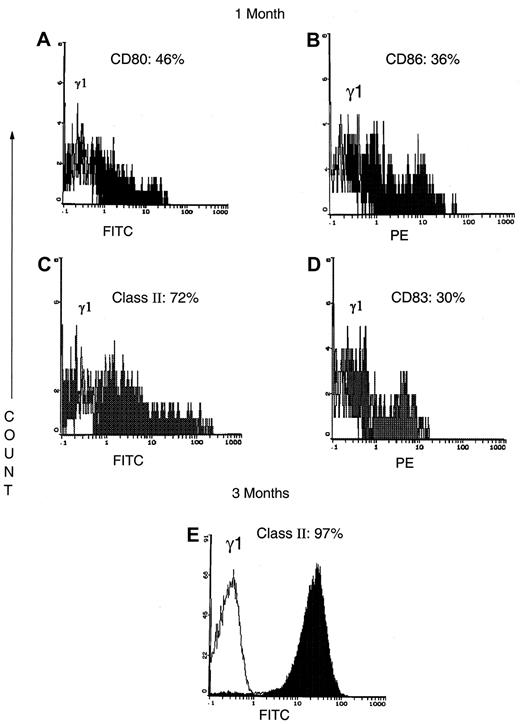
The APC population contained CD14+ monocytes that represented approximately 40% of the cells at 2 weeks and declined to less than 10% of the APCs by 1 month of culture. The cultured APCs were reactive with antibodies that detected costimulatory molecules (Figure 4A, CD80: 46%; Figure 4B, CD86: 36%). At 1 month, the APCs were 72% class II reactive (Figure 4C). The maturing CD83+ DC population represented 30% (Figure4D) of the APCs. When APCs were tested after 3 months in culture, the majority of the APCs expressed higher levels of class II (Figure 4E). Further studies (Table 1) showed that at 3 weeks only 7% of the DCs were mature as shown by CD83 expression on the cell membrane and cytoplasmic reactivity with DC-LAMP (9% positive cells). After 2 months of cultivation of cells from the same patient, the levels of CD83 expression (15%) and DC-LAMP+ (36%) had increased 2- to 4-fold, indicating continued DC maturation. In 3-month-old cultures from another patient with CTCL, 70% of the DCs expressed membrane CD83 or cytoplasmic DC-LAMP or both. Overall, at 1 to 2 months of culture the APCs were predominantly immature cells admixed with approximately 30% mature DCs. By 3 months of culture, substantial DC maturation had occurred at a time point that preceded the imminent demise of both the CTCL cells and the APCs.
Flow cytometry phenotype of the APC at 1 and 3 months of culture.
(A-D) At 1 month of culture, the APCs from a representative CTCL patient are a mixture of immature APCs that express costimulatory molecules CD80 (A) and CD86 (B); class II MHC molecules (C); and mature DC that express CD83 (D). (E) By 3 months of culture, the majority of the APCs from a representative patient express higher levels of class II MHC molecules.
Flow cytometry phenotype of the APC at 1 and 3 months of culture.
(A-D) At 1 month of culture, the APCs from a representative CTCL patient are a mixture of immature APCs that express costimulatory molecules CD80 (A) and CD86 (B); class II MHC molecules (C); and mature DC that express CD83 (D). (E) By 3 months of culture, the majority of the APCs from a representative patient express higher levels of class II MHC molecules.
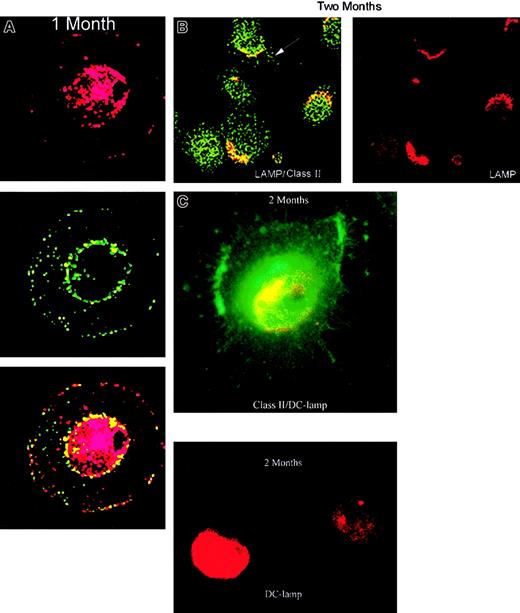
Confocal microscopic demonstration of DC class II sequestration
The DCs obtained from 1- and 2-month cultures were freed of CTCL cells and the expression of the cytoplasmic lysosomal marker lamp and class II MHC molecules monitored. After 1 month of culture (Figure5A), confocal microscopy revealed an intermediate DC phenotype7 with some lysosomal colocalization of LAMP and class II molecules and a punctate pattern of nonlysosomal class II vesicles (CIIV-like) beneath the plasma membrane. This phenotype has been reported after 1 day to 1 week of culture7 and was still evident in many DCs after 1 month of coculture with CTCL cells, demonstrating delayed maturation. By 2 months of culture (Figure 5B), the punctate pattern of CIIV-like structures and some lysosomal colocalization was still present. Migration of class II molecules to dendritic extensions of the cell membrane also developed indicating increased maturation from the intermediate phenotype toward a late DC phenotype. Fluorescent microscopy confirmed (Figure 5C) that some DCs had maturated at 2 months with expression of a starfishlike morphology, class II on the cell membrane and DC-LAMP in the cytoplasm.
Confocal microscopic evaluation of DCs from 1- and 2-month cultures.
(A) DCs were purified from 1-month cultures by CD2-bead depletion of CTCL cells. The DCs were stained for cytoplasmic expression of the lysosomal marker LAMP (red) and class II MHC (green). The 2 images were merged to reveal colocalization of lysosomal compartments containing class II MHC (yellow). (B) DCs purified from 2- month cultures demonstrate class II colocalization (yellow) with LAMP (red) in lysosomal compartments and migration of class II molecules (green) to dendritic extensions of the cell membrane (arrow). (C) Fluorescent microscopic observation demonstrates cytoplasmic DC-LAMP staining (red) and membrane class II staining (green) on dendritic appendages of mature DCs with starfish morphology at 2 months of culture. Original magnification × 630.
Confocal microscopic evaluation of DCs from 1- and 2-month cultures.
(A) DCs were purified from 1-month cultures by CD2-bead depletion of CTCL cells. The DCs were stained for cytoplasmic expression of the lysosomal marker LAMP (red) and class II MHC (green). The 2 images were merged to reveal colocalization of lysosomal compartments containing class II MHC (yellow). (B) DCs purified from 2- month cultures demonstrate class II colocalization (yellow) with LAMP (red) in lysosomal compartments and migration of class II molecules (green) to dendritic extensions of the cell membrane (arrow). (C) Fluorescent microscopic observation demonstrates cytoplasmic DC-LAMP staining (red) and membrane class II staining (green) on dendritic appendages of mature DCs with starfish morphology at 2 months of culture. Original magnification × 630.
Increased DC maturation enhances MLC stimulation
The ability to stimulate an MLC response in normal alloreactive T cells was tested to evaluate DC maturation. Mature DCs are potent stimulators in MLCs due to their expression of high levels of cell membrane class II MHC molecules, whereas immature DCs retain class II molecules in the cytoplasm and are less stimulatory in MLCs.7 The ability of CTCL cell–depleted DCs from 1- and 2-month cultures to stimulate a proliferative response in serially diluted normal control T-cell responders was tested. DCs purified from 2-month cultures were significantly more stimulatory in MLCs (P ≤ .001) in comparison to DCs obtained from 1-month cultures or normal control B cells and monocytes, at all ratios tested (100 × 103, 50 × 103, 10 × 103 CTCL cells: 1 × 103 DCs; results not shown). DC immaturity at 1 month was confirmed by their inability to stimulate a response, at all ratios tested, that was significantly different from that of normal control B cells and monocytes. Therefore, as indicated by the immunophenotype results, at 1 month of culture the majority of the DCs were immature cells that can mature into immunostimulatory DCs at 2 months of culture.
Cytokines produced by the cultured cells may influence DC maturation
Supernatants from 2-month cocultures contained cytokines that could affect CTCL cell/APC interaction, including TNF-α, IFN-γ, and IL-10 (Table 2). IL-15, which has been reported to prolong CTCL cell growth, was not found in the culture supernatants.16 Although CTCL cell production of TNF-α and IFN-γ may promote DC maturation,17 18IL-10 production could inhibit DC differentiation.
Cytokines produced in the supernatants of the cocultivated CTCL cells and APCs
| Cytokine (pg/mL) . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| IFN-γ | 24.6 ± 1.1 | 200 ± 4.0 | 0 |
| TNF-α | 64.6 ± 0.5 | 30.0 ± 1.30 | 30.0 ± 1.40 |
| IL-10 | 198.0 ± 14.0 | 0 | 0 |
| Cytokine (pg/mL) . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| IFN-γ | 24.6 ± 1.1 | 200 ± 4.0 | 0 |
| TNF-α | 64.6 ± 0.5 | 30.0 ± 1.30 | 30.0 ± 1.40 |
| IL-10 | 198.0 ± 14.0 | 0 | 0 |
The addition of γ-irradiated apoptotic CTCL cells alone or in conjunction with a neutralizing antibody to IL-10 was tested for an effect on DC maturation. The results showed that although apoptotic tumor cells slightly increased DC differentiation, a larger effect (as measured by membrane CD83 and coexpression of CD83/class II) was found when a neutralizing anti–IL-10 antibody was added (Table3). These studies suggest that in some patients with CTCL production of IL-10 may prevent DC maturation.
Influence of apoptotic tumor cells and anti–IL-10 neutralizing antibody on DC maturation
| Patient 1 . | No treatment CTCL cells cocultured APCs (%) . | + γ-irradiated CTCL cells (%) . | + γ-irradiated CTCL cells + αIL10 (%) . |
|---|---|---|---|
| CD83 | 12 | 9 | 34 |
| Class II/CD83 | 1 | 15 | 37 |
| Patient 1 . | No treatment CTCL cells cocultured APCs (%) . | + γ-irradiated CTCL cells (%) . | + γ-irradiated CTCL cells + αIL10 (%) . |
|---|---|---|---|
| CD83 | 12 | 9 | 34 |
| Class II/CD83 | 1 | 15 | 37 |
Effect of monoclonal antibodies on proliferation
To determine whether the effect of antibodies that prevented CTCL cell and DC interaction, monoclonal antibodies were added to the cocultures and proliferation monitored. CTCL cell proliferation was inhibited by the addition of antibodies that bound to the clonotypic TCR (Vβ antibody) or an anti-CD40 antibody, which reduced the access of the CTCL cell CD40 ligand to the APC CD40 molecule (Figure6). CD40 binding may increase DC maturation and thereby reduce the population of immature DCs, which are critical for continued CTCL cell proliferation.
Antibodies that interfere with direct membrane contact of the CTCL cells and the APC block proliferation.
The cocultures were incubated with antibodies to the clonotypic TCR (Vβ5c), CD40 on the APC or an isotype control (IgG1), and proliferation measured by uptake of [3H]-thymidine. Interference with the TCR or the interaction of CD40 with its ligand on the CTCL cells inhibited proliferation. The results represent the mean ± SD of 5 replicate samples.
Antibodies that interfere with direct membrane contact of the CTCL cells and the APC block proliferation.
The cocultures were incubated with antibodies to the clonotypic TCR (Vβ5c), CD40 on the APC or an isotype control (IgG1), and proliferation measured by uptake of [3H]-thymidine. Interference with the TCR or the interaction of CD40 with its ligand on the CTCL cells inhibited proliferation. The results represent the mean ± SD of 5 replicate samples.
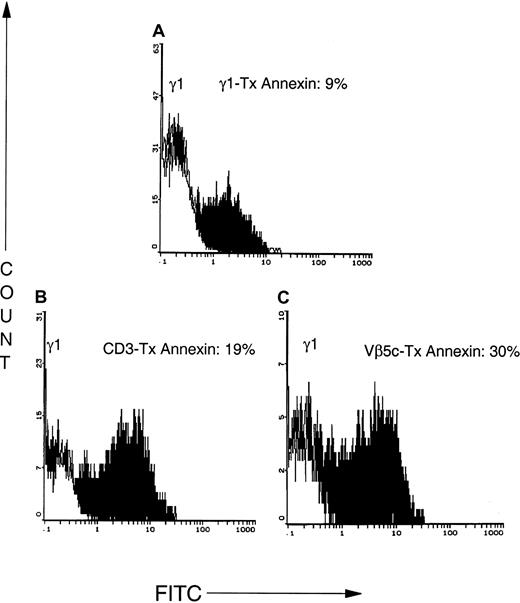
CD3 antibody causes apoptotic tumor cell death and secondary necrosis
Although resting T cells are stimulated to divide after CD3-TCR triggering, T cells that have entered the cell cycle become apoptotic after CD3 signaling.19 To determine whether the inhibitory effect of CD3 binding on CTCL cell proliferation was due to blocking of TCR interaction or resulted from T-cell depletion, we examined whether binding of anti-CD3 antibody or a clone-specific Vβ-reactive antibody mediated CTCL cell apoptosis. The basal rate of apoptosis in the CTCL cell population was 9% (Figure 7) as shown by the binding of annexin V after incubation with an isotype control. In addition, 6% of the lymphocytes became secondarily necrotic. Treatment of CD2–bead-purified CTCL cells with CD3 antibody for 1 hour resulted in the apoptotic cell death of 19% of the tumor cells (15% necrosis), whereas an antibody to the clonotypic TCR caused 30% of the CTCL cells to undergo programmed cell death (23% necrosis). Therefore, approximately one third of the antibody-treated CTCL cells in the cultures undergo rapid apoptotic cell death and more than 20% of the CTCL cells become necrotic. The necrotic cell death that ensued as a consequence of antibody-mediated apoptosis may result in the release of inflammatory cytokines contributing to DC maturation.20
Antibodies that bind to CD3 or the clonotypic TCR cause rapid apoptosis of CTCL cells.
CTCL cells were CD2–bead purified from 2-month cultures and incubated for 1 hour with an isotype control (A; IgG1), CD3 (B), or an antibody to the variable region of the β chain of the clonotypic TCR (C; Vβ5c). Apoptosis was assessed by the binding of annexin V–FITC, a marker of early apoptotic cells. Tx indicates treatment.
Antibodies that bind to CD3 or the clonotypic TCR cause rapid apoptosis of CTCL cells.
CTCL cells were CD2–bead purified from 2-month cultures and incubated for 1 hour with an isotype control (A; IgG1), CD3 (B), or an antibody to the variable region of the β chain of the clonotypic TCR (C; Vβ5c). Apoptosis was assessed by the binding of annexin V–FITC, a marker of early apoptotic cells. Tx indicates treatment.
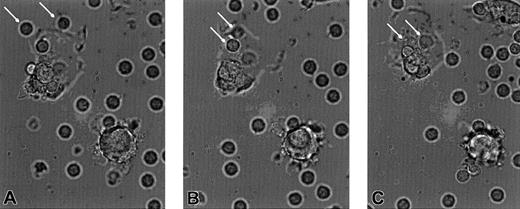
Real-time motion reveals rapid DC phagocytosis of apoptotic CTCL cells
To determine the rate of CTCL cell ingestion by autologous cultured DC, we filmed a quick-time phase contrast movie (to view download apple quick time viewer from Netscape download site for both MAC and PC). Cultures from 2 CTCL patients (patient no. 4, 2 months in culture; patient no. 1, 1 month) received CD3 antibody at time 0 and filming continued for 1 hour. The compressed movie demonstrates that as early as 8 minutes (Figure8A, 8 minutes freeze frame) CTCL cells approximate to the DC membrane, are engulfed by a pseudopodal extension of the DC membrane (Figure 8B, 9 minutes), and are fully internalized by 10 minutes (Figure 8C). Viewing the entire movie demonstrates that some of the DCs are aggressively phagocytic even at 2 months of culture and ingest multiple CTCL cells. The CTCL cells appear to be attracted to the DCs possibly through a chemokine gradient produced by the DC. The results of this very rapid phagocytosis is engorgement of the DC to the point where their cytoplasm becomes distended and appears to be full of apoptotic CTCL cells.
Freeze-frame photomicrographs demonstrate that the APC are avidly phagocytic and ingest the apoptotic CTCL cells.
(A) CTCL cells (arrows) begin to adhere to the cell membrane of an APC, at 8 minutes after the addition of CD3 antibody. (B) The CTCL cells (arrows) become surrounded by a pseudopodial extension of the APC membrane, at 9 minutes. (C) The CTCL cells (arrows) have become fully internalized in the cytoplasm of the APC, at 10 minutes. Original magnification × 400.
Freeze-frame photomicrographs demonstrate that the APC are avidly phagocytic and ingest the apoptotic CTCL cells.
(A) CTCL cells (arrows) begin to adhere to the cell membrane of an APC, at 8 minutes after the addition of CD3 antibody. (B) The CTCL cells (arrows) become surrounded by a pseudopodial extension of the APC membrane, at 9 minutes. (C) The CTCL cells (arrows) have become fully internalized in the cytoplasm of the APC, at 10 minutes. Original magnification × 400.
Uptake of apoptotic CTCL cells drives DC differentiation
CD3-treated CTCL cells were rendered apoptotic as demonstrated by the binding of APO-2, a marker of early apoptotic cells (Figure 9A). Twenty minutes after binding of the CD3 antibody to purified CTCL cells (T20), 29% of the cells were apoptotic. In control cocultures, neither CD4 antibody, an irrelevant isotype-matched Vβ antibody (Vβ18), nor an isotype control caused apoptosis. The impact of this enhanced apoptotic cell death on DC maturation was studied.
CD3 mediates apoptosis of CTCL cells that are ingested by the APC, resulting in maturation to CD83+ DCs.
(A) Two-color cytofluorimetry of sequential samples obtained from the cocultured cells at 20 minutes after the addition of CD3 antibody demonstrate that 30% of the CTCL cells have become apoptotic as determined by their coexpression of CD3 (detected with a secondary antimouse antibody conjugated to FITC) and APO-PE (a marker of early apoptotic cells). Apoptosis of the CTCL cells increases at 35 minutes and begins to decline by 50 minutes. The number of DCs containing apoptotic material from the CTCL cells increased from 10% at 20 minutes to 30% of the APCs after overnight incubation, demonstrating maturation of the APC driven by phagocytosis of apoptotic tumor cells. (B) Immunofluorescent staining of sequential specimens from the CD3-treated cocultures demonstrates that DC maturation as measured by CD83 expression increases after overnight incubation with the apoptotic CTCL cells, as measured by flow cytometry.
CD3 mediates apoptosis of CTCL cells that are ingested by the APC, resulting in maturation to CD83+ DCs.
(A) Two-color cytofluorimetry of sequential samples obtained from the cocultured cells at 20 minutes after the addition of CD3 antibody demonstrate that 30% of the CTCL cells have become apoptotic as determined by their coexpression of CD3 (detected with a secondary antimouse antibody conjugated to FITC) and APO-PE (a marker of early apoptotic cells). Apoptosis of the CTCL cells increases at 35 minutes and begins to decline by 50 minutes. The number of DCs containing apoptotic material from the CTCL cells increased from 10% at 20 minutes to 30% of the APCs after overnight incubation, demonstrating maturation of the APC driven by phagocytosis of apoptotic tumor cells. (B) Immunofluorescent staining of sequential specimens from the CD3-treated cocultures demonstrates that DC maturation as measured by CD83 expression increases after overnight incubation with the apoptotic CTCL cells, as measured by flow cytometry.
Twenty minutes after the addition of CD3, sequentially timed samples were taken from the DCs, and fixed and permeabilized to allow detection of internalized T cells. In Figure 9A, 2-color flow cytometry demonstrated that at early time points only small numbers of CD3+ apoptotic blebs from CTCL cells were found in the cytoplasm of mature CD83+ DCs. After overnight incubation, 24% of the DC had matured, were membrane CD83+, and contained CD3+ CTCL-derived material. The increase in membrane CD83 expression after overnight incubation with apoptotic CTCL cells was confirmed by 1-color flow cytometry (Figure 9B). An antibody to CD4, which binds to the CTCL cells and is the same isotype as the CD3 antibody (IgG1), did not cause rapid apoptosis or enhanced phagocytic uptake, indicating that Fc receptor-mediated uptake of the antibody-positive CTCL cells did not play a significant role in the observed phagocytosis.
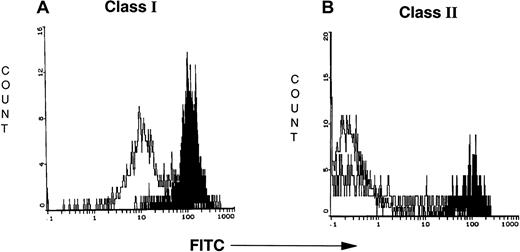
DC phagocytosis of CTCL cells increases membrane class I and II expression
To examine the impact on the DC of CTCL cell ingestion, MHC class I and II expressions were monitored. Class I (Figure10A) and class II (Figure 10B) expression on the DCs was substantially increased 24 hours after ingestion of apoptotic CTCL cells. Sequential samples were taken from purified DCs (T0) prior to overnight incubation and after 24 hours of incubation (T24) with autologous purified CTCL cells that had been rendered apoptotic by CD3 binding. DCs from patient 1 (3-week culture) increased the intensity of their class I expression 5-fold and their class II expression 1.8-fold. DCs from 2 patients markedly increased the intensity of their MHC class I and II expression after phagocytosis of apoptotic CTCL cells (Table 4).
Uptake of apoptotic CTCL cells increases class I and II MHC expression on the maturing DCs.
(A,B) Flow cytometry demonstrates increased class I and II MHC expression on the maturing DCs after overnight incubation with CTCL cells that had been rendered apoptotic by CD3 antibody. The white curve demonstrates class I or II binding at T0. The black curve demonstrates enhanced class I or II expression after overnight incubation with apoptotic CTCL cells.
Uptake of apoptotic CTCL cells increases class I and II MHC expression on the maturing DCs.
(A,B) Flow cytometry demonstrates increased class I and II MHC expression on the maturing DCs after overnight incubation with CTCL cells that had been rendered apoptotic by CD3 antibody. The white curve demonstrates class I or II binding at T0. The black curve demonstrates enhanced class I or II expression after overnight incubation with apoptotic CTCL cells.
Increased expression of MHC molecules on DCs after phagocytosis of apoptotic CTCL cells
| Patient . | Antibody (%/mean fluorescence intensity) . | |||
|---|---|---|---|---|
| Class I . | Class II . | |||
| T0 . | T24 . | T0 . | T24 . | |
| 1 (3-wk culture) | 93/15.3 | 96/82.3 | 26/34.0 | 49/61.0 |
| 2 (5-wk culture) | 78/12.4 | 99/122.8 | 18/1.72 | 50/96.8 |
| Patient . | Antibody (%/mean fluorescence intensity) . | |||
|---|---|---|---|---|
| Class I . | Class II . | |||
| T0 . | T24 . | T0 . | T24 . | |
| 1 (3-wk culture) | 93/15.3 | 96/82.3 | 26/34.0 | 49/61.0 |
| 2 (5-wk culture) | 78/12.4 | 99/122.8 | 18/1.72 | 50/96.8 |
Discussion
The pathognomonic hallmark of CTCL is the intraepidermal Pautrier microabscess, composed of large clusters of CTCL cells that are malignant and in mitosis, surrounding Langerhans cells (an immature DC) both in the epidermis and in dermal infiltrates.21Although the diagnostic importance of this disease feature has been known for decades, the implications for the immunobiology of CTCL have remained obscure. The ability to investigate the dynamic interaction between the CD4+ CTCL cell and the DC has been limited by difficulties in cultivating CTCL cells. We have established long-term cultures that permit dissection of the interaction between the CTCL cell and the DC. The ability to study both cell types will have significant ramifications for our understanding of the factors that drive the malignant T-cell proliferation in CTCL and may permit the design of innovative therapies.
Our studies are the first to report the establishment of long-term cultures of CTCL cells that preserve the phenotype of the initial malignant clone and can be reproducibly propagated, from the leukapheresis of all patients with CTCL tested. Although others have reported CTCL cell growth in vitro,11,16 22 the ability to culture CTCL cells has been limited to selected patients with unusual phenotypes, or HTLV-1 virally transformed cells and the life span of these cell lines was short. In our cultures, the growth, viability, and survival of the CTCL cells is dependent on direct membrane contact with autologous, immature DCs. Separation of the cells by a membrane or the transfer of supernatant fluid from proliferating cultures does not support growth of either isolated cell type for a prolonged period.
The prolonged survival of the DCs and their ability to proliferate and remain aggressively phagocytic is another remarkable feature of the culture system. Normally, DCs cultured with GM-CSF and IL-4 do not proliferate extensively and do not remain viable beyond 1 month in vitro.12 At 1 to 2 months in culture with CTCL cells, approximately two thirds of the DCs were immature cells capable of aggressive phagocytosis and were admixed with mature DCs. After 3 months in culture the majority of the DCs had matured and begun to degranulate and die.
The immaturity of the DC is central to the prolonged growth of the CTCL cells in the cultures. The CTCL cell may aid in the maintenance of DC survival, through ligation of CD40 on the DC by CD40 ligand on the tumor cell, an interaction that has been shown to prolong the survival of both DCs and antigen-stimulated T cells through enhanced DC maturation, which increases their ability to present antigen to T cells.23 In some patients, secretion of the maturation inhibitory cytokine IL-10 may serve to promote DC immaturity. In contrast, other cytokines such as TNF-α and IFN-γ are also produced by the CTCL cells and may affect the long-term growth of the DCs and aid in their maturation. IL-15, which has been reported to be important in CTCL cell survival,16 was not produced in the culture supernatants, although IL-15 may play a role in supporting CTCL cell growth in vivo.
Maturation of DCs is associated with a loss of phagocytic ability.24 The immaturity of the cultured DCs was demonstrated by their ability even after 1 to 2 months of culture to rapidly engulf apoptotic tumor cells. The tumor cells were rendered apoptotic by TCR triggering with CD3 or an antibody to the variable region of the clonotypic TCR. The ability to provoke CD3-mediated apoptotic cell death may reflect the activated status of the proliferating CTCL cells that have entered the cell cycle.19 Although resting T cells proliferate in response to CD3 stimulation, activated T cells undergo apoptotic cell death. We have previously demonstrated that isolated CTCL cells cultured with IL-2 and IL-7 in the absence of DCs do not proliferate and do not undergo apoptosis when stimulated with CD3.25 In contradistinction, the CTCL cells cultured with DCs are proliferating as shown by radioisotope incorporation and CFSE staining, and after antibody-mediated TCR triggering, at least 30% of the CTCL cells will undergo programmed cell death.
In the cultured cells, in the absence of antibody-driven apoptosis, gradual DC maturation over a 3-month period was observed and may be due to the uptake of tumor cells that have reached the limit of their proliferative capacity and died. It is possible that DC uptake of dying CTCL cells drives the long-term proliferation of CTCL through 2 potential mechanisms. Maturing DCs express higher levels of class II MHC molecules and murine models have shown that association of mature CD4+ T cells with class II+ DCs supports the survival of the CD4 T cells.26 The DCs may process peptides derived from the engulfed apoptotic CTCL cells and present them in class II MHC molecules to resting CTCL cells, thereby promoting their proliferation. Alternatively, the DCs may release a nonspecific growth factor that drives the CTCL cells to proliferate. However, supernatants from the cultured cells do not support the growth of isolated CTCL cells, indicating that any secreted growth stimulant acts directly on the CTCL cells, does not penetrate a 0.4-μm membrane, and does not survive transfer. Ultimately, when the all the DCs mature and die no further stimulus is provided for the CTCL cells and they die as well.
The maturation level of the DCs may have profound influences on the type of effector CD4 T-cell response initiated. In an alloreactive model, using naive CD4 T cells isolated from cord blood, stimulation with immature DCs skewed the T-cell population toward IL-10, producing IL-2, IL-4, IFN-γ nonproducing inhibitory T cells (Tr1 cells) that failed to proliferate.27 Recent studies have shown that some features of this immunosuppressive T-cell subset resemble the CTCL cells that are growing in our long-term cultures, including production of IL-10 and growth supported by the cytokines IL-2 and IL-4 (for a review, see Shevach28). Although Tr1 cells express CD25 and class II MHC molecules and cytoplasmic CTLA-4, only occasional expression of these molecules is found when CTCL cells from some patients are examined, but this phenotype is not a consistent feature of the malignant T cells. Using the in vitro cultures, it will be possible to determine whether the CD4+ CTCL cells are derived from a subset of Tr1-like T cells and whether the immunosuppressive capacities of this cell type explain some of the features of this malignancy.
The quick-time movie images indicate that apoptotic CTCL cells are drawn to the DCs by a mechanism that remains to be elucidated but probably represents a chemoattractive gradient. Epidermal Langerhans cells, migrating from skin after sensitization with antigen, have been shown to up-regulate the expression of macrophage-derived chemokine as they mature,29 drawing antigen-specific T cells to the DCs by chemoattraction. The results obtained in our cultures may be used to construct a theoretical model of how CTCL grows both in vitro and in vivo. In the cultures and in the skin of patients with CTCL, some of the CTCL cells may have already encountered antigen and are actively proliferating. When this proliferative population of CTCL cells undergoes programmed cell death and is engulfed by immature DCs, they drive DC maturation, which may potentiate DC presentation of CTCL peptides to both CD4 and CD8 T cells. The nonproliferating CTCL population may be drawn to the maturing DCs by chemokine expression and exposed to class II presented self-peptide that can drive their entry into the cell cycle, thereby maintaining the growth of the malignancy. As long as the DCs are immature, perpetuated in vivo by renewal from the bone marrow,30 CTCL cell growth will be promoted, suggesting that therapies such as psoralen and UV-A light in the epidermis may mediate their beneficial effect through the depletion of the immature DC population, as well as by a direct impact on CTCL cell proliferation.31,32 Skin Langerhans cells that contain Birbeck granules and express E-cadherin and Lag may represent a separate population from the monocyte-derived DCs studied in our culture system.33 Comparative studies of the impact on CTCL cell growth of these 2 DC subsets will be pursued in future experiments.
The restricting element that presents the antigen that stimulates CTCL cell proliferation in the cultures may be class II MHC molecules but is not CD1a, which is expressed by epidermal Langerhans cells but is not found on the cultured immature DCs. The nature of the putative antigen remains to be elucidated. Peptides derived from the CTCL cells themselves are likely candidates and include the clonotypic TCR, which is recognized by autologous CD8 T cells when presented in class I MHC molecules on an APC.34 Transforming retroviruses have been implicated in the ontogeny of CTCL. Reports suggest that HTLV-1–related sequences may be found in the DNA of lymphocytes from some patients with CTCL and in keratinocytes from noninvolved skin.35 The nonproductive retroviral sequences could provide a source of peptide for Langerhans cell presentation to CTCL cells. Alternatively, retroviral products from an unknown virus that infects Langerhans cells or keratinocytes may serve as a source of peptide.
It is clear that the long-term cultures will allow investigators to delve more deeply into the factors that drive the proliferation and progression of CTCL. For the first time it will be possible to study the interaction of the malignant CTCL cells and APCs in vitro and measure the factors that either promote or retard the growth of the malignancy. Based on our preliminary observations, we postulate that CTCL may represent an antigen-driven malignancy of CD4 T cells that fails to respond to immunoregulation, due at least in part, to the impact of the malignant T cells on APC maturation. Beyond the significance of these results for normal and tumor cell immunobiology, they raise major opportunities for the design and development of improved immunotherapy. Titration of dose, schedule of administration, and cellular impact of radiation, chemotherapy, antibody, cytokine, and oligonucleotide administration can now be finely tuned in vitro prior to institution in vivo.
Supported by the New York Cardiac Foundation (C.B.), the Dermatology Foundation (D.H.), and grant CA81138 from the National Institutes of Health (to M.D.).
C.L.B. and D.H. have contributed equally to this article and should be considered co–first authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carole L. Berger, Department of Dermatology, Yale University, School of Medicine, 333 Cedar St, New Haven, CT 06510; e-mail: carole.berger@yale.edu.

![Fig. 1. CTCL cells proliferate only in the presence of APCs. / CTCL cells were obtained from 1- (▪) and 3-month (▨) cultures of 2 representative patients and were freed of APCs by CD2–magnetic bead enrichment. The purified CTCL cells were readded after overnight culture to γ-irradiated autologous APCs that had been CTCL cell bead-depleted. After 3 additional days of culture, only CTCL cells cocultivated with APCs proliferated, as shown by uptake of [3H]-thymidine. The results presented are the means of 5 replicate samples ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/8/10.1182_blood.v99.8.2929/4/m_h80822428001.jpeg?Expires=1763471988&Signature=OBn0bQHq4eqNFYRx7oVfykdl94b3we1E79Zrfx4ui~P3ByxI6nH-BooCUtAGmq1P6x2UQ9JQyVE8sZtaMsswT~gdsl92YJkwHUpxvOe1cMYxVpeH7xgpfwCRLV-AFiJ87bBlGNUm~ZIonX2mvp48x-y6sps28OoSyR3VtoEaERZ1PFVsZ9DgTuFSS8yxZvUItiErWd8zawugKKKPZGB4s4LxSXdmsCNvEHcDSQ1wxBoKJXPWfnOqnN3T0feHpchIJmOUstMf-cmDpQjXEM2bLWMZsuXv2DRj1xZQ7-3Aa2kjtD46EF-YGUpY2TdHnPJMY5JSrNLOdBkY4gflev-sMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Antibodies that interfere with direct membrane contact of the CTCL cells and the APC block proliferation. / The cocultures were incubated with antibodies to the clonotypic TCR (Vβ5c), CD40 on the APC or an isotype control (IgG1), and proliferation measured by uptake of [3H]-thymidine. Interference with the TCR or the interaction of CD40 with its ligand on the CTCL cells inhibited proliferation. The results represent the mean ± SD of 5 replicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/8/10.1182_blood.v99.8.2929/4/m_h80822428006.jpeg?Expires=1763471988&Signature=oRm6vTsbNLVMWMNN0lKIKszAaXljftYBmQGZFIjRSQfARVm0OuaONpK-aLDujCCBqWsYhFEjBZ6uNk~w~J3C8vaqbIDarMRJqEdAFBxftV3tmUxs8tEWhGTiacWqe5jRd3oHKPQlhaz0747EAO4p-IZjpOQKjnPU0XeBQEy8aNfSZ0YGpZciUUwFUPu0nOYTJ2-mhuBT0FJpvAMwztONy7WHWLVcK2m0YWc1ppBxu6VamVyv7~scKVPsmGvp897sOVpa~tvRpBzJPi0SoT5WQk~IxpHU~9yhBvO~WROVEQ5pn4SiPt8UvRiXbqp5o9UTvOYEFVKkTvhQ8Hxeg7iCQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal