Abstract
Autoantibodies of the cryoprecipitating IgG3 isotype have been shown to play a significant role in the development of murine lupus–like autoimmune syndrome. At present, the structural basis of IgG3 cryoprecipitation and its role in autoantibody pathogenicity remain to be defined. Using molecular variants of an IgG3 monoclonal rheumatoid factor, 6-19, derived from an autoimmune MRL-Faslpr mouse, we have investigated the implication of charged residues in the heavy-chain variable (VH) region, potential CH3-linked oligosaccharides, and galactosylation of CH2-linked oligosaccharides in its cryoglobulin activity. The cryoglobulin activity of the IgG3 6-19 mutant bearing more negatively charged residues at VH 6 and 23 was found to be reduced but still highly significant, whereas that of the mutant lacking a potential CH3 glycosylation site remained unchanged. In marked contrast, IgG3 6-19 variants obtained from 6-19 heavy-chain transgenic mice displayed barely detectable cryoglobulin activity associated with an increased level of galactosylation in the CH2 oligosaccharide side chains. Thus, our data strongly suggest that the cryoglobulin activity of IgG3 6-19 autoantibody is critically determined by levels of galactosylation in the CH2 oligosaccharide side chains, whereas VH residues play a secondary role in 6-19 IgG3 cryoglobulin activity.
Introduction
Cryoglobulins are a heterogeneous group of immunoglobulins (Ig) which precipitate upon cooling and redissolve on warming. Most cryoglobulins are either intact monoclonal Ig or Ig complexes in which one component, usually IgM, has rheumatoid factor activity.1,2 Monoclonal cryoglobulins are mainly associated with various lymphoproliferative disorders, whereas mixed cryoglobulins are often found in serum from patients with autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis or with chronic infectious diseases. The presence of cryoglobulins can result in a wide range of vascular, renal, and neurologic complications, probably depending on their concentrations, their temperature-dependent solubility behavior, and the nature and type of proteins involved.2 However, the precise pathogenic mechanisms involved in the induction of these tissue lesions have not been well defined.
In humans and mice, antibodies of the IgG3 isotype have a unique physicochemical property, which allows them to self-associate via Fc-Fc interactions, independently of their specificities.3-5This property is necessary, but not sufficient in itself to confer cryoglobulin activity, since not all monoclonal IgG3 proteins in humans6-8 and mice5,9,10 exhibit cyroglobulin activity. However, the molecular basis responsible for the cryoglobulin formation by IgG3 molecules is still unclear. A study comparing nucleotide sequences of the variable (V) regions of cryogenic and noncryogenic IgG3 monoclonal antibodies (mAbs) has claimed a role of framework residues 6 and 23 of the heavy-chain V (VH) domain in IgG3 cryoprecipitation. These residues were more positively charged in cryogenic IgG3 than in their noncryogenic counterparts.11In addition, it has been suggested that oligosaccharide chains potentially present in the CH3 domain of murine IgG3, because of an additional asparagine-linked glycosylation site in this domain,12 might be involved in the cryoglobulin activity by promoting self-association of IgG3.13 Moreover, there is remarkable heterogeneity in galactosylation levels of carbohydrate side chains attached at residue 297 (asparagine) on the CH2 domain of IgG,14 and these structural differences may play an additional role for the cryoglobulin activity of self-associating IgG3 complexes. Most biantennary oligosaccharide chains are nonsialylated, and end with either 2 terminal galactose residues, 1 terminal galactose and 1 terminal N-acetylglucosamine, or 2 terminalN-acetylglucosamines.14 It has been shown that the level of terminal galactosylation is substantially diminished in some diseases,14-16 although it is still unknown whether this association has a pathogenic significance.
We have previously established that an IgG3 anti-IgG2a cryoglobulin derived from an autoimmune MRL-Faslpr mouse is able to induce lupuslike glomerulonephritis and skin vasculitis.10,17 Using this model, we aimed to define the respective roles of VH residues 6 and 23, of potential CH3 oligosaccharides, and of galactosylation levels of CH2 oligosaccharide side chains in cryoglobulin activity of IgG3 mAb. Therefore, we first generated 2 different 6-19 mutant rheumatoid factor (RF) mAbs either by replacing VH residues 6 and 23 with those commonly found in noncryogenic IgG3 mAbs, or by eliminating the potential CH3 glycosylation site, and compared their cryoglobulin activity with that of the wild-type 6-19 RF mAb. Second, 3 “6-19” IgG3 RF mAbs were established from mice bearing a transgene encoding the 6-19 IgG3 heavy chain, which spontaneously produce IgG3 “6-19” mAbs by combining the transgenic 6-19 IgG3 heavy chains with endogenous light chains. We expected that these “6-19” mAbs, identical in the amino-acid sequences of their heavy and light chains to those of the original 6-19 mAb, bore potentially different galactosylation patterns because of their synthesis in different cells, as shown previously.18Thus, we should be able to study the role of galactosylation in the CH2 oligosaccharides for the cryogenic potential of the 6-19 mAb. The analysis of these 6-19 IgG3 RF variants revealed that the cryoglobulin activity of the 6-19 mAb was primarily determined by the galactose content of CH2 oligosaccharide chains, with a secondary involvement of VH residues 6 and 23.
Materials and methods
Monoclonal antibodies
The 6-19 IgG3 anti-IgG2a RF and its IgG1 switch variant, SS2F8, derived from an autoimmune-prone MRL-Faslprmouse, were described previously.10,17 The 6-19Q6E/K23A mutant mAb at VH positions 6 (glutamine to glutamic acid) and 23 (lysine to alanine) and the 6-19N471T mutant mAb at position 471 (asparagine to threonine), thus lacking the potential glycosylation site in the CH3 domain, were generated by transfecting a 6-19 heavy-chain loss cell line with respective LVDJH6-19–Cγ3 mutant plasmids, together with a plasmid containing the hygromycin-resistant gene, as described previously.18 IgG3 anti-IgG2a RF mAbs bearing the 6-19 idiotype, including A6C, P11C, and Y12C mAb, bearing heavy and light chains identical to those of the 6-19 mAb were established by fusion of X63-Ag8.653 myeloma cells with spleen cells from mice expressing a transgene encoding the 6-19 IgG3 heavy chain. The details of the 6-19 heavy-chain transgenic mice will be described elsewhere. The 411H12 IgG3 anti-IgG1 RF mAb obtained from A/J mice immunized with P-azophenylarsonate–coupled Limulus polyphemus hemocyanin was provided by Dr D. Nemazee (Scripps Research Institute, La Jolla, CA). Other IgG3 mAbs in use were DP7, S57, S106,19 AM16,20 7B6.8, 1G3,211G2,22 R4A12,23 11F8,242-6D,25 SH11,26 and C11M.9 mAbs were purified from culture supernatants by protein A affinity column chromatography. The purity was more than 90% as documented by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Oligonucleotide-directed mutagenesis
The 6-19 VH gene27 and a 4.6-kb XbaI fragment encoding the CH3 domain of the γ3 constant (Cγ3) region, derived from the Cγ3 genomic pJW7 clone,12 served as templates for mutagenesis. Mutants were synthesized using a mutagenesis kit from Amersham Pharmacia Biotech (Dübendorf, Switzerland). After verifying the presence of the mutations by sequencing the 6-19 VH and CH3 gene segments, the LVDJH6-19–Cγ3 plasmid containing the complete 6-19 IgG3 heavy-chain mutant gene was constructed as described previously.18 The presence of the mutations was also confirmed by sequence analysis of cDNA derived from a reverse transcriptase–polymerase chain reaction (RT-PCR) amplification of mRNA isolated from the transfected cells secreting either 6-19Q6E/K23A or 6-19N471T mutant mAb.
RT-PCR and cDNA sequencing
RNA was prepared from hybridomas by RNeasy Mini Kit (Qiagen AG, Basel, Switzerland). The first strand of cDNA was synthesized with an oligo(dT) primer and total RNA. For amplification with PfuDNA polymerase (Stratagene Cloning Systems, La Jolla, CA), the following pairs of oligonucleotides were used: VH1BACK primer (5′-AGGTSMARCTGCAGSAGTCWGG-3′)28 and Cγ3-CH1 primer (5′-CGATAGACAGATGG-3′) for the IgG3 VH region, 3′-untranslated 6-19 VH primers (5′-CACTGACTTTCACCATG-3′) and 3′-untranslated Cγ3 primer (5′-GACCCGAGGAATGGC-3′) for the 6-19 IgG3 heavy-chain, and Vk6-19 leader primer (5′-GTTGCCTCCTCAAATG-3′) and Ck primer (5′-TGGATGGTGGGAAGATG-3′) for the light chain. The nucleotide sequence was determined by the dideoxynucleotide chain termination method.29
Enzyme-linked immunosorbent assay
Cryoglobulin activity
A quantity of 0.1 mL purified IgG mAbs or sera was placed in conical glass tubes at 4°C for 48 hours. The samples were centrifuged at 3000 rpm at 4°C for 30 minutes. The precipitates were washed 3 times with cold phosphate-buffered saline (PBS) and resolubilized in 4 M urea. The concentrations of IgG in cryoprecipitates were determined by ELISA.
IgG3 self-association assay
The ability of the 6-19 mAb and its variants to form IgG3-IgG3 self-associating complexes was determined by a radioimmunoassay as described previously.10 Briefly, various amounts of mAbs were incubated with 125I-labeled 6-19 mAb (10 ng) at 4°C overnight in the presence of 10 μL normal mouse serum. After 1 hour of incubation at 4°C with 1 mL of 7.5% polyethylene glycol (Mr 6000; Merck, Dietikon, Switzerland), IgG3 complexes formed with radiolabeled 6-19 mAb were precipitated by centrifugation at 3000 rpm at 4°C for 30 minutes. The precipitates were washed once with 7.5% polyethylene glycol. Results are expressed as a percentage of labeled 6-19 mAb precipitated specifically after correction of the nonspecific precipitation (< 10%) obtained in the presence of normal mouse serum alone.
Analysis of oligosaccharide structures
The analysis of the structure of carbohydrate side chains present in the IgG3 samples was carried out as previously described.18 Briefly, IgG3 mAbs were purified from cultured supernatants by protein A column chromatography, followed by fast-performance liquid chromatography (FPLC) using a Superdex 200 pg column (Pharmacia, Uppsala, Sweden). The purified IgG3 mAbs were then subjected to gas-phase hydrazinolysis for 3 hours at 90°C using Hydraclub S204 (Honen, Tokyo, Japan) followed byN-acetylation to quantitatively liberate N-linked oligosaccharides.31 The oligosaccharides liberated from the IgG3 samples were purified by cellulose column chromatography32 and then labeled withP-aminobenzoic acid ethyl ester (ABEE; Nacalai Tesque, Kyoto, Japan) by reductive amination.33 The ABEE-labeled oligosaccharides were subjected to high-performance liquid chromatography (HPLC) using a COSMOGEL diethylaminoethyl column (Nacalai Tesque), and separated into neutral, monosialo, and disialo oligosaccharide fractions. Neutral oligosaccharide mixtures obtained by sialidase treatment of ABEE-oligosaccharide fraction were applied to HPLC using a Wakosil 5C18-200 octadecylsilane column (Wako Pure Chemical Industries, Kyoto, Japan). Desialylated ABEE-oligosaccharides were separated into 8 oligosaccharide fractions which were eluted as a series of authentic biantennary complex-type oligosaccharides with or without fucosylation.
Results
VH residues 6 and 23 influence but do not critically determine the cryoglobulin activity of murine IgG3
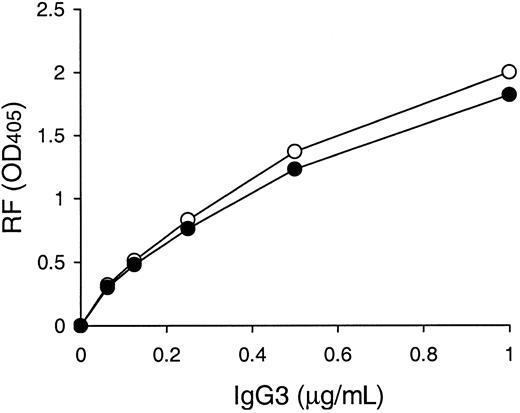
It has been reported that neutral glutamine and cationic lysine are found at VH positions 6 and 23, respectively, in 6 murine IgG3 cryoglobulins, including the 6-19 RF mAb, whereas their counterparts in 5 noncryoglobulins are more negatively charged at these residues: anionic glutamic acid at position 6 and neutral amino acid (alanine, threonine, or valine) at position 23.11 To specifically assess the influence of the VH residues 6 and 23 on the IgG3 cryoglobulin activity, we determined the effect of mutations of these 2 residues on the cryogenic potential of the 6-19 IgG3 mAb. A comparative analysis with the wild-type 6-19 mAb showed that the cryoglobulin activity of the 6-19Q6E/K23A mutant bearing more negatively charged residues at VH positions 6 (glutamic acid instead of glutamine) and 23 (alanine instead of lysine) was approximately 2 times lower than that of the wild-type 6-19 mAb, but still highly significant (Table1). Notably, anti-IgG2a RF activity of the 6-19Q6E/K23A mutant was comparable with that of the wild-type 6-19 RF mAb (Figure 1).
Cryoglobulin activity and N-linked oligosaccharide content of 6-19, 6-19Q6E/K23A, and 6-19N471T mAbs
| mAb . | Ig isotype . | Vh6/23* . | Cryoglobulin† . | Oligosaccharide‡ . |
|---|---|---|---|---|
| 6-19 | IgG3 | Q/K | 29 | 112 |
| 6-19Q6E/K23A | IgG3 | E/A | 14 | 112 |
| 6-19N471T | IgG3 | Q/K | 27 | 108 |
| SS2F8 | IgG1 | Q/K | < 1 | 115 |
| mAb . | Ig isotype . | Vh6/23* . | Cryoglobulin† . | Oligosaccharide‡ . |
|---|---|---|---|---|
| 6-19 | IgG3 | Q/K | 29 | 112 |
| 6-19Q6E/K23A | IgG3 | E/A | 14 | 112 |
| 6-19N471T | IgG3 | Q/K | 27 | 108 |
| SS2F8 | IgG1 | Q/K | < 1 | 115 |
mAb indicates monoclonal antibody; Ig, immunoglobulin; Q, glutamine; E, glutamic acid; K, lysine; A, alanine.
Amino acid residues at positions 6 and 23 of heavy chains.
Concentrations of IgG (μg/mL) in cryoprecipitates recovered following the incubation of mAb (1 mg/mL) for 48 hours at 4°C.
Amounts of N-linked oligosaccharides liberated from different 6-19 mAbs (unit/mg of IgG3). One unit of oligosaccharides was designated as 1 μL of ABEE-oligosaccharide solution with the absorbance at 304 nm equals 1.0.
Anti-IgG2a RF activities of 6-19 and 6-19Q6E/K23A mAbs.
Anti-IgG2a RF activity of purified 6-19 (○) and 6-19Q6E/K23A (●) mAb was determined by ELISA, and results are expressed as OD at 405 nm.
Anti-IgG2a RF activities of 6-19 and 6-19Q6E/K23A mAbs.
Anti-IgG2a RF activity of purified 6-19 (○) and 6-19Q6E/K23A (●) mAb was determined by ELISA, and results are expressed as OD at 405 nm.
To extend this observation, we carried out analysis of an additional 8 cryoprecipitating and 6 noncryoprecipitating IgG3 mAbs. Although the VH residues 6 and 23 are in general more positively charged in the cryoprecipitating populations, this is not always the case (Table2). DP7 anti-DNA, S57 anti-DNA, and 411H12 anti-IgG1 mAbs bearing more positively charged VH residues 6 (glutamine) and 23 (lysine) like cryogenic IgG3 fail to cryoprecipitate. To the contrary, SH11 anti-DNA and C11M anti-DNP mAbs with residues like noncryoprecipitating IgG3 exhibit a cryoglobulin activity.
Vh residues at positions 6 and 23 in various IgG3 mAbs with or without cryoglobulin activity
| mAb . | Vh6* . | Vh23* . | Vhfamily . | Specificity . | Cryoglobulin† . |
|---|---|---|---|---|---|
| 6-19 | Q | K | J558 | IgG2a | + |
| 7B6.8 | Q | K | J558 | DNA | + |
| 1G3 | Q | K | J558 | DNA | + |
| 1G2 | Q | K | J558 | DNA | + |
| 8A4 | Q | K | J558 | Hydrogenase | + |
| AM16 | Q | K | Vgam3.8 | IgG2a | + |
| DP7 | Q | K | J558 | DNA | − |
| S57 | Q | K | J558 | DNA | − |
| 411H12 | Q | K | J558 | IgG1 | − |
| R4A12 | E | A | S107 | DNA | − |
| S106 | E | A | 7183 | DNA | − |
| 2-6D | E | T | 7183 | Nuclear antigen | − |
| 11F8 | E | T | Q52 | DNA | − |
| SH11 | E | P | VH10 | DNA | + |
| C11M | E | S | 36-60 | DNP | + |
| mAb . | Vh6* . | Vh23* . | Vhfamily . | Specificity . | Cryoglobulin† . |
|---|---|---|---|---|---|
| 6-19 | Q | K | J558 | IgG2a | + |
| 7B6.8 | Q | K | J558 | DNA | + |
| 1G3 | Q | K | J558 | DNA | + |
| 1G2 | Q | K | J558 | DNA | + |
| 8A4 | Q | K | J558 | Hydrogenase | + |
| AM16 | Q | K | Vgam3.8 | IgG2a | + |
| DP7 | Q | K | J558 | DNA | − |
| S57 | Q | K | J558 | DNA | − |
| 411H12 | Q | K | J558 | IgG1 | − |
| R4A12 | E | A | S107 | DNA | − |
| S106 | E | A | 7183 | DNA | − |
| 2-6D | E | T | 7183 | Nuclear antigen | − |
| 11F8 | E | T | Q52 | DNA | − |
| SH11 | E | P | VH10 | DNA | + |
| C11M | E | S | 36-60 | DNP | + |
mAb indicates monoclonal antibody; Q, glutamine; E, glutamic acid; K, lysine; A, alanine; T, threonine; P, proline; S, serine.
Self-association and cryoglobulin activity of murine IgG3 is independent of potential oligosaccharide chains on the CH3 domain
A unique feature of the murine IgG3 isotype is the presence of a potential asparagine-linked glycosylation site at residues 471-473 (asparagine-leucine-serine) in the CH3 domain.12 Although it is unknown whether murine IgG3 is indeed glycosylated in the CH3 domain, CH3 oligosaccharide chains, if present, might play a role in the cryoglobulin activity of murine IgG3 by modifying its self-associating property, as suggested by Panka.13 To verify this possibility, we generated a 6-19N471T mutant mAb, in which asparagine at position 471 was replaced by threonine, thus eliminating the potential CH3 N-linked glycosylation site. When various amounts of 6-19 or 6-19N471T mutant mAb were incubated with radiolabeled 6-19 protein, both mAbs exhibited a concentration-dependent formation of IgG3-IgG3 complexes in a quantitatively identical manner (Figure2). In addition, the ability of the 6-19N471T mutant to generate cryoprecipitates following a 48-hour incubation at 4°C was indistinguishable from that of the wild-type 6-19 mAb (Table 1). In contrast, no significant IgG3 complex and cryoglobulin formation was observed with the 6-19 IgG1 switch variant, SS2F8 (Figure 2 and Table 1). Significantly, the content ofN-linked oligosaccharides liberated from the 6-19N471T mutant was essentially identical to that of the wild-type 6-19 and 6-19Q6E/K23A mutant, which was not higher than that of its IgG1 variant, SS2F8, (Table 1), strongly arguing against the possible glycosylation on the IgG3 CH3 domain.
IgG3 self-association assay.
Various amounts of 6-19 (○), 6-19N471T (●), and 6-19 IgG1 variant, SS2F8 (▪), were incubated with 125I-labeled 6-19 mAb (10 ng) in the presence of 10 μL normal mouse serum. Results are expressed as a percentage of labeled 6-19 precipitated specifically.
IgG3 self-association assay.
Various amounts of 6-19 (○), 6-19N471T (●), and 6-19 IgG1 variant, SS2F8 (▪), were incubated with 125I-labeled 6-19 mAb (10 ng) in the presence of 10 μL normal mouse serum. Results are expressed as a percentage of labeled 6-19 precipitated specifically.
Lack of cryoglobulin activity of 6-19 somatic variants derived from 6-19 heavy-chain transgenic mice
The extent of the terminal galactosylation of oligosaccharide side chains on the IgG CH2 domain is highly variable14-16 and depends on the cellular environment.34 We therefore explored the possibility that different levels of galactosylation influence the cryoprecipitating potential of IgG3 self-associating complexes, using 6-19 variants identical in the amino-acid sequences of their heavy and light chains, but different in galactosylation patterns because of their synthesis in different hybridoma cells. To obtain such variants, a series of IgG3 anti-IgG2a RF mAbs expressing the 6-19 idiotype were generated from 6-19 heavy-chain transgenic mice, which spontaneously produce substantial amounts of 6-19–like IgG3 anti-IgG2a RF, resulting from the combination of the transgenic 6-19 heavy chains with endogenous light chains. The 6-19 RF mAb, derived from MRL-Faslpr mice (Ighj), utilizes the Jk2′ gene segment,27 an Ighj-linked allelic form of the Jk2 gene. Therefore, 6-19 heavy-chain transgenic mice were crossed once with MRL mice to introduce the Ighj allotype. Using their spleen cells, 12 IgG3 anti-IgG2a RF mAbs bearing the 6-19 idiotype were established. Nucleotide sequence analysis of light-chain cDNA revealed that all 6-19–like mAbs utilize the Vk1c germ-line gene, identical to that of the 6-19 mAb,27 but differentJk segments (Table 3). Among them, light chains of 3 mAbs (A6C, P11C, and Y12C), established from 3 independent fusions, use the Jk2′ segment identical to that of the 6-19 mAb.
Light chain Jk gene usage and Jk sequences of 6-19–like IgG3 anti-IgG2a RF mAb derived from 6-19 heavy-chain transgenic mice
| Fusion . | mAb3-150 . | Jk . | Jk sequence† . |
|---|---|---|---|
| 6-19 | Jk2′ | YTFGSGTKLEIK | |
| I | A6C | Jk2′ | ------------ |
| G1H | Jk2 | ----G------- | |
| S1B | Jk4 | F----------- | |
| C12A | Jk1 | P---G------- | |
| II | P11C | Jk2′ | ------------ |
| E21A | Jk1 | R---G------- | |
| Q4C | Jk1 | W---G------- | |
| R11A | Jk4 | ●---T-------- | |
| L10H | Jk4 | F---T------- | |
| D6G | Jk5 | L---A-----L- | |
| III | Y12C | Jk2′ | ------------ |
| Z5C | Jk1 | W---G------- |
| Fusion . | mAb3-150 . | Jk . | Jk sequence† . |
|---|---|---|---|
| 6-19 | Jk2′ | YTFGSGTKLEIK | |
| I | A6C | Jk2′ | ------------ |
| G1H | Jk2 | ----G------- | |
| S1B | Jk4 | F----------- | |
| C12A | Jk1 | P---G------- | |
| II | P11C | Jk2′ | ------------ |
| E21A | Jk1 | R---G------- | |
| Q4C | Jk1 | W---G------- | |
| R11A | Jk4 | ●---T-------- | |
| L10H | Jk4 | F---T------- | |
| D6G | Jk5 | L---A-----L- | |
| III | Y12C | Jk2′ | ------------ |
| Z5C | Jk1 | W---G------- |
The 6-19 monoclonal antibody (mAb) is derived from an MRL-Faslpr mouse.17 The 6-19–like IgG3 anti-IgG2a RF mAbs bearing the 6-19 idiotype, resulting from the combination of the transgenic 6-19 heavy chains with endogenous light chains, were established from 3 independent fusions by using spleen cells from 6-19 heavy-chain transgenic mice, and all of them utilize the Vk1c gene segment identical to that of the 6-19 mAb. †Dashes refer to amino acid residue identical to the 6-19 Jk sequence, and a gap is indicated by a dot.
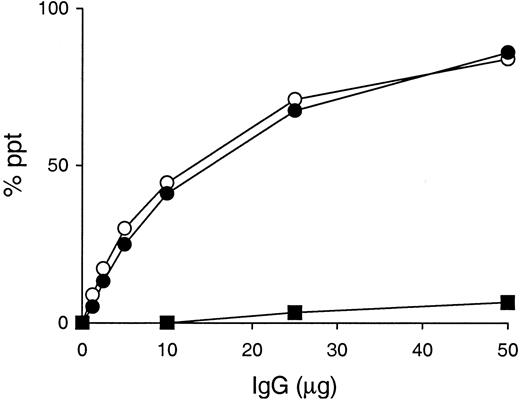
Using these 3 6-19 derived variants, A6C, P11C, and Y12C, cryoglobulin activity was assessed in vitro following a 48 hour incubation at 4°C. Strikingly, none of the A6C, P11C, and Y12C mAbs showed evidence for cryoprecipitation (Figure 3A). Superdex 200HR gel filtration chromatography and SDS-PAGE analysis excluded the possibility that the lack of cryoglobulin activity by A6C, P11C, and Y12C mAbs was due to the formation of an abnormal truncated form or an aberrant assembly of their heavy and light chains in the hybridoma cells (data not shown). Notably, nucleotide sequence analysis of their heavy-chain cDNA confirmed that these 3 mAbs bear heavy chains identical to that of the 6-19 RF mAb, consistent with the fact that their anti-IgG2a RF activities were comparable with that of the 6-19 mAb (Figure 3B). Noteworthy, the self-associating capacities of these 3 mAbs were indistinguishable from that of the 6-19 mAb (Figure 3C).
Cryoglobulin, anti-IgG2a RF, and IgG3 self-associating activities of A6C, P11C, and Y12C IgG3 mAbs established from 6-19 heavy-chain transgenic mice.
(A) 0.1 mL of various concentrations of purified 6-19 (○), A6C (●), P11C (■), and Y12C (▴) mAbs were incubated for 48 hours at 4°C. Results are expressed concentrations of IgG3 in cryoprecipitates. Note the lack of cryoprecipitation of A6C, P11C, and Y12C mAbs, in contrast to the generation of significant amounts of cryoglobulins by the 6-19 mAb even at a concentration of 0.25 mg/mL. (B) Anti-IgG2a RF activity of purified 6-19 (○), A6C (●), P11C (■), and Y12C (▴) mAbs was determined by ELISA, and results are expressed as OD at 405 nm. (C) Various amounts of 6-19 (○), A6C (●), P11C (■), Y12C (▴), and 6-19 IgG1 variant, SS2F8 (▪), were incubated with125I-labeled 6-19 mAb (10 ng) in the presence of 10 μL normal mouse serum. Results are expressed as a percentage of labeled 6-19 precipitated specifically.
Cryoglobulin, anti-IgG2a RF, and IgG3 self-associating activities of A6C, P11C, and Y12C IgG3 mAbs established from 6-19 heavy-chain transgenic mice.
(A) 0.1 mL of various concentrations of purified 6-19 (○), A6C (●), P11C (■), and Y12C (▴) mAbs were incubated for 48 hours at 4°C. Results are expressed concentrations of IgG3 in cryoprecipitates. Note the lack of cryoprecipitation of A6C, P11C, and Y12C mAbs, in contrast to the generation of significant amounts of cryoglobulins by the 6-19 mAb even at a concentration of 0.25 mg/mL. (B) Anti-IgG2a RF activity of purified 6-19 (○), A6C (●), P11C (■), and Y12C (▴) mAbs was determined by ELISA, and results are expressed as OD at 405 nm. (C) Various amounts of 6-19 (○), A6C (●), P11C (■), Y12C (▴), and 6-19 IgG1 variant, SS2F8 (▪), were incubated with125I-labeled 6-19 mAb (10 ng) in the presence of 10 μL normal mouse serum. Results are expressed as a percentage of labeled 6-19 precipitated specifically.
Different levels of galactosylation in the cryoprecipitating 6-19 mAb and noncryoprecipitating 6-19 somatic variants
To determine whether the lack of the cryoglobulin activity of the 3 6-19 variants, A6C, P11C, and Y12C, can be attributed to a possible structural difference in the carbohydrate side chains present in the CH2 domain, the oligosaccharide structures liberated from these 3 mAbs were compared with those of the cryoprecipitating 6-19, 6-19Q6E/K23A, and 6-19N471T mAbs. Analysis by diethylaminoethyl-HPLC showed comparable molar ratios of neutral asialo-, acidic monosialo-, and acidic disialo-oligosaccharides, in which neutral nonsialylated oligosaccharides amounted to approximately 90% of total oligosaccharides (Table 4).
Structural analysis of N-linked oligosaccharide chains purified from 6-19 mAb and its variants
| IgG . | Sialylated glycoforms4-150 . | Galactosylated glycoforms4-151 . | G0/(G1 + G2) . | ||||
|---|---|---|---|---|---|---|---|
| N . | A1 . | A2 . | G0 . | G1 . | G2 . | ||
| 6-19 | 90.0 | 9.0 | 1.0 | 48.4 | 40.3 | 11.3 | 0.94 |
| 6-19Q6E/K23A | 86.2 | 11.5 | 2.3 | 53.7 | 36.7 | 9.6 | 1.16 |
| 6-19N471T | 89.6 | 10.0 | 0.4 | 47.5 | 42.1 | 10.4 | 0.90 |
| A6C | 85.1 | 12.8 | 2.1 | 31.6 | 44.5 | 23.9 | 0.46 |
| P11C | 84.3 | 13.7 | 2.0 | 32.5 | 45.9 | 21.6 | 0.48 |
| Y12C | 87.4 | 10.8 | 1.8 | 33.6 | 45.3 | 21.1 | 0.51 |
| IgG . | Sialylated glycoforms4-150 . | Galactosylated glycoforms4-151 . | G0/(G1 + G2) . | ||||
|---|---|---|---|---|---|---|---|
| N . | A1 . | A2 . | G0 . | G1 . | G2 . | ||
| 6-19 | 90.0 | 9.0 | 1.0 | 48.4 | 40.3 | 11.3 | 0.94 |
| 6-19Q6E/K23A | 86.2 | 11.5 | 2.3 | 53.7 | 36.7 | 9.6 | 1.16 |
| 6-19N471T | 89.6 | 10.0 | 0.4 | 47.5 | 42.1 | 10.4 | 0.90 |
| A6C | 85.1 | 12.8 | 2.1 | 31.6 | 44.5 | 23.9 | 0.46 |
| P11C | 84.3 | 13.7 | 2.0 | 32.5 | 45.9 | 21.6 | 0.48 |
| Y12C | 87.4 | 10.8 | 1.8 | 33.6 | 45.3 | 21.1 | 0.51 |
mAb indicates monoclonal antibody; Ig, immunoglobulin.
Percent molar ratios of neutral asialo (N), acidic monosialo (A1), and acidic disialo (A2) oligosaccharides were calculated on the basis of their ultraviolet (UV) absorbance at 304 nm on diethylaminoethyl–high-performance liquid chromatography (HPLC).
Percent molar ratios of non (G0), mono (G1), and digalactosylated (G2) glycoforms in desialylated, neutral oligosaccharides were calculated on the basis of their UV absorbance at 304 nm on octadecylsilane-HPLC.
Octadecylsilane-HPLC analysis of the neutral oligosaccharides obtained after sialidase treatment of total oligosaccharides showed no measurable differences in the level of fucosylation among the different 6-19 mAbs (Figure 4), fucosylated oligosaccharides amounting to more than 95% of neutral oligosaccharides. In contrast, cryogenic and noncryogenic 6-19 mAbs differed in galactosylation patterns. The content of nongalactosylated (G0; no galactose, terminating in N-acetylglucosamine) oligosaccharides was uniformly elevated in the cryogenic 6-19, 6-19Q6E/K23A, and 6-19N471T mAbs, as compared with the noncryogenic A6C, P11C, and Y12C mAbs (peak 8 in Figure 4 and Table 4). Moreover, the cryogenic 6-19 mAbs had approximately 2-fold lower content of digalactosylated (G2; 2 terminal galactose residues) oligosaccharides than the noncryogenic 6-19 variants (peak 5 in Figure 4 and Table 4). Consequently, the ratio of the nongalactosylated (G0) versus galactosylated (G1 + G2) oligosaccharides was 2 times higher in the cryoprecipitating 6-19 mAbs than in the noncryoprecipitating 6-19 variants.
Separation of desialylated oligosaccharides of cryogenic 6-19, 6-19Q6E/K23A, and 6-19N471T, and noncryogenic A6C, P11C, and Y12C IgG3 mAbs by octadecylsilane-HPLC.
Desialylated ABEE-oligosaccharides of the IgG samples were separated into 8 oligosaccharide fractions which were eluted as a series of authentic biantennary complex-type oligosaccharide structures (positions 1 to 8) of ± Galβ1-4GlcNAcβ1-2Manα1-6(±Galβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-4(± Fucα1-6)GlcNAc shown in the right column. Peaks 1 and 5 correspond to digalactosylated (G2) oligosaccharide chains without or with a fucose residue; peaks 2, 3, 6, and 7 correspond to monogalactosylated (G1) oligosaccharide chains without or with a fucose residue; and peaks 4 and 8 correspond to nongalactosylated (G0) oligosaccharide chains without or with a fucose residue. G and Gal indicate galactose; GN and GlcNAc, N-acetylglucosamine; M and Man, mannose; F and Fuc, fucose.
Separation of desialylated oligosaccharides of cryogenic 6-19, 6-19Q6E/K23A, and 6-19N471T, and noncryogenic A6C, P11C, and Y12C IgG3 mAbs by octadecylsilane-HPLC.
Desialylated ABEE-oligosaccharides of the IgG samples were separated into 8 oligosaccharide fractions which were eluted as a series of authentic biantennary complex-type oligosaccharide structures (positions 1 to 8) of ± Galβ1-4GlcNAcβ1-2Manα1-6(±Galβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-4(± Fucα1-6)GlcNAc shown in the right column. Peaks 1 and 5 correspond to digalactosylated (G2) oligosaccharide chains without or with a fucose residue; peaks 2, 3, 6, and 7 correspond to monogalactosylated (G1) oligosaccharide chains without or with a fucose residue; and peaks 4 and 8 correspond to nongalactosylated (G0) oligosaccharide chains without or with a fucose residue. G and Gal indicate galactose; GN and GlcNAc, N-acetylglucosamine; M and Man, mannose; F and Fuc, fucose.
Discussion
The present study was designed to investigate the molecular basis of the cryogenic potential of murine IgG3 by using several different variants of an 6-19 IgG3 RF mAb. Our analysis has demonstrated that the cryoglobulin activity of the 6-19 mAb was reduced, but still highly significant in its Q6E/K23A mutant, whereas it was hardly detectable in its somatic variants bearing an increased content of galactose residues in the oligosaccharide side chains present in the CH2 domain of their heavy chains. Our results indicate that the level of IgG galactosylation critically determines the IgG3 cryoglobulin activity, with a secondary role of VH residues.
A previous analysis of a panel of IgG3 mAbs (6 cryoglobulins and 5 noncryoglobulins) has shown that cryogenic IgG3 mAbs are more positively charged at VH residues 6 and 23 than noncryogenic IgG3 mAbs.11 However, the present analysis of 14 additional IgG3 mAbs failed to show a complete correlation of the presence of more positive residues at VH 6 and 23 with the cryoglobulin activity. Furthermore, we have observed that the 6-19Q6E/K23A mutant mAb, VH residues 6 and 23 of which were mutated to noncryoglobulinlike residues, still exhibited significant cryoglobulin activity. Strikingly, A6C, P11C, and Y12C mAbs, identical in the amino-acid sequences of their heavy and light chains to those of the 6-19 mAb, were found to be hardly cryoprecipitable. These results clearly indicate that VH residues 6 and 23 are not the only factor determining the cryoglobulin activity of murine IgG3.
Most significantly, comparative analysis of the oligosaccharide structures of different 6-19 mAbs revealed that the level of galactosylation was substantially decreased in the cryogenic 6-19 mAbs, as compared with those of the noncryogenic 6-19 variants, A6C, P11C, and Y12C. Thus, the galactose content of CH2 oligosaccharide side chains plays a key role in determining the cryoglobulin activity of the 6-19 IgG3 mAb. At present, we lack a good explanation for the role of galactosylation in cryoglobulin activity of murine IgG3 mAbs. The terminal galactose residue apparently forms a tight interaction with a lectinlike binding pocket on the CH2 domain of IgG, which partly plays a role in maintaining the relative geometry of the 2 CH2 domains.35,36 Thus, the absence of the terminal galactose could lead to alterations in the conformation of IgG3 molecules, thereby promoting their cryoprecipitating potential. It should, however, be mentioned that we have recently observed that the 6-19J mAb bearing the 6-19 heavy chains and J558 λ1 light chains was still cryogenic despite an even higher galactose content than noncryogenic 6-19 variants.18 This suggests that the amino acid sequence of the light chains plays an additional role in IgG3 cryoglobulin activity, since both variable and constant regions are highly different between the J558 λ1 and 6-19 k light chains. In fact, a recent study reported that the light-chain V region's amino-acid sequence substantially influenced the cryoprecipitating potential of an IgG3 antihydrogenase fromWalinella succinogenes.37 Thus, the cryoglobulin activity of murine IgG3 mAbs is apparently controlled by several factors, such as the level of galactosylation and the V region's amino-acid sequences of both heavy and light chains.
Because of the presence of an additional potential N-linked glycosylation site in the CH3 domain of murine IgG3 isotype,12 it has been speculated that possible CH3 oligosaccharide chains may play a role in the self-associating property of murine IgG3 and hence cryoglobulin activity.13 However, this possibility is excluded for 2 reasons. First, the 6-19N471T mutant mAb lacking this potential glycosylation site is able to self-associate and generate cryoglobulins as efficiently as the wild-type 6-19 mAb. Second, the content of N-linked oligosaccharides of the 6-19N471T mutant was not different from that of the wild-type 6-19 mAb as well as that of its IgG1 variant mAb lacking the potential to self-associate and cryoprecipitate. Collectively, this indicates the absence of glycosylation at the CH3 domain of murine IgG3. The present results contrast with those described by Panka, who claimed that the self-associating ability of IgG3 was significantly reduced in a similarN-linked glycosylation site-loss mutant.13 This discrepancy could be due to the assay used by Panka's study, in which the self-associating activity was measured by a multistep, solid-phase absorbent assay, instead of a direct binding assay in a liquid-phase system, as originally proposed5 and used in the present and previous studies.10 11 It should be also mentioned that asparagine at position 471 was replaced by threonine in our mutant, instead of serine in the study by Panka. Thus, we cannot totally exclude the possibility that the change of asparagine to serine, but not threonine, may affect the self-associating property of murine IgG3.
Our demonstration that a decrease in the relative content of galactose residues in the CH2 oligosaccharide chains is associated with IgG3 cryoglobulin activity suggests that the level of IgG galactosylation may be important in determining the pathogenic potential of autoantibodies with cryoglobulin activity. We observed the development of cryoglobulinemia associated with glomerulonephritis and skin leukocytoclastic vasculitis in mice implanted with transfectoma cells secreting 6-19Q6E/K23A mutant mAbs, as in the case of 6-19 hybridoma-injected mice, whereas no lesions were induced in mice transplanted with hybridoma cells secreting the 6-19 variants, A6C, P11C, or Y12C, lacking cryoglobulin activity (A.K., S.K., Y.K., et al, unpublished data, March 2001). However, the interpretation of these results was difficult, because serum levels of IgG3 concentrations in the latter group of mice have never exceeded more than 1 mg/mL, in contrast with the concentrations (4 mg/mL-5 mg/mL) obtained with mice injected with 6-19 hybridoma or 6-19Q6E/K23A transfectoma cells. In fact, the capacity of A6C, P11C, and Y12C hybridoma cells to secrete IgG3 mAbs was approximately 5 to 10 times less than that of the 6-19 hybridoma or 6-19Q6E/K23A transfectoma cells. The experiment with a hybridoma secreting sufficient amounts of IgG3 “6-19” mAbs lacking cryoglobulin activity could define the role of cryoglobulin activity in the pathogenic potential of IgG3 monoclonal cryoglobulins. Clearly, further elucidation of the pathogenic role of cryoglobulin activity and decreased IgG galactosylation would help develop new diagnostic and therapeutic approaches for cryoglobulin- and autoantibody-mediated disorders.
We thank Mr T. Moll for critically reading the manuscript, and Ms G. Leyvraz, Mr G. Brighouse, Mr G. Celetta, and Ms P. Henchoz for their excellent technical help.
Supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture and from the Ministry of Health and Welfare of Japan, Tokai University, and the Swiss National Foundation for Scientific Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shozo Izui, Department of Pathology, CMU, 1211 Geneva 4, Switzerland; e-mail: shozo.izui@medecine.unige.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal