Abstract
Long-lived antibody-secreting plasma cells are formed in the secondary lymphoid organs and subsequently home to the bone marrow, although the mechanisms that control this migration remain primarily unknown. In this study, we show that IgG plasma cells constitute a significant fraction of cervical lymph node cells from older mice deficient in both E- and P-selectin (E/P−/−), and that these cells can be prospectively isolated by phenotype. These IgG plasma cells were polyclonal, cytoplasmic Ig+, spontaneously secreted antibody, were in the G0/G1 phase of the cell cycle, and failed to express multiple B-cell surface markers. The plasma cells exhibited up-regulated cell surface expression of multiple adhesion molecules, including α4 and leukocyte function-associated antigen 1 (LFA-1) integrins, CD44, and P-selectin glycoprotein ligand 1 (PSGL-1). IgG plasma cells bound to vascular cell adhesion molecule 1 (VCAM-1) significantly better than IgM+B cells, indicating that the α4 integrins were constitutively active. A subset of IgG plasma cells also bound hyaluronic acid, the ligand for CD44. In addition, the IgG plasma cells interacted strongly with E-selectin, but poorly with P-selectin, despite elevated levels of PSGL-1 protein. The preferential interaction of plasma cells with E-selectin, but not P-selectin, correlated with elevated α1,3-fucosyltransferase-VII messenger RNA levels, but selective down-regulation of core 2 β1-6-N-glucosaminyltransferase levels, compared to B cells. These results demonstrate a unique adhesion profile for murine IgG plasma cells. Furthermore, the E/P−/− mice represent a novel system to isolate and purify significant numbers of primary IgG plasma cells.
Introduction
Long-lived plasma cells have recently been identified as an important component of B-cell memory,1,2and the majority of long-lived plasma cells are localized in the bone marrow.2,3 Migration of newly formed plasma cells from the lymph nodes and spleen to bone marrow is therefore an important step in the maintenance of a long-term antibody response to a pathogen. Adoptive transfer of bone marrow containing plasma cells from mice 4 months after lymphocytic choriomeningitis virus (LCMV) infection into naive recipients results in significant long-term LCMV-specific serum IgG levels, suggesting that long-lived plasma cells may maintain their capacity to home to bone marrow.1Plasma cells have also been identified at sites of chronic inflammation in diseases such as rheumatoid arthritis.4 It is unknown to what extent plasma cells are generated locally in these inflammatory tissues, or if plasma cells are formed in lymphoid organs and home to these sites. Thus, although the formation of plasma cells in an immune response has been extensively studied, little is known about the molecular mechanisms and adhesion molecules that govern normal plasma cell migration.
The selectins are a family of carbohydrate-binding adhesion molecules that mediate the earliest steps of leukocyte interaction with the vessel wall.5 E-selectin and P-selectin are members of the selectin family that are inducibly expressed in most tissues during an inflammatory response, but are also constitutively expressed in a limited number of tissues.5 For example, E-selectin is constitutively expressed in human and murine bone marrow.6,7 Bone marrow endothelial cells support rolling of fetal liver hematopoietic progenitor cells (HPCs) and an HPC-like cell line.8 This rolling was mediated by both E-selectin and P-selectin and vascular cell adhesion molecule 1 (VCAM-1). E-selectin, P-selectin, and α4/VCAM-1 interactions were also found to be important in homing of HPCs to the bone marrow after irradiation and transplantation.9 Furthermore, α4-null chimeric mice demonstrate that α4integrins play a role in the attachment and transmigration of pre-B cell lines beneath bone marrow stroma, and these chimeric mice also display reduced myeloid and B-lymphoid progenitor numbers in the bone marrow.10 Whether similar mechanisms control the migration of plasma cells to bone marrow is unknown.
E-selectin and P-selectin double-deficient (E/P−/−) mice spontaneously develop mucocutaneous infections and exhibit a severe reduction in leukocyte rolling, strongly elevated blood leukocyte counts, and an absence of early neutrophil migration in induced peritonitis.7,11 E/P−/− mice also display severe cervical lymphadenopathy, including expanded numbers of lymphocytes, macrophages, and most notably, plasma cells.11 In addition, serum IgG levels are increased about 10-fold.11 In this report, we show that E/P−/− mice represent a novel source from which to purify significant numbers of primary IgG plasma cells and demonstrate that these cells exhibit a unique constellation of adhesion receptors.
Materials and methods
Mice
The E-selectin/P-selectin double-deficient mice,11backcrossed 5 generations to C57BL/6, were provided by Dr Dan Bullard (University of Alabama-Birmingham). Wild-type C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in our mouse facility. E/P−/− mice were generally 3 to 12 months of age when used.
Cell isolation
For isolation of the B-cell compartment by depletion of T cells and myeloid cells, E/P−/− or wild-type cervical lymph node cells were incubated for 15 minutes at 4°C with anti-CD5 and anti-CD11b microbeads (Miltenyi Biotec, Auburn, CA). Cells were then passed through a CS depletion column (Miltenyi Biotec) according to the manufacturer's protocol. For plasma cell isolation, cervical lymph node cells were incubated with anti-CD5, anti-Mac-1, anti-IgM, and anti-B220 microbeads before the depletion protocol. A purity of more than 94% B220-IgM plasma cells was achieved. Wild-type IgM+ B cells used in Western blot analysis and adhesion assays were isolated from wild-type C57BL/6 spleens using anti-IgM microbeads and LS+ selection columns (Miltenyi Biotec).
Fluorescence-activated cell sorting, DNA content staining, and immunohistochemistry
Fluorescence-activated cell sorting (FACS) analysis was performed as described elsewhere.12 For 3-color FACS analysis of E/P−/− B-cell compartments, various biotinylated monoclonal antibodies (mAbs) followed by a phycoerythrin (PE)–streptavidin second step were used in conjunction with allophycocyanin (APC)–conjugated B220 and fluorescein isothiocyanate (FITC)–conjugated anti-IgM. Data were collected using a FACSort or FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). The following antibodies plus isotype controls were purchased from Pharmingen (San Diego, CA): APC-RA3/6B2 (anti-CD45R/B220), biotin-1D3 (anti-CD19), biotin-281.2 (anti-syndecan-1/CD138), biotin-90 (anti-CD38), biotin-KH74 (anti–major histocompatibility complex [MHC] class II I-Ab), biotin-104 (anti-CD45.2), biotin-C71/16 (anti-CD18), biotin-M17/4 (anti-CD11a), biotin-R1-2 (anti-CD49d), PE-MEL-14 (anti-CD62L/L-selectin), and PE-IM7 (anti-CD44). FITC-conjugated and biotinylated anti-IgM (b76), and biotinylated anti-CD43 (S7 and S11) were provided by Dr Thomas Waldschmidt (University of Iowa, Iowa City). Anti–P-selectin glycoprotein ligand 1 (PSGL-1) mAb 3C12 was provided by Immunex (Seattle, WA). FITC-conjugated hyaluronic acid (HA) was provided by Dr Mark Siegelman (University of Texas Southwestern Medical School, Dallas). PE-conjugated streptavidin was purchased from Southern Biotechnology (Birmingham, AL) and APC-conjugated streptavidin was purchased from Pharmingen.
For morphology analysis and immunohistochemistry, isolated plasma cells were cytospun onto slides and fixed with ice-cold 95% ethanol/5% glacial acetic acid. The fixed cells were then stained with either hematoxylin and eosin, or with mAb specific for Igκ, Igλ, IgA, or with polyclonal anti-IgG or anti-IgM (Biosource, Camarillo, CA).
For cell cycle analysis, plasma cells were resuspended in 0.5 mL stain solution, then passed through an 18-gauge needle and incubated in a shaker for 20 minutes at 37°C. Then 0.5 mL of hypertonic solution was added, the cells were again passed through an 18-gauge needle, wrapped in aluminum foil, and stored at 4°C for 6 hours to overnight prior to FACS analysis.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blot analysis
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis of plasma cell and IgM+ B-cell lysates were performed as described.13 Cell lysates were made from equal numbers of cells with the following high-salt RIPA lysis buffer: 50 mM Tris, pH 8, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate (DOC), 0.1% SDS, 1 mM EDTA, with protease inhibitors. Rabbit antimouse Blimp-1 antiserum was kindly provided by Dr Mark Davis (Stanford University, Stanford, CA) and rabbit antimouse BCL-6 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Horseradish-peroxidase–conjugated antirabbit Ig second step was purchased from Biosource and nitrocellulose membranes were developed using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ). The murine plasmacytoma cell line, J558, was used as a positive control for Blimp-1 expression, and BJAB cells were used as a positive control for BCL-6.
ELISPOT analysis
In 96-well Unifilter plates (Whatman, Clifton, NJ), a mAb specific for κ-light chain (Southern Biotechnology) was plated at 5 μg/mL overnight at 4°C in a humidified chamber. Prior to adding the cells, the plates were washed 4 times with phosphate-buffered saline (PBS) and blocked for at least 1 hour with Dulbecco modified Eagle medium (DMEM)/1% bovine serum albumin (BSA) at room temperature. The cells were incubated on the plates for 3 to 4 hours at 37°C. For purified plasma cells, 50 cells were added per well, and 4000 spleen, bone marrow, or peripheral blood cells were added per well. Each sample was analyzed in triplicate. Following 3 washes with PBS and 4 washes with PBS/Tween, 1:2000, 100 μL of the appropriate biotinylated detecting antibody in PBS/Tween 1:2000/1% BSA was added at 5 μg/mL and incubated overnight at 4°C in a humidified chamber. The plates were then washed 4 times with PBS/Tween and incubated with alkaline phosphatase conjugated-antibiotin antibody (Vector Labs, Burlingame, CA) at a 1:1000 dilution in PBS/Tween/BSA for 2 hours at room temperature. Following 4 washes with PBS, 200 μL developer (0.3 mg/mL nitroblue tetrazolium [NBT; Bio-Rad, Hercules, CA] and 0.15 mg/mL 5-bromo-4-chloro-3-inodolyl phosphate [BCIP; Sigma, St Louis, MO] in Tris buffer) was added per well for approximately 15 minutes, and the plates were washed 4 times with PBS, air dried, and counted using an ELISPOT plate reader and software (Cellular Technologies, Cleveland, OH).
Low-shear L/VCAM-1 adhesion assay
L cells stably transfected with human VCAM-1 were plated in 35-mm tissue culture plates at 150 000 cells/plate and allowed to grow to near confluence. Then 0.70 × 106 to 1.0 × 106 E/P−/− plasma cells or wild-type IgM+ B cells were resuspended in 600 μL unsupplemented RPMI and incubated with or without 50 nM phorbol myristate acetate (PMA) for 15 minutes at 37°C. After each L-cell plate was washed 3 times with RPMI, the appropriate cells were added and incubated for 15 minutes on a continuously rocking platform at room temperature. Following incubation, each plate was washed 5 times with RPMI and fixed with cold 0.74% formaldehyde/RPMI solution. Where indicated, untransfected L cells were used. For the blocking experiments, the plasma cells were incubated with 2 μg anti-α4 antibody (R1-2) in a volume of 100 μL for 10 minutes, following treatment with 50 nM PMA or media alone. The volume was then increased to 600 μL and the cells were immediately added to the L-cell plates and the assay was continued as above. Cells bound per field were counted for 20 fields. The T-lymphoblastoid cell line, Jurkat, which binds well to VCAM-1, was used as a positive control.
Parallel plate flow assay
Monolayers of Chinese hamster ovary (CHO) cells stably transfected with either E-selectin or P-selectin were grown in 35-mm tissue culture plates and served as the rolling substrate. Wild-type bone marrow cells, wild-type IgM+ B cells, or E/P−/− plasma cells were introduced into the flow chamber (Glycotech, Rockville, MD) at a concentration of 0.5 × 106 cells/mL in RPMI supplemented with 0.1% serum. Wild-type bone marrow cells (about 50% Gr-1+neutrophils) roll well on both E-selectin and P-selectin and served as the positive control. The shear stress in the flow chamber was maintained constant at 1.5 dynes/cm2 using a syringe pump (Harvard Apparatus, Holliston, MA), and images were obtained using a Nikon Eclipse TE300 inverted microscope (Nikon, Melville, NY). Data analysis was performed using Celltrak software developed by Compix (Cranberry Township, PA), as previously described.14Briefly, a rolling event is defined as a rolling cell that can be tracked between sequential images separated by a 2-second time delay. The total number of rolling events was collected for 50 to 100 sequential images and the percentage of control events was calculated. Data are represented as the mean percentage control of 3 experiments.
Glycosyltransferase reverse transcriptase–polymerase chain reaction
Analysis of α1,3-fucosyltransferase-VII (FucT-VII), core 2 β1-6-N-glucosaminyltransferase (C2GlcNAcT-I), and dihydrofolate reductase (DHFR) messenger RNA (mRNA) expression was performed using reverse transcriptase–polymerase chain reaction (RT-PCR).15 Total cellular RNA was isolated using Trizol (Life Technologies, Rockville, MD) from wild-type bone marrow cells, wild-type IgM+ B cells, and E/P−/−plasma cells, and was used as a template in a 20-μL RT reaction. RNA from equal numbers of cells, ranging from 0.3 × 106 to 0.8 × 106 cells, was used in the RT reactions. Wild-type bone marrow cells served as a positive control for all genes, and DHFR served as an internal normalization control. Reactions performed in the absence of RT were used as negative controls. A 50-μL PCR reaction was performed with cycling parameters 94°C/60°C/72°C for 30 seconds each with 32, 28, and 28 cycles for FucT-VII, C2GlcNAcT-I, and DHFR, respectively, with primers as described.15 PCR products were run out on 1.4% agarose gels, transferred to nitrocellulose, and Southern blotted.
Statistical analysis
Differences between groups in the adhesion assays were analyzed using a Student t test, with P < .05 being considered statistically significant.
Results
Cellular expansion and differentiation within the B-cell compartment of E/P−/− cervical lymph nodes
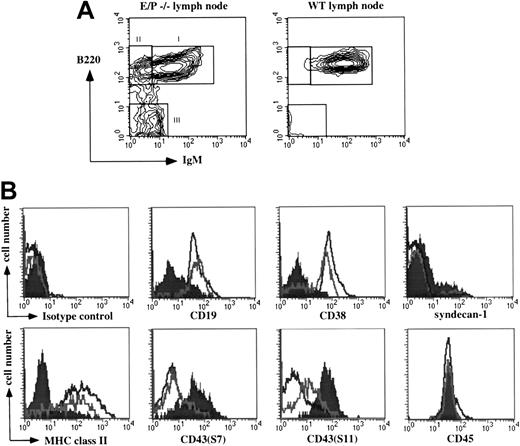
The lymphadenopathy and the presence of morphologically defined plasma cells observed in the cervical lymph nodes of E/P−/− mice11 prompted us to investigate in detail the cellular composition of these lymph nodes. Total cell numbers from the E/P−/− cervical lymph nodes were greatly increased compared to wild-type; mean ± SD was 54 × 106 ± 19.9 × 106 (n = 86 mice, 22 isolations) for E/P−/− mice, and ranged from 17.8 × 106 to 101.3 × 106 per mouse. For wild-type, the mean ± SD per mouse was 5.3 × 106 ± 3.2 × 106 (n = 36 mice, 7 isolations). Although the expansion involved several cell types, including T cells and macrophages, we focused our analysis on the B-cell compartment. Depletion of non-B (CD5+/Mac-1+) cells revealed an expanded B-cell compartment comprising 17.1% ± 9.9% (n = 6) of total cervical lymph node cells. Three main populations defined by correlated B220 and surface IgM expression were evident (Figure1A). Population I cells were B220hi and IgM+, corresponding to the single population of normal B cells present in wild-type mice (Figure 1A). As expected, these cells were CD19+, CD38+, MHC class II+, syndecan-1/CD138−, and mostly negative for 2 CD43 epitopes, S7 and S11, with a subset of positive cells for each (Figure1B). Population II cells, not detectable in wild-type mice, were B220hi and IgM− (Figure 1A). These cells were also uniformly CD19+, MHC class II+, S7−, and syndecan-1−, but contained a distinct subset of CD38− cells, and showed an increase in S11 expression relative to the IgM+ cells (Figure 1B). In the mouse, CD38 expression is lost in germinal centers, but is regained in the transition to memory cells.16 Furthermore, the B220hi/IgM− cells were surface IgG+ (data not shown). Taken together, this phenotype of the B220hi/IgM− population is consistent with a combination of class-switched IgG memory cells and germinal center cells.
Expression of lineage markers within the expanded E/P−/− lymph node B cell compartment.
(A) Non-B (CD5+/Mac-1+) cells were depleted from E/P−/− and control cervical lymph nodes as described in “Materials and methods.” Examination of B220 and surface IgM expression by flow cytometry revealed 3 distinct B-cell populations present in E/P−/− lymph nodes (indicated as I, II, and III), but only the normal IgM+ B cells in wild-type lymph nodes. (B) Three-color flow cytometry analysis was performed to further characterize the 3 E/P−/− B-cell populations. The histograms show expression of CD19, CD38, syndecan-1, MHC class II, CD43 (S7), CD43 (S11), CD45, and an isotype control antibody on population I (black line), population II (gray line), and population III (filled). A representative experiment of at least 6 is shown. Fourteen total experiments examined syndecan-1 expression of population III cells resulting in an overall mean of 42.5% ± 25.9% positive. One representative experiment is shown.
Expression of lineage markers within the expanded E/P−/− lymph node B cell compartment.
(A) Non-B (CD5+/Mac-1+) cells were depleted from E/P−/− and control cervical lymph nodes as described in “Materials and methods.” Examination of B220 and surface IgM expression by flow cytometry revealed 3 distinct B-cell populations present in E/P−/− lymph nodes (indicated as I, II, and III), but only the normal IgM+ B cells in wild-type lymph nodes. (B) Three-color flow cytometry analysis was performed to further characterize the 3 E/P−/− B-cell populations. The histograms show expression of CD19, CD38, syndecan-1, MHC class II, CD43 (S7), CD43 (S11), CD45, and an isotype control antibody on population I (black line), population II (gray line), and population III (filled). A representative experiment of at least 6 is shown. Fourteen total experiments examined syndecan-1 expression of population III cells resulting in an overall mean of 42.5% ± 25.9% positive. One representative experiment is shown.
Purified B220−/surface IgM− cells are IgG plasma cells
Population III cells were negative for both B220 and surface IgM (Figure 1A). In contrast to the 2 B220hi populations, these cells were CD38−, MHC class II−, and CD19 expression was retained by only a small subset (Figure 1B). In addition, these cells were both S7hi and S11hi, and a subset (42.5% ± 25.9%, n = 14) expressed syndecan-1. Despite absent B220, the cells in population III expressed equal levels of CD45 as the 2 B220hi populations (Figure 1B). These cells also exhibited increased forward light scatter (data not shown). This phenotype suggested that these cells represent the plasma cells previously observed in E/P−/− cervical lymph nodes.11
We isolated the B220−/IgM− population III cells to confirm that this population consisted of plasma cells. These purified B220−/IgM− cells displayed classical plasma cell morphology, with abundant dark staining cytoplasm and eccentrically placed nuclei (Figure 2A). Furthermore, they were virtually all cytoplasmic (c) Ig+, with about 97% cIgG+κ+ (Figure 2B), and only less than 4% cIgA+, cIgλ+ or cIgμ+ cells detectable (Figure 2B and data not shown). This profile is consistent with the previous observation of increased IgG, but not IgM or IgA, levels in E/P−/−serum,11 and the approximately 20-fold higher frequency of κ-expressing versus λ-expressing B cells in normal mice.17 18 ELISPOT analysis revealed that the plasma cells spontaneously secreted each of the murine IgG subclasses, with the majority of cells secreting IgG1, IgG2a, or IgG2b, and a small number of IgG3-secreting cells (Figure 2C). Furthermore, cell cycle analysis using DNA content staining with propidium iodide (PI) showed that approximately 97% of the cells were in the G0/G1 phase of the cell cycle (Figure 2D). Taken together, the surface phenotype, characteristic morphology, lack of cycling, and spontaneous secretion of antibody clearly demonstrate that the B220−/IgM− population present in the E/P−/− cervical lymph nodes consists of IgG plasma cells.
B220−/IgM− population III cells are IgG-secreting plasma cells.
(A) B220−/IgM− cells were isolated as described in “Materials and methods,” cytocentrifuged onto slides, and stained with hematoxylin and eosin. Original magnification × 40. (B) B220−/IgM− cells were purified, cytocentrifuged onto slides, fixed, stained with FITC-conjugated anti-Igλ, anit-Igκ, anti-Igμ, or anti-Igγ and examined by both phase and confocal microscopy. Original magnification × 40. (C) The number of plasma cells secreting the various IgG isotypes was determined by ELISPOT. The data are represented as the mean (± SEM) number of secreting cells per 50 cells for 4 independent plasma cell isolations. In each individual experiment, each isotype was performed in triplicate. (D) Purified plasma cells were fixed and stained with PI as described in “Materials and methods,” and analyzed by flow cytometry. A representative experiment of 3 is shown. Cell lines examined in parallel showed significant fractions in the S and G2/M phases of the cell cycle (not shown), whereas the plasma cells were nearly all G0/G1.
B220−/IgM− population III cells are IgG-secreting plasma cells.
(A) B220−/IgM− cells were isolated as described in “Materials and methods,” cytocentrifuged onto slides, and stained with hematoxylin and eosin. Original magnification × 40. (B) B220−/IgM− cells were purified, cytocentrifuged onto slides, fixed, stained with FITC-conjugated anti-Igλ, anit-Igκ, anti-Igμ, or anti-Igγ and examined by both phase and confocal microscopy. Original magnification × 40. (C) The number of plasma cells secreting the various IgG isotypes was determined by ELISPOT. The data are represented as the mean (± SEM) number of secreting cells per 50 cells for 4 independent plasma cell isolations. In each individual experiment, each isotype was performed in triplicate. (D) Purified plasma cells were fixed and stained with PI as described in “Materials and methods,” and analyzed by flow cytometry. A representative experiment of 3 is shown. Cell lines examined in parallel showed significant fractions in the S and G2/M phases of the cell cycle (not shown), whereas the plasma cells were nearly all G0/G1.
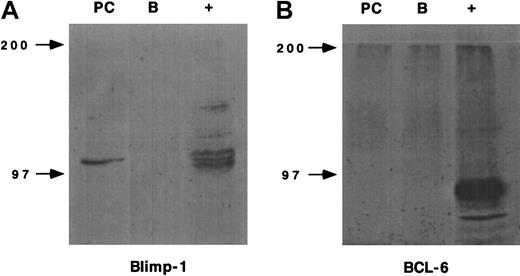
IgG plasma cells express Blimp-1 but not BCL-6
We also examined the expression of 2 transcription factors associated with different stages of B-cell differentiation, Blimp-1 and BCL-6, by Western blot analysis.19-22 As expected, the E/P−/− plasma cells expressed Blimp-1, which is thought to be an important transcription factor involved in plasma cell differentiation,19,20 whereas IgM+ B cells from wild-type spleens did not (Figure3). In contrast, neither the plasma cells nor the IgM+ B cells expressed the transcriptional repressor BCL-6, which is expressed principally in germinal center cells (Figure 3).21,23 24 The lack of BCL-6 provides further evidence that the plasma cells have completely exited the germinal center reaction.
Plasma cell expression of transcription factors.
The expression of Blimp-1 and BCL-6 by E/P−/− plasma cells or wild-type IgM+ B cells was determined by Western blot using rabbit polyclonal antiserums. The murine plasmacytoma cell line, J558, served as the positive control (+) for Blimp-1 expression and BJAB cells served as the positive control (+) for BCL-6 expression. A representative experiment of 2 independent plasma cell and IgM+ B cell isolations is shown.
Plasma cell expression of transcription factors.
The expression of Blimp-1 and BCL-6 by E/P−/− plasma cells or wild-type IgM+ B cells was determined by Western blot using rabbit polyclonal antiserums. The murine plasmacytoma cell line, J558, served as the positive control (+) for Blimp-1 expression and BJAB cells served as the positive control (+) for BCL-6 expression. A representative experiment of 2 independent plasma cell and IgM+ B cell isolations is shown.
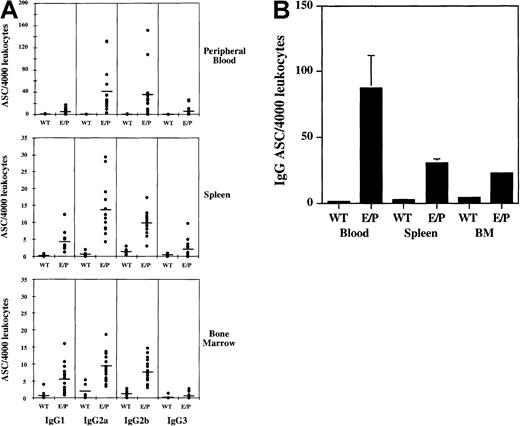
IgG-secreting plasma cells are present in multiple E/P−/− tissues
To quantify the extent of IgG plasma cell accumulation in other E/P−/− lymphoid tissues, bone marrow, spleen, and peripheral blood from E/P−/− mice were analyzed by ELISPOT. Plasma cells secreting antibodies of all 4 murine IgG subclasses were detected at increased levels compared with wild-type from each of these E/P−/− tissues (Figure4A). Interestingly, the total frequency of IgG-secreting plasma cells is highest and most elevated versus wild-type in the peripheral blood compared with the spleen and bone marrow (Figure 4B), suggesting an inability of these plasma cells to exit the circulation due to the lack of the endothelial selectins.
Increased frequency of plasma cells in other E/P−/− tissues.
ELISPOT analysis was performed to quantify plasma cell numbers in E/P−/− peripheral blood, spleen, and bone marrow. (A) Four thousand leukocytes of each tissue type were added per well and each dot represents the mean number of antibody-secreting cells (ASCs) from triplicate wells for an individual mouse. For peripheral blood and spleen, wild-type (WT) n = 6 and E/P n = 14, and for bone marrow, wild-type n = 9 and E/P n = 19. The mean for each isotype is represented by a horizontal line. Note the scale difference for peripheral blood. (B) The total number of IgG-secreting plasma cells for wild-type versus E/P−/− tissues is represented as the mean (± SEM) ASCs per 4000 input leukocytes per mouse. Error bars are present for each group, although SEM is too small to visualize for wild-type tissues and E/P−/− bone marrow.
Increased frequency of plasma cells in other E/P−/− tissues.
ELISPOT analysis was performed to quantify plasma cell numbers in E/P−/− peripheral blood, spleen, and bone marrow. (A) Four thousand leukocytes of each tissue type were added per well and each dot represents the mean number of antibody-secreting cells (ASCs) from triplicate wells for an individual mouse. For peripheral blood and spleen, wild-type (WT) n = 6 and E/P n = 14, and for bone marrow, wild-type n = 9 and E/P n = 19. The mean for each isotype is represented by a horizontal line. Note the scale difference for peripheral blood. (B) The total number of IgG-secreting plasma cells for wild-type versus E/P−/− tissues is represented as the mean (± SEM) ASCs per 4000 input leukocytes per mouse. Error bars are present for each group, although SEM is too small to visualize for wild-type tissues and E/P−/− bone marrow.
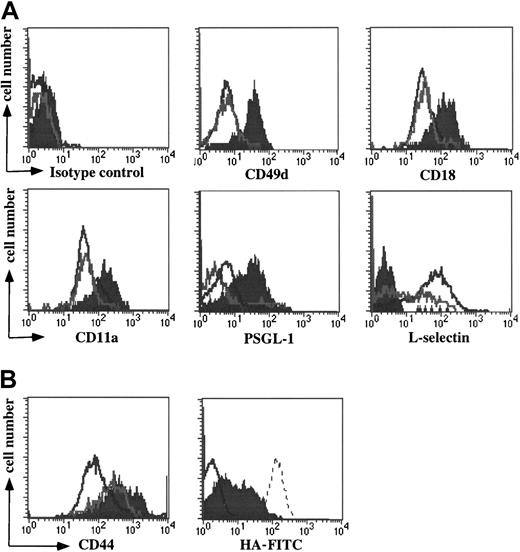
IgG plasma cells up-regulate expression of multiple leukocyte adhesion molecules
To investigate the adhesion characteristics of plasma cells, we first examined the surface expression of leukocyte adhesion molecules by the 3 B-cell subpopulations present in the cervical lymph nodes of E/P−/− mice. Three-color FACS analysis demonstrated that the B220hi IgM+ population I and B220hi IgM− population II cells had equally low levels of leukocyte function-associated antigen (LFA-1; CD11a/CD18) and α4 integrins (Figure5A). Compared to the IgM+cells, the B220hi IgM− population had a larger subset of L-selectin cells, consistent with previous activation, and a modest decrease of PSGL-1 (Figure 5A). In contrast, the plasma cells showed a strong up-regulation of α4 integrins, LFA-1, and PSGL-1, and failed to express L-selectin (Figure 5A). Activation of B cells is therefore not uniformly associated with up-regulation of leukocyte integrins, in contrast to T cells. These results suggest that up-regulation of both leukocyte integrins and PSGL-1 is characteristic of commitment to plasma cell differentiation, and not solely a consequence of B-cell activation.
Up-regulation of leukocyte adhesion molecules by plasma cells.
(A) Three-color flow cytometry analysis of the E/P−/−cervical lymph node B-cell populations (Figure 1) shows the relative expression of CD49d (α4), CD18 and CD11a (LFA-1), PSGL-1, L-selectin, and an isotype control antibody. The populations are as in Figure 1. A representative experiment of 6 is shown. (B) On the left the relative expression of CD44 on the 3 B-cell populations is shown. On the right a subset of purified plasma cells bind HA-FITC. A representative experiment of 8 is shown. HA-FITC staining is depicted on purified plasma cells (filled) versus the entire B-cell compartment (CD5−, Mac-1−; negative control, gray line). For the experiment shown, plasma cells constituted a small fraction of the entire B-cell compartment. An HA-binding T cell line, BW5147, was used as a positive control (dotted line).
Up-regulation of leukocyte adhesion molecules by plasma cells.
(A) Three-color flow cytometry analysis of the E/P−/−cervical lymph node B-cell populations (Figure 1) shows the relative expression of CD49d (α4), CD18 and CD11a (LFA-1), PSGL-1, L-selectin, and an isotype control antibody. The populations are as in Figure 1. A representative experiment of 6 is shown. (B) On the left the relative expression of CD44 on the 3 B-cell populations is shown. On the right a subset of purified plasma cells bind HA-FITC. A representative experiment of 8 is shown. HA-FITC staining is depicted on purified plasma cells (filled) versus the entire B-cell compartment (CD5−, Mac-1−; negative control, gray line). For the experiment shown, plasma cells constituted a small fraction of the entire B-cell compartment. An HA-binding T cell line, BW5147, was used as a positive control (dotted line).
Cell surface expression of CD44 was increased on population II cells, as expected, and on plasma cells (Figure 5B). CD44 is involved in progenitor cell–stromal cell interactions in bone marrow,25,26 as well as in homing of T cells to sites of inflammation.27 Increased expression of CD44 is characteristic of prior activation for both B and T cells, but does not predict the HA-binding properties of the cells. To examine the functionality of the enhanced CD44 expression by the plasma cells, we analyzed the binding of FITC-conjugated hyaluronic acid (HA-FITC) to the plasma cells using flow cytometry.27,28 Previous work has demonstrated that binding of HA-FITC correlates well with the formation of adhesive interactions, including rolling interactions under flow.28 A subset of purified plasma cells (24.8% ± 16.4%, n = 8) stained positively with HA-FITC (Figure5B), similar to activated T cells,28 suggesting that these cells may be more capable of homing to bone marrow or sites of inflammation than their HA-nonbinding counterparts. In contrast, no significant HA binding was detected in the B-cell compartment before plasma cell purification (Figure 5B). Acquisition of HA binding is therefore also plasma cell specific.
Plasma cells spontaneously bind to VCAM-1
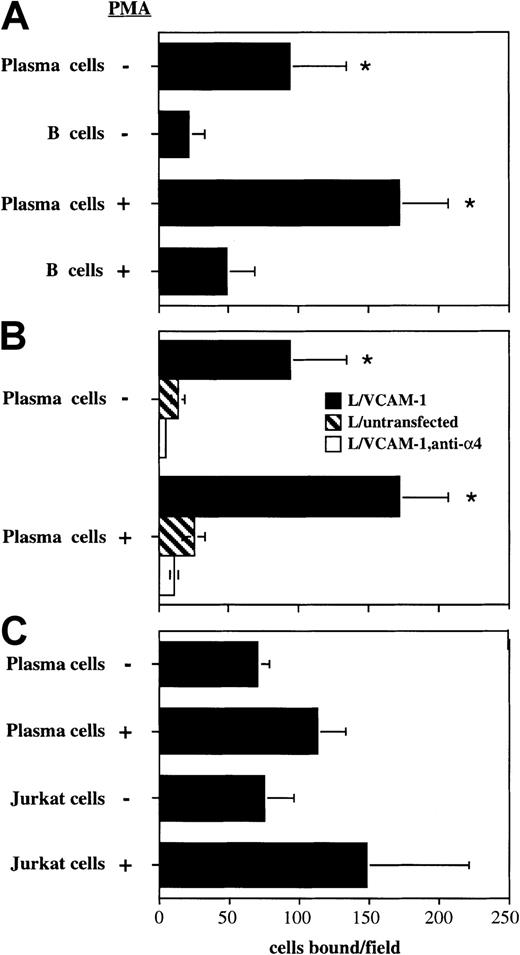
To investigate the functionality of the up-regulated α4 on the plasma cells, we performed a low-shear adhesion assay using L cells stably transfected with VCAM-1. Isolated plasma cells bound about 5-fold better than IgM+ B cells (Figure6A). Following activation with PMA, plasma cell binding to the VCAM-1/L cells increased by 70% to 80% and was still about 3.5-fold better than IgM+ B cells (Figure6A). Binding was specific because plasma cell binding was reduced to background levels following the introduction of an anti-α4 blocking antibody or if untransfected L cells were used (Figure 6B). Plasma cell binding to VCAM-1–expressing cells was similar to the binding of the Jurkat T lymphoblastoid cell line, with or without activation with PMA (Figure 6C). These data demonstrate that α4 integrins up-regulated by plasma cells are constitutively active and can mediate binding of plasma cells to VCAM-1–expressing cells.
Enhanced binding of plasma cells to VCAM-1 through α4 integrins.
(A) Binding of plasma cells and IgM+ B cells to VCAM-1/L cells was analyzed using a low shear rocking adhesion assay. Where indicated, cells were incubated with 50 nM PMA for 15 minutes prior to the assay. (B) Plasma cell binding to untransfected L cells (striped bars), or in the presence of 2 μg of the anti-α4blocking antibody R1-2 (empty bars). Data are represented as the mean number of bound cells per field ± SD for 20 fields of view. A representative experiment of 3 is shown. The asterisk indicates statistically different (P < .05) from part A, the corresponding group (with or without PMA) of plasma cells; or in part B, plasma cells binding to VCAM-1 in the absence of blocking mAb. (C) The relative binding of plasma cells and Jurkat cells. The mean ± SD of 2 independent experiments is shown.
Enhanced binding of plasma cells to VCAM-1 through α4 integrins.
(A) Binding of plasma cells and IgM+ B cells to VCAM-1/L cells was analyzed using a low shear rocking adhesion assay. Where indicated, cells were incubated with 50 nM PMA for 15 minutes prior to the assay. (B) Plasma cell binding to untransfected L cells (striped bars), or in the presence of 2 μg of the anti-α4blocking antibody R1-2 (empty bars). Data are represented as the mean number of bound cells per field ± SD for 20 fields of view. A representative experiment of 3 is shown. The asterisk indicates statistically different (P < .05) from part A, the corresponding group (with or without PMA) of plasma cells; or in part B, plasma cells binding to VCAM-1 in the absence of blocking mAb. (C) The relative binding of plasma cells and Jurkat cells. The mean ± SD of 2 independent experiments is shown.
Selective rolling of plasma cells on E-selectin
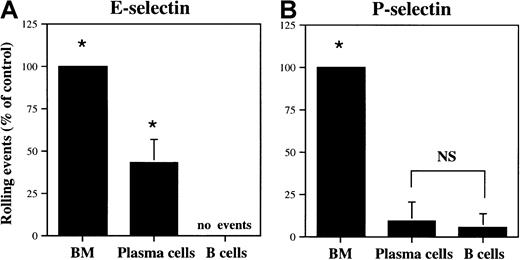
We examined the interaction of plasma cells with E-selectin and P-selectin under flow conditions in a parallel plate flow chamber at a shear stress of 1.5 dynes/cm2. The isolated plasma cells rolled well on E-selectin, with the number of interactions equal to 43.1% ± 13.7% (n = 3) of bone marrow cells (about 50% neutrophils; Figure 7A). Bone marrow leukocytes represent a population capable of significant rolling interactions on E-selectin and P-selectin.5 In contrast, very little plasma cell rolling was observed on P-selectin (9.3% ± 11.2% of control, n = 3; Figure 7B), despite the elevated levels of PSGL-1 protein (Figure 5A). B cells did not roll detectably on E-selectin and rolled only slightly on P-selectin (5.3% ± 8.4% of control, n = 5; Figure 7). This E-selectin binding/P-selectin nonbinding phenotype is distinct from that of activated T cells, which bind well to both endothelial selectins.15 29
Plasma cells interact selectively with E-selectin.
Plasma cells and IgM+ B cells were analyzed for interactions with E-selectin (A) or P-selectin (B) transfected CHO cells in a parallel plate flow chamber, as described in “Materials and methods.” The data are represented as a mean percent control interactions ± SD (n = 3), with wild-type bone marrow cells serving as positive control. Asterisk indicates statistically different (P < .05) from IgM+ B cells; NS, not statistically different.
Plasma cells interact selectively with E-selectin.
Plasma cells and IgM+ B cells were analyzed for interactions with E-selectin (A) or P-selectin (B) transfected CHO cells in a parallel plate flow chamber, as described in “Materials and methods.” The data are represented as a mean percent control interactions ± SD (n = 3), with wild-type bone marrow cells serving as positive control. Asterisk indicates statistically different (P < .05) from IgM+ B cells; NS, not statistically different.
Plasma cells up-regulate FucT-VII but down-regulate C2GlcNAcT-I
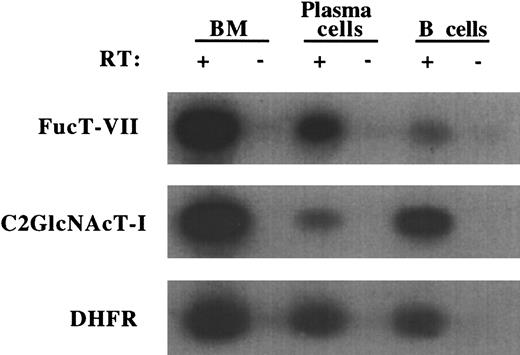
To determine the basis for this unique selectin-binding profile, we examined the expression of glycosyltransferases involved in selectin ligand biosynthesis. Expression of FucT-VII is absolutely required for formation of both E-selectin and P-selectin ligands,30whereas C2GlcNAcT-I is essential for P-selectin binding,31,32 but is not required for E-selectin binding under these conditions.33 We therefore analyzed the mRNA levels of these enzymes in purified plasma cells by RT-PCR. The plasma cells exhibited significantly higher levels of FucT-VII mRNA relative to IgM+ B cells (Figure 8). In contrast, the plasma cells exhibited sharply decreased levels of C2GlcNAcT-I mRNA (Figure 8), consistent with decreased surface expression of B220, a C2GlcNAcT-I–dependent epitope.32These results indicate that the selective interaction of plasma cells with E-selectin under flow conditions is a function of the enhanced levels of FucT-VII expression and that C2GlcNAcT-I is not required for this interaction.
Plasma cells up-regulate FucT-VII but down-regulate C2GlcNAcT-I.
Expression of FucT-VII and C2GlcNAcT-I mRNA by E/P−/−plasma cells and wild-type IgM+ B cells was determined by RT-PCR. Wild-type bone marrow was used as a positive control for all 3 genes, and DHFR served as a normalization control. Presence or absence of RT in the RT-PCR reactions is indicated. A representative experiment of 3 is shown.
Plasma cells up-regulate FucT-VII but down-regulate C2GlcNAcT-I.
Expression of FucT-VII and C2GlcNAcT-I mRNA by E/P−/−plasma cells and wild-type IgM+ B cells was determined by RT-PCR. Wild-type bone marrow was used as a positive control for all 3 genes, and DHFR served as a normalization control. Presence or absence of RT in the RT-PCR reactions is indicated. A representative experiment of 3 is shown.
Discussion
Tissue-specific leukocyte homing is mediated by the regulated expression of adhesion molecules as well as differential responsiveness to chemotactic signals and has been studied extensively for multiple cell types. For example, Th1 and Th2 effector cells display distinct homing patterns resulting in part from differential expression of glycosyltransferases and consequently selectin ligands.29 34 However, less is known about the homing of B-cell effector cell types. Specifically, the mechanisms that control plasma cell migration and anatomic localization remain largely unknown. To examine plasma cell adhesion and homing molecules we isolated IgG plasma cells from the cervical lymph nodes of E/P−/−mice.
The analysis of the E/P−/− cervical lymph nodes revealed a B-cell compartment comprised of cells at multiple stages of differentiation (Figure 1). In addition to the unactivated IgM+ B cells (population I) corresponding to the single population in wild-type mice, 2 additional major populations were identified in the E/P−/− mice. Population II cells were B220hi and IgM−, expressed CD19, MHC class II, surface IgG, and consisted of CD38+ and CD38−subsets as well as L-selectin+ and L-selectin−subsets (Figures 1 and 5A, and data not shown). This phenotype is consistent with a combination of germinal center cells and postgerminal center IgG memory cells. Population III consisted of plasma cells, which were negative for B220 and surface IgM, as well as CD38 and MHC class II (Figure 1). These plasma cells were essentially all cIgG+, spontaneously secreted antibody, and were similar morphologically (Figure 2). The presence of B cells at distinct stages of differentiation ranging from IgM+ unactivated cells to IgG-secreting plasma cells within the E/P−/− lymph nodes provides a unique view into antigen-driven B-cell differentiation.
The E/P−/− plasma cells exhibited heterogeneity with respect to a few markers. For instance, several subclasses of IgG antibodies were represented within the plasma cell population (Figure2C). The diversity of IgG isotypes is expected due to the bacterial infections, which are persistent in E/P−/−mice.7,11 Additionally, only a subset of these IgG plasma cells expressed syndecan-1 (42.5% ± 25.9%, n = 14; Figure 1B). Syndecan-1 has been used previously as a marker for IgM-secreting plasma cells in vitro and in vivo, and for IgG1-secreting plasma cells in the spleen and bone marrow following immunization with the hapten (4-hydroxy-3-nitrophenyl) acetyl (NP).35-37 Our results suggest that syndecan-1 may not be expressed on all plasma cells, or on plasma cells at all stages of differentiation. Furthermore, a small subset of plasma cells retained CD19 expression. The incomplete loss of CD19 expression has been observed previously for antibody-secreting cells following immunization with NP.37 Taken together, the retention of CD19 expression by a fraction of cells and the heterogeneous expression of syndecan-1 imply the existence of multiple distinct stages of plasma cell differentiation. The plasma cells were also heterogeneous for binding of HA (Figure 5B). It is therefore attractive to speculate that expression of syndecan-1 or the ability to bind HA may be a marker for long-lived plasma cells destined to home to and reside in bone marrow.
High numbers of plasma cells have also been reported in the lymph nodes of mice deficient in CD18 or CXCR2. Similar to the E/P−/−mice, CD18−/− and CXCR2−/− mice display highly elevated leukocyte counts and severe defects in neutrophil recruitment to sites of inflammation, in addition to an accumulation of plasma cells in their cervical lymph nodes.38 39 Both the E/P−/− and CD18−/− mice also suffer from progressive skin lesions that are secondary to unresolved dermal infections with endogenous bacteria. Consequently, mice with targeted mutations in different genes, each of which results in severely compromised neutrophil traffic, result in strikingly similar phenotypes. This observation supports the idea that the large expansion and differentiation of the B-cell compartment, as well as other lymph node populations, in these animals results from persistent bacterial infections that cannot be effectively eliminated, due to severely impaired recruitment of neutrophils. Thus, persistently high levels of antigen and, presumably, various bacterial products, continuously drives an intense immune response.
In addition to the presence of plasma cells in the cervical lymph nodes, increased IgG-secreting plasma cells were detected in spleen, bone marrow, and peripheral blood of E/P−/− mice (Figure4). In wild-type mice, the highest frequency of IgG plasma cells was in the bone marrow, with extremely low levels detected in wild-type peripheral blood, as expected. In contrast, peripheral blood from E/P−/− mice contained a higher frequency of IgG plasma cells than either E/P−/− spleen or bone marrow. These data illustrate that enhanced plasma cell differentiation or accumulation in E/P−/− mice is not restricted to the cervical lymph nodes. Furthermore, the substantial increase in peripheral blood plasma cells in E/P−/− mice serves as preliminary evidence for a possible role of E-selectin or P-selectin in plasma cell migration.
Our results demonstrate that IgG plasma cells display a unique array of adhesion molecules involved in leukocyte traffic. Specifically, these plasma cells showed a greatly increased expression of α4integrins, LFA-1, CD44, and PSGL-1 (Figure 5). For the leukocyte integrins and PSGL-1, these were plasma cell–specific changes not specifically associated with activation, because they were not present on B220hi/IgM−/IgG+ B cells. Furthermore, the α4 integrins on plasma cells were shown to mediate substantial binding of plasma cells to VCAM-1 (Figure 6), and a subset of plasma cells bound HA (Figure 5B), indicating that these molecules were active and functional. Up-regulation of leukocyte integrins and CD44 is also characteristic of activated/memory T cells, but up-regulation of PSGL-1 has not been previously observed on normal activated lymphocytes.
Of equal importance, the purified plasma cells interacted selectively with E-selectin but not P-selectin under flow conditions, despite the higher levels of PSGL-1 protein (Figures 5 and 7). This is in sharp contrast to activated T cells, particularly Th1 cells, which exhibit high levels of rolling on both endothelial selectins.15,29,34 Plasma cells also showed an up-regulation of FucT-VII mRNA (Figure 8), an enzyme required for E-selectin and P-selectin ligand biosynthesis,30 but this increased expression of FucT-VII mRNA was accompanied by a sharp decrease in C2GlcNAcT-I mRNA levels (Figure 8). Recent work revealed an absence of rolling on P-selectin but unimpaired rolling on E-selectin of C2GlcNAcT-I−/− leukocytes under the in vitro conditions used here,33 demonstrating that C2GlcNAcT-I is selectively required for functional P-selectin ligands. The preferential up-regulation of FucT-VII accompanied by a down-regulation of C2GlcNAcT-I by the plasma cells also contrasts with the up-regulation of both of these enzymes in activated T cells, particularly Th1 cells.29 The down-regulation of an enzyme selectively required for binding to P-selectin but not E-selectin represents a novel mechanism for control of lymphocyte traffic, and these plasma cells represent the first such example.
E-selectin, P-selectin, and VCAM-1 are important in progenitor cell rolling in bone marrow microvessels and recruitment of HPCs to bone marrow following irradiation and transplantation.8,9 Both human and murine bone marrow endothelial cells constitutively express VCAM-1,6,40,41 human bone marrow endothelial cells express E-selectin,6 and E-selectin has been detected in murine bone marrow using RT-PCR.7 In vitro rolling experiments performed here revealed that purified plasma cells roll well on E-selectin, but not P-selectin (Figure 7), and expressed up-regulated α4 integrins that supported binding to VCAM-1 (Figures 5and 6). Taken together, this suggests that the interaction of plasma cells with E-selectin and VCAM-1 are likely to be important in the bone marrow localization of these cells. In fact, the selective interaction of the plasma cells with E-selectin, and not P-selectin, may represent a mechanism for preventing the migration of plasma cells to acute inflammatory sites, thereby directing them to the bone marrow. Intact α4/VCAM-1 interactions, still present in E/P−/− mice, as well as interactions mediated by other adhesion molecules, such as CD44, most likely underlie the increased frequency of IgG plasma cells in the bone marrow of E/P−/− mice in the absence of E-selectin (Figure 4). In vivo homing experiments using IgG plasma cells purified from E/P−/− mice will provide more information regarding the cooperative effects of these multiple adhesion molecules in plasma cell bone marrow localization.
In conclusion, we have demonstrated that IgG plasma cells display a unique constellation of leukocyte adhesion molecules. Within the B-cell compartment, an adhesion phenotype specific to plasma cell differentiation was observed, including up-regulation of integrin expression, integrin activation, HA binding, and rolling interactions with E-selectin. IgG plasma cells additionally exhibited a pattern of glycosyltransferases consistent with the preferential selectin mediated rolling capabilities observed for these cells. Taken together, this plasma cell–specific spectrum of adhesion molecules described here likely underlies the distinct homing and anatomic localization of antibody-secreting plasma cells. The further analysis of the IgG plasma cells, and in particular plasma cell subsets, generated in E/P−/− mice will offer further insight into plasma cell differentiation mechanisms and possible markers for bone marrow homing or long-lived plasma cells.
We thank Dr Tom Waldschmidt, University of Iowa, Iowa City, for FACS reagents and helpful discussions, and Dr Mark Davis, Stanford University, for Blimp-1 antiserum. We also thank Dr Mark Siegelman, University of Texas Southwestern Medical Center, Dallas, for FITC-HA.
Supported by NIH grants HL58710 (to G.S.K.) and AG13874 and K07 AG00997 (to P.L.W.). G.S.K. was an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Geoffrey S. Kansas, Department of Microbiology-Immunology, Northwestern Medical School, 303 E Chicago Ave, Chicago, IL 60611; e-mail: gsk@northwestern.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal