Abstract
Plasmin, the primary fibrinolytic enzyme, has a broad substrate spectrum and is implicated in biologic processes dependent upon proteolytic activity, such as tissue remodeling and cell migration. Active plasmin is generated from proteolytic cleavage of the zymogen plasminogen (Plg) by urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA). Here, we have investigated the role of plasmin in C2C12 myoblast fusion and differentiation in vitro, as well as in skeletal muscle regeneration in vivo, in wild-type and Plg-deficient mice. Wild-type mice completely repaired experimentally damaged skeletal muscle. In contrast, Plg−/− mice presented a severe regeneration defect with decreased recruitment of blood-derived monocytes and lymphocytes to the site of injury and persistent myotube degeneration. In addition, Plg-deficient mice accumulated fibrin in the degenerating muscle fibers; however, fibrinogen depletion of Plg-deficient mice resulted in a correction of the muscular regeneration defect. Because we found that uPA, but not tPA, was induced in skeletal muscle regeneration, and persistent fibrin deposition was also reproducible in uPA-deficient mice following injury, we propose that fibrinolysis by uPA-dependent plasmin activity plays a fundamental role in skeletal muscle regeneration. In summary, we identify plasmin as a critical component of the mammalian skeletal muscle regeneration process, possibly by preventing intramuscular fibrin accumulation and by contributing to the adequate inflammatory response after injury. Finally, we found that inhibition of plasmin activity with α2-antiplasmin resulted in decreased myoblast fusion and differentiation in vitro. Altogether, these studies demonstrate the requirement of plasmin during myogenesis in vitro and muscle regeneration in vivo.
Introduction
The proteolytic conversion of the ubiquitous zymogen plasminogen (Plg) to the active protease plasmin is an extensively used mechanism for the generation of extracellular proteolytic activity. This conversion is exerted by 2 physiologic plasminogen activators (PAs), tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA). Because unrestrained generation of the broad-spectrum protease plasmin may be hazardous to the cells, plasmin activity is tightly controlled at the levels of PAs by plasminogen activator inhibitors (PAI-1 and PAI-2) and at the level of plasmin by α2-antiplasmin (reviewed by Irigoyen et al1 and Collen and Lijnen2). Plasmin is the major enzyme responsible for the dissolution of fibrin at both intravascular and extravascular sites. In addition, plasmin has been proposed to lead to matrix degradation indirectly via activation of matrix metalloproteinases (MMPs). Furthermore, plasmin can activate several latent growth factors in vitro, including latent transforming growth factor-β and latent basic fibroblast growth factor, and these activities have been proposed to be crucial for cell migration and tissue remodeling in vivo. Thus, the plasmin system has been shown to play a role in a wide variety of physiopathological processes involving extracellular matrix degradation, tissue remodeling, and cell migration, including ovulation, trophoblast invasion, postlactational mammary involution, neurite outgrowth, wound healing, inflammation, angiogenesis, and tumor cell invasion (reviewed by Irigoyen et al1 and Collen and Lijnen2).
Extracellular proteolysis takes place during skeletal muscle formation and pathological muscle regeneration, in which satellite cells play a major role. In response to muscle injury, damaged tissue is infiltrated by fibroblasts, inflammatory cells, and macrophages.3Necrotic tissue is removed, revascularization starts, and proliferation of satellite cells is initiated. A number of proteolytic enzymes have been proposed to play a role during muscle regeneration, either in the inflammatory response and/or in the migration of myoblasts across the basal lamina and in their further fusion to form the terminal muscle fiber.4,5 Metalloproteinases such as MMP-2 and MMP-9, meltrin-α, and cathepsin B seem to be required for myotube formation in vitro.5-8 Moreover, the expression of MMP-2 and MMP-9 has also been reported in the degeneration-regeneration process of myofibers in vivo.9 The mechanism of MMP activation in most cell types has been found to involve a proteolytic activation cascade initiated by uPA. Most MMPs then can be directly activated by cleavage to a lower molecular weight protein by plasmin.10Expression of uPA and tPA has been reported in cell cultures from chicken, mouse, rat, and human muscle.11-13 We showed that inhibition of uPA proteolytic activity with an anti-uPA antibody abrogated migration, fusion, and differentiation of murine myoblasts in vitro.14 Moreover, we recently demonstrated that uPA activity is required for efficient skeletal muscle regeneration in vivo.15 However, the role of plasmin in skeletal myogenesis in vitro and in muscle regeneration in vivo has never been investigated. In this study we show that inhibition of plasmin activity with α2-antiplasmin abrogates myoblast fusion and differentiation in vitro. Moreover, using mice genetically deficient in Plg (Plg−/−)16 we show that the regeneration capacity of muscle tissue in Plg-deficient mice is severely impeded. Because degenerating muscles of Plg-deficient mice show a dramatic accumulation of fibrin, and because fibrinogen depletion restores the onset of normal muscle regeneration in these mice, we propose that plasmin-mediated fibrinolysis is required for efficient muscle regeneration in vivo.
Materials and methods
Cell culture
C2C12 murine myoblastic cell line was obtained from the American Type Culture Collection and maintained in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), designated as growth medium. Fusion and differentiation were initiated by replacing growth medium with serum-free DMEM supplemented with 170 nM insulin (Sigma, St Louis, MO), designated as differentiation medium. When indicated, DMEM plus insulin was supplemented with 10 μM SB203580 (Calbiochem, Darmstadt, Germany), 100 nM α2-antiplasmin (kindly provided by Dr R. Lijnen, Leuven, Belgium), or 0.2% bovine serum albumin (BSA) (Sigma). All media were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin.
Animals
The mice used were 9- to 12-week-old C57Bl16 control strain and the corresponding Plg-deficient mice.16 For all breading, heterozygous Plg+/− mice were used. The genotypes of mice were established by polymerase chain reaction (PCR) of tail biopsy DNA. The wild-type (WT) Plg allele was detected with the primers PLG-1: 5′-TCAGCAGGGCAATGTCACGG-3′ and PLG-2: 5′-CTCTCTGTCTGCCTTCCATGG-3′. These primers generate a 450 base pair (bp) product. The targeted Plg allele was detected with the primers Neo-1: 5′-ATGATTGAACAAGATGGATTGCACG-3′ and Neo-2: 5′-TTCGTCCAGATCATCCTGATCGAC-3′. These primers generate a 480 bp product. All mice were housed in standard facilities and kept at room temperature with a natural night-day cycle. Prior to manipulation, mice were anesthetized by an intraperitoneal injection of dormicum/droperidol.
Induction of muscle regeneration
Regeneration of skeletal muscle was induced by intramuscular injection of 50 μL of 50% glycerol (vol/vol) in the gastrocnemius muscle group of the mice, as described by Kawai et al.17The experiments were performed in right hind-limb muscles, and contralateral intact muscles were used as control. Morphologic and biochemical examinations were performed at 2, 4, 7, 10, 30, and 50 days after injury (AI). Four animals were used for each time point.
Histologic and immunohistochemical analysis
At selected times, muscles of control and Plg−/−mice were removed after cervical dislocation. They were carefully dissected, frozen in isopentane-chilled liquid nitrogen, and stored at −80°C prior to sectioning. Transverse cryostat sections (10-μm thick) were stained with hematoxylin/eosin (H/E). Immunohistochemistry was performed using the Vectastain Elite kit (Vector Laboratories, Burlingame, CA) and diaminobenzidine for single-labeling experiments. The following antibodies were used: mouse monoclonal antibody against myosin developmental-type heavy chain (MHCd) 1:50 (Novocastra, Newcastle, United Kingdom) and rabbit antimouse fibrinogen 1:1000 (kindly provided by Dr K. Dano, Finsenlab, Denmark). For immunofluorescence staining, sections were incubated with rat monoclonal antibodies anti–Mac-1 1:30 (Pharmingen, San Diego, CA), anti–Gr-1 1:30 (Pharmingen), and anti-T11 1:50 (Coulter Immunology, Hialeah, FL) conjugated with fluorescein and mounted with Vectashield (Vector Laboratories). Sections were photographed using an Olympus BX60 microscope (Tokyo, Japan). Control experiments without primary antibody demonstrated that immunofluorescence signals observed were specific (not shown).
RNA analysis
Total RNA was extracted and purified from either C2C12 cells or freshly isolated muscle tissue according to the procedure of Chomczynski and Sacci.18 Five micrograms of total RNA was subjected to Northern analysis, and blots were hybridized with radiolabeled complementary DNA probe corresponding to murine myogenin, as previously described.14 19 To normalize signal intensity, blots were later rehybridized with a radiolabeled 18S oligonucleotide probe. For reverse transcription (RT)–PCR analysis, 2 μg total RNA was reverse transcribed using the first-strand complementary DNA synthesis kit (Pharmacia, Uppsala, Sweden) in a 35-μL reaction. Amplification parameters were denaturation at 95°C for 45 seconds, annealing for 2 minutes at 58°C, and extension at 72°C for 1 minute. Primers for detection of Plg reverse transcriptase product were derived from murine Plg complementary DNA sequence: forward primer: 5′-TCAGCAGGGAATGTCACGG-3′; reverse primer: 5′-CTCTCTGTCGCCTTCCATGG-3′. Expected RT-PCR product size was 410 bp.
Preparation of muscle extracts
Gastrocnemius muscles were dissected out and sectioned, weighed, and pounded in an ice-cold Potter tube with 0.1 mM Tris-HCl buffer, pH 7.6, containing 2 mM ethylenediaminetetraacetic acid (EDTA) and 0.4% Triton X-100. The resulting extracts were centrifuged for 20 minutes at 4°C at 12 000g, and the supernatants were stored as aliquots at −80°C until use. Protein concentration was determined in the supernatants using the Bio Rad (Herts, United Kingdom) protein assay.
Analysis of myogenin protein expression
Tissue extracts from noninjured or injured muscle were prepared as described above, and myogenin expression was analyzed by Western blotting using a rabbit polyclonal antimyogenin antibody 1:200 (sc-576; Santa Cruz Biotechnology, Santa Cruz, CA). Immunoblots were performed and developed with the ECL detection system (Amersham, Buckinghamshire, United Kingdom).
Plasmin kinetics
Plasmin content in 20 μL conditioned media (5-fold concentrated, using Centricon, Pall Filtron, Ann Arbor, MI) from C2C12 cells in DMEM or DMEM supplemented with insulin was measured using the S-2251–based assay, which was done in triplicate in microtitration plates at 37°C and calibrated with purified plasmin (Chromogenix, Milan, Italy). The chromogenic substrate selective for plasmin, S-2251 (Chromogenix), was used to follow the initial rate of Plg activation by measuring p-nitroaniline generation. Conditioned media were mixed with a buffer containing 0.1 M Tris-HCl and 2 mM EDTA, pH7.6, and 1.6 mM S-2251 as substrate. The generation of plasmin was detected by measuring the p-nitroaniline release from the substrate as indicated above.
Zymography
Sixty micrograms of muscle extracts or 40 μL C2C12 conditioned media were size-fractionated on a 10% nonreducing sodium dodecyl sulfate (SDS) acrylamide gel, which was washed for 30 minutes in 2.5% Triton X-100/phosphate-buffered saline and for 30 minutes in distilled water. The gel was subsequently placed in contact with a casein gel containing 2% (wt/vol) nonfat dry milk, 0.25 mM Tris-HCl (pH 7.6), 1% (wt/vol) agarose, 0.25 × phosphate-buffered saline, and 15 μg/mL Plg (Chromogenix) and incubated in a humid chamber at 37°C until caseinolytic bands were visualized and photographed.
Systemic defibrinogenation
Plg-deficient mice were anesthetized, and 14-day mini-osmotic pumps (model 1002, Alza, Palo Alto, CA), filled with a buffered solution of 500 U/mL ancrod (Sigma Chemical), were implanted subcutaneously into their backs (one minipump per animal). The insertion sites were then closed by sutures. The pumps deliver 0.25 μL/h, so the mice received 3 U ancrod/d. In control animals, saline-filled minipumps were implanted. On day 3 of ancrod or saline infusion, injury was induced by intramuscular injection of 50% glycerol. Ten days after injury, mice were killed and gastrocnemius muscles were dissected, frozen, and analyzed by H/E staining. Fibrinogen levels in citrated blood from ancrod- or saline-treated mice were analyzed by SDS–polyacrylamide gel electrophoresis (PAGE) (6% polyacrylamide gel) followed by Western blotting using an antimouse fibrinogen antibody 1:1000.
Statistical analysis
The Student t test was used to determine whether there were significant (P < .05) differences between WT and Plg-deficient mice.
Results
C2C12 muscle cells produce uPA-dependent plasmin activity
Previous studies have reported that myogenic cells express Plg activators in vitro.11,12,14 However, the presence of the downstream protease plasmin in muscle cells has never been documented. Thus, we investigated whether plasmin activity was generated by C2C12 cells (a myoblast cell line derived from murine satellite cells), which have been extensively used as a model for myogenesis in vitro.20 First, we demonstrated by RT-PCR that Plg messenger RNA was synthesized in C2C12 cells (Figure1A). Using a specific chromogenic substrate for the activity of plasmin, we observed that plasmin activity was detected both in conditioned medium of proliferating preconfluent C2C12 myoblasts (lane 1) (Figure 1B) and in conditioned medium of fusing myotubes (lane 2) (Figure 1B). To analyze whether the generation of plasmin by C2C12 cells was uPA- or tPA-dependent, we analyzed the activity of both PAs in these cells by zymography. This assay allowed clear distinction between uPA and tPA, which migrated at 45 and 72 kd, respectively. As shown in Figure 1C, uPA proteolytic activity was clearly observed in C2C12-conditioned medium of both myoblasts (lane 1) and myotubes (lane 2), whereas tPA activity was practically undetectable. These results suggested that the generation of plasmin in C2C12 muscle cells was mediated mainly by uPA.
C2C12 myoblasts produce uPA-dependent plasmin activity.
(A) C2C12 muscle cells express Plg. RT-PCR was performed to detect the expression of Plg in murine liver (positive control, lane 2) and differentiating C2C12 cells (lane 3). Lane 1 corresponds to PCR-negative control. Expected RT-PCR product size was 410 bp. (B) Plasmin activity is produced by C2C12 myoblasts. Histograms show plasmin specific activity (SA) (expressed as mOD/min/mg protein) in conditioned medium of C2C12 cells: preconfluent myoblasts (lane 1) and fusing myotubes (lane 2). No plasmin activity is detected in DMEM medium (lane 3), used as a negative control. The assays were performed in 0.1 M Tris-HCl and 2 mM EDTA, pH 7.6, containing the chromogenic substrate S-2251. (C) C2C12 cells produce uPA proteolytic activity. Conditioned media from preconfluent myoblasts (lane 1) and fusing myotubes (lane 2) cells were analyzed, after SDS-PAGE, for uPA and tPA activity, by PA zymography. The 45-kd approximate molecular mass species indicated by an arrow corresponds to murine uPA and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane and stained with Coomassie blue. The photographs were taken after overnight incubation at 4°C and 4 hours at 37°C.
C2C12 myoblasts produce uPA-dependent plasmin activity.
(A) C2C12 muscle cells express Plg. RT-PCR was performed to detect the expression of Plg in murine liver (positive control, lane 2) and differentiating C2C12 cells (lane 3). Lane 1 corresponds to PCR-negative control. Expected RT-PCR product size was 410 bp. (B) Plasmin activity is produced by C2C12 myoblasts. Histograms show plasmin specific activity (SA) (expressed as mOD/min/mg protein) in conditioned medium of C2C12 cells: preconfluent myoblasts (lane 1) and fusing myotubes (lane 2). No plasmin activity is detected in DMEM medium (lane 3), used as a negative control. The assays were performed in 0.1 M Tris-HCl and 2 mM EDTA, pH 7.6, containing the chromogenic substrate S-2251. (C) C2C12 cells produce uPA proteolytic activity. Conditioned media from preconfluent myoblasts (lane 1) and fusing myotubes (lane 2) cells were analyzed, after SDS-PAGE, for uPA and tPA activity, by PA zymography. The 45-kd approximate molecular mass species indicated by an arrow corresponds to murine uPA and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane and stained with Coomassie blue. The photographs were taken after overnight incubation at 4°C and 4 hours at 37°C.
Inhibition of plasmin activity reduces myotube formation and muscle differentiation in vitro
To investigate the role of plasmin in C2C12 myogenesis, we analyzed whether inhibition of plasmin activity affected myoblast fusion and differentiation in vitro. C2C12 cells cultured in DMEM plus 10% FBS were transferred to DMEM containing insulin for 3 days, to induce myoblast fusion and differentiation, in the absence or presence of the physiologic plasmin inhibitor, α2-antiplasmin, and myotube formation and differentiation were analyzed consequently. As shown in Figure 2A,B, in the absence of α2-antiplasmin, C2C12 cells fused extensively; in contrast, fewer myotubes formed in cultures that had been treated with α2-antiplasmin; as an experimental control, we observed that SB203580 (a specific inhibitor of p38 mitogen–activated protein kinase) significantly reduced myoblast fusion, as previously reported,21 22 while no decrease in myotube formation was observed in the presence of BSA. The effect of α2-antiplasmin on myotube formation was determined microscopically by counting the fraction of nuclei present in myotubes. The results represented in the graph (Figure 2A) show that α2-antiplasmin reduced myotube formation by 45%. SB203580 reduced myotube formation by 80%, while treatment of cells with BSA had practically no effect. To establish whether the inhibition of plasmin activity also affected myogenic differentiation, RNA was obtained from cells treated or not with α2-antiplasmin, SB203580, or BSA and analyzed for the expression of the myogenic differentiation marker myogenin. As shown in Figure 2C, myogenin transcripts were absent in preconfluent myoblasts grown in DMEM plus 10% FBS (lane 1), while their accumulation was induced when C2C12 cells were switched to DMEM plus insulin for 3 days, coinciding with extensive myotube formation (lane 2). When differentiating C2C12 cells were incubated with α2-antiplasmin (lane 3) or SB203580 (lane 4) for 3 days, the levels of myogenin messenger RNA were significantly decreased; in contrast, no alteration in the levels of these transcripts was observed when BSA was added to the cells (lane 5). Furthermore, expression of myogenin was already detected 18 hours following incubation of C2C12 cells in differentiation medium, at a stage when no myotubes had been formed (Figure 2D, lane 1), while in the presence of α2-antiplasmin or SB203580 the levels of myogenin were reduced (lanes 2 and 3, respectively). Altogether, these results indicate that plasmin activity is required for complete myoblast fusion and differentiation in vitro.
Inhibition of plasmin activity with α2-antiplasmin decreases myotube formation and differentiation.
C2C12 myoblasts were grown in DMEM plus 10% FBS and then switched to DMEM supplemented with insulin for 3 days (A, B, C), to induce myotube formation and differentiation, or 18 hours (D), to induce only myogenic differentiation, in the presence or absence of α2-antiplasmin, SB203580, or BSA. (A) The α2-antiplasmin decreases myotube formation. Quantitative effects of plasmin inhibition on myoblast fusion. Near-confluent C2C12 cells were switched to DMEM plus insulin to promote fusion in the absence (○) or presence of α2-antiplasmin (▵), SB203580 (▪), or BSA (■). Fusion is represented as percentage of nuclei in myotubes. Values are mean of 3 independent experiments. (B) Lower-power views of representative fields. Preconfluent cells in DMEM/10% FBS (i), confluent cells in DMEM/insulin (ii), confluent cells in DMEM/insulin plus α2-antiplasmin (iii). Magnifications are × 100. (C) The α2-antiplasmin decreases myogenic differentiation. Northern blot analysis of 5 μg total RNA from C2C12 cells grown as myoblasts in DMEM plus 10% FBS (lane 1) and then switched to DMEM plus insulin for 3 days to induce myoblast differentiation and fusion, in the absence (lane 2) or presence of α2-antiplasmin (lane 3), SB203580 (lane 4), or BSA (lane 5). The probe for each hybridization is indicated. (D) Inhibition of plasmin activity with α2-antiplasmin decreases myogenin expression prior the onset of myotube formation. Northern blot analysis of 10 μg total RNA from C2C12 cells grown as myoblasts in DMEM plus 10% FBS and the switched for 18 hours to DMEM plus 170 nM insulin to induce myogenic differentiation, in the absence (lane 1) or presence of 100 nM α2-antiplasmin (lane 2) or 10 μM SB203580 (lane 3). The probe for each hybridization is indicated.
Inhibition of plasmin activity with α2-antiplasmin decreases myotube formation and differentiation.
C2C12 myoblasts were grown in DMEM plus 10% FBS and then switched to DMEM supplemented with insulin for 3 days (A, B, C), to induce myotube formation and differentiation, or 18 hours (D), to induce only myogenic differentiation, in the presence or absence of α2-antiplasmin, SB203580, or BSA. (A) The α2-antiplasmin decreases myotube formation. Quantitative effects of plasmin inhibition on myoblast fusion. Near-confluent C2C12 cells were switched to DMEM plus insulin to promote fusion in the absence (○) or presence of α2-antiplasmin (▵), SB203580 (▪), or BSA (■). Fusion is represented as percentage of nuclei in myotubes. Values are mean of 3 independent experiments. (B) Lower-power views of representative fields. Preconfluent cells in DMEM/10% FBS (i), confluent cells in DMEM/insulin (ii), confluent cells in DMEM/insulin plus α2-antiplasmin (iii). Magnifications are × 100. (C) The α2-antiplasmin decreases myogenic differentiation. Northern blot analysis of 5 μg total RNA from C2C12 cells grown as myoblasts in DMEM plus 10% FBS (lane 1) and then switched to DMEM plus insulin for 3 days to induce myoblast differentiation and fusion, in the absence (lane 2) or presence of α2-antiplasmin (lane 3), SB203580 (lane 4), or BSA (lane 5). The probe for each hybridization is indicated. (D) Inhibition of plasmin activity with α2-antiplasmin decreases myogenin expression prior the onset of myotube formation. Northern blot analysis of 10 μg total RNA from C2C12 cells grown as myoblasts in DMEM plus 10% FBS and the switched for 18 hours to DMEM plus 170 nM insulin to induce myogenic differentiation, in the absence (lane 1) or presence of 100 nM α2-antiplasmin (lane 2) or 10 μM SB203580 (lane 3). The probe for each hybridization is indicated.
Plasmin production in regenerating muscle is dependent on uPA activity
We next investigated the functional relevance of plasmin during skeletal muscle regeneration in vivo. Muscle regeneration was induced in mice by intramuscular injection of 50% glycerol.17Using the chromogenic substrate S-2251 for the activity of plasmin, we had shown previously that plasmin generation was increased during skeletal muscle regeneration in WT mice, with a peak of activity occurring 2 to 4 days AI and decreasing by day 10.15
To investigate whether the generation of plasmin from Plg is uPA- or tPA-dependent, we analyzed uPA and tPA activities in regenerating muscle tissues at different times AI. Tissue extracts were prepared from noninjured muscle (0 days AI); from 2-, 4-, and 7-day–injured muscle (2, 4, and 7 days AI); and analyzed by zymography (Figure 3). As mentioned above, this assay allows clear distinction between uPA and tPA, which migrate at 45 and 72 kd, respectively. No uPA activity was detectable in tissue extracts from noninjured muscle. However, it was induced in the regenerating muscle samples, reaching a peak at 4 days AI, as demonstrated by the appearance of a casein degradation band of 45 kd, corresponding to murine uPA active enzyme. Under the same conditions, tPA activity was undetectable in all muscle extracts analyzed, while purified human uPA (55 kd), utilized as a control for activity (C), was detected in an adjacent lane. These results suggested that uPA, rather than tPA, might be involved in Plg activation during skeletal muscle regeneration in vivo.
The proteolytic activity of uPA is increased in regenerating muscle after injury.
Forty micrograms of proteins from muscle extracts of noninjured (0 days AI) and injured skeletal muscle (2, 4, and 7 days AI). were analyzed after SDS-PAGE by PA zymography. Two nanograms of purified human uPA (55 kd) were utilized as a control for activity (C). The 45-kd approximate molecular mass species corresponds to murine uPA and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane and stained with Coomassie blue. The photographs were taken after overnight incubation at 4°C and 4 hours at 37°C.
The proteolytic activity of uPA is increased in regenerating muscle after injury.
Forty micrograms of proteins from muscle extracts of noninjured (0 days AI) and injured skeletal muscle (2, 4, and 7 days AI). were analyzed after SDS-PAGE by PA zymography. Two nanograms of purified human uPA (55 kd) were utilized as a control for activity (C). The 45-kd approximate molecular mass species corresponds to murine uPA and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane and stained with Coomassie blue. The photographs were taken after overnight incubation at 4°C and 4 hours at 37°C.
Expression of myogenin is reduced in regenerating muscle tissue of Plg-deficient mice
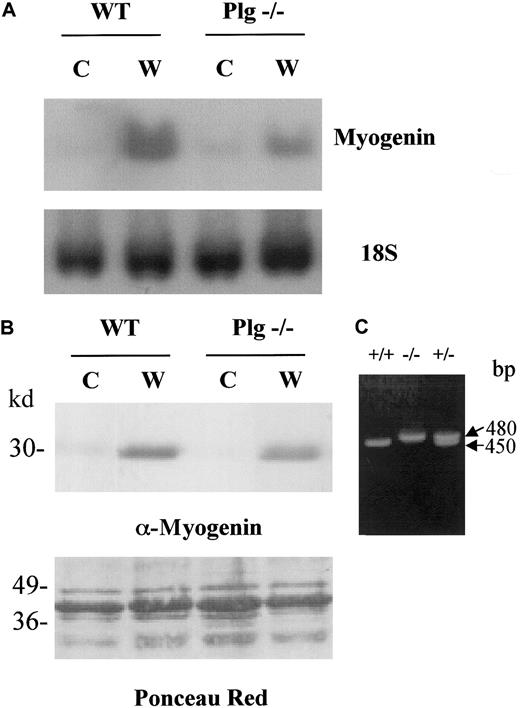
To evaluate the consequences of loss of plasmin in the skeletal muscle regeneration process, we induced muscle injury in WT and Plg−/− mice by glycerol injection, and the expression myogenin was analyzed because the expression of this transcription factor is known to be induced in satellite cells during muscle regeneration in vivo. RNA and protein lysates were prepared from muscles on the fourth day after injury (W, wound) as well as from noninjured muscles (C, control). As shown by Northern blotting, myogenin RNA levels were very low in quiescent muscle from WT mice and were dramatically induced in injured muscle (Figure4A, compare C and W in WT), indicating that the regeneration process was proceeding normally. However, myogenin transcript levels were induced to a lesser extent in Plg-deficient mice than in WT mice after injury (Figure 4A, compare W in WT and Plg−/−), while the 18S RNA level was comparable in all lanes. Similarly, following muscle injury, myogenin protein levels were induced to a greater extent in WT mice than in Plg-deficient mice (Figure 4B, compare W in WT and Plg−/−). These results indicated that Plg is required for efficient expression of muscle regeneration specific gene products. As a control for the the Plg−/− genotype, PCR genotyping of WT, Plg−/−, and Plg+/− mice was performed using specific primers. As shown in Figure 4C, PCR products of 450 and 480 bp, corresponding to WT Plg allele and targeted allele, respectively, were obtained.
Distinct up-regulation of myogenin in skeletal muscle of WT and Plg-deficient mice after freeze-crush injury.
RNA and tissue extracts were obtained from the gastrocnemious muscle of WT and Plg−/− mice at 0 days AI (C, control) or 4 days AI (W, wound). (A) Analysis of myogenin messenger RNA in regenerating muscle of WT and Plg−/− mice. Northern analysis was performed to detect expression of myogenin and 18S. Myogenin transcript levels were induced in regenerating muscle of WT mice. Expression of myogenin was lower in regenerating muscle of Plg-deficient mice compared with regenerating muscle of WT mice, whereas levels of 18S were similar in all lanes. (B) Analysis of myogenin protein in regenerating muscle of WT and Plg−/− mice. Western analysis was performed to detect expression of myogenin using an antimyogenin antibody. Ponceau Red staining was performed to check for equal protein loading. Myogenin was induced in both WT and Plg-deficient mice, although induction was greater in the former mice. (C) PCR genotyping of Plg+/+, Plg−/−, and Plg+/− mice. PCR products of 450 and 480 bp correspond to WT Plg allele and targeted allele, respectively.
Distinct up-regulation of myogenin in skeletal muscle of WT and Plg-deficient mice after freeze-crush injury.
RNA and tissue extracts were obtained from the gastrocnemious muscle of WT and Plg−/− mice at 0 days AI (C, control) or 4 days AI (W, wound). (A) Analysis of myogenin messenger RNA in regenerating muscle of WT and Plg−/− mice. Northern analysis was performed to detect expression of myogenin and 18S. Myogenin transcript levels were induced in regenerating muscle of WT mice. Expression of myogenin was lower in regenerating muscle of Plg-deficient mice compared with regenerating muscle of WT mice, whereas levels of 18S were similar in all lanes. (B) Analysis of myogenin protein in regenerating muscle of WT and Plg−/− mice. Western analysis was performed to detect expression of myogenin using an antimyogenin antibody. Ponceau Red staining was performed to check for equal protein loading. Myogenin was induced in both WT and Plg-deficient mice, although induction was greater in the former mice. (C) PCR genotyping of Plg+/+, Plg−/−, and Plg+/− mice. PCR products of 450 and 480 bp correspond to WT Plg allele and targeted allele, respectively.
Plg-deficient mice show a severe regeneration defect with enhanced fibrosis and myotube degeneration
To analyze the functional significance of the increased plasmin activity in skeletal muscle after damage, we performed glycerol-induced muscle injury in WT mice and in Plg−/−mice, and we analyzed comparatively the histopathological changes induced by the lesion in both types of mice. Skeletal muscle of Plg-deficient mice showed a dramatic regeneration defect after glycerol-induced injury, whereas regeneration in WT mice proceeded normally (Figure 5A). This regeneration defect in Plg−/− mice was apparent by 4 days AI but was most striking 10 to 30 days after injury. Analysis of H/E-stained cross-sections of WT and Plg-deficient mice 2 days after injury showed that muscles of all mice were edematous, presenting fibrotic infiltrates within the enlarged intercellular space separating the necrotic myofibers. Cross-sections of muscles 4 days after injury already showed features of ongoing regeneration in WT mice, with many new myofibers characterized by small size and single nuclei, and with a reduction in fibrotic infiltrates (Figure 5A). In contrast, in Plg-deficient mice, at 4 days AI the muscle appeared edematous and no new uninucleated, small myofibers were detected yet. In WT mice, at 7 days AI most injured fibers regenerated into groups of centrally nucleated myotubes, an indication of advanced regeneration, and a very small number of necrotic fibers could be observed; in addition, the size of newly formed myofibers had increased in the WT animals. In contrast, in Plg-deficient mice, the muscle appeared necrotic, showing still extensive fibrosis 7 days after damage. Ten days after injury, the muscle of WT mice was completely regenerated; no sign of previous damage was detectable in WT mice, except for the presence of centrally located nuclei inside the regenerated fibers (Figure 5A). In Plg-deficient mice, however, a high number of degenerated myotubes were still visible. Thirty days after injury, the lesion was no longer noticeable in WT mice, except for the central myonuclei. In Plg−/− mice, however, the muscle presented extensive fibrosis with high numbers of degenerated myotubes. Notably, extensive muscle degeneration was still observed in Plg-deficient mice 50 days after injury, suggesting that regeneration is not only retarded, but dramatically impaired, in mice deficient in Plg (see Figure5A).
Skeletal muscle regeneration is defective in Plg-deficient mice.
(A) Glycerol-induced injuries result in impaired skeletal muscle regeneration of Plg-deficient mice. Frozen sections of muscles from WT and Plg-deficient mice were stained with H/E after 2, 4, 7, 10, 30, and 50 days AI, as indicated. Contralateral control muscles were also stained with H/E (0 days AI). In WT mice, advanced regeneration is visible after 4 days, and regeneration is complete after 10 days. In Plg-deficient mice, a regeneration defect is already visible after 4 days AI but is most striking after 10 days. In WT mice, virtually no sign of the previous injury was detectable after 30 and 50 days, except for the centrally located myonuclei. Original magnifications × 400. (B) Persistence of MHCd-positive myofibers 10 days after glycerol injury in Plg-deficient mice. Cryostat frozen sections from WT and Plg-deficient mice were reacted with a monoclonal antibody against MHCd at 4 and 10 days AI, as indicated. Large numbers of MHCd-expressing myofibers are visible in regenerating muscle of Plg-deficient mice 4 and 10 days after intramuscular glycerol injection. In WT mice, MHCd-positive fibers are observed 4 days AI, whereas no MHCd-expression is detected 10 days AI. Original magnification × 400.
Skeletal muscle regeneration is defective in Plg-deficient mice.
(A) Glycerol-induced injuries result in impaired skeletal muscle regeneration of Plg-deficient mice. Frozen sections of muscles from WT and Plg-deficient mice were stained with H/E after 2, 4, 7, 10, 30, and 50 days AI, as indicated. Contralateral control muscles were also stained with H/E (0 days AI). In WT mice, advanced regeneration is visible after 4 days, and regeneration is complete after 10 days. In Plg-deficient mice, a regeneration defect is already visible after 4 days AI but is most striking after 10 days. In WT mice, virtually no sign of the previous injury was detectable after 30 and 50 days, except for the centrally located myonuclei. Original magnifications × 400. (B) Persistence of MHCd-positive myofibers 10 days after glycerol injury in Plg-deficient mice. Cryostat frozen sections from WT and Plg-deficient mice were reacted with a monoclonal antibody against MHCd at 4 and 10 days AI, as indicated. Large numbers of MHCd-expressing myofibers are visible in regenerating muscle of Plg-deficient mice 4 and 10 days after intramuscular glycerol injection. In WT mice, MHCd-positive fibers are observed 4 days AI, whereas no MHCd-expression is detected 10 days AI. Original magnification × 400.
To confirm further the muscular regeneration defect in Plg−/− mice, we performed an immunohistochemical analysis of muscle sections of WT and Plg-deficient mice at 4 and 10 days after injury using a monoclonal antibody against MHCd. The expression of this MHC isoform occurs exclusively in embryonic and early neonatal life and upon muscle regeneration in adult life. As shown in Figure 5B, the regenerating myofibers of both WT and Plg-deficient mice expressed MHCd 4 days AI; in contrast, at 10 days AI MHCd expression was detected in Plg−/− mice but not in WT mice. These results clearly indicated that the muscles of Plg-deficient mice present an ongoing regeneration/degeneration process at stages when muscle regeneration is almost complete in WT mice.
Plg deficiency alters the inflammatory response after muscle injury
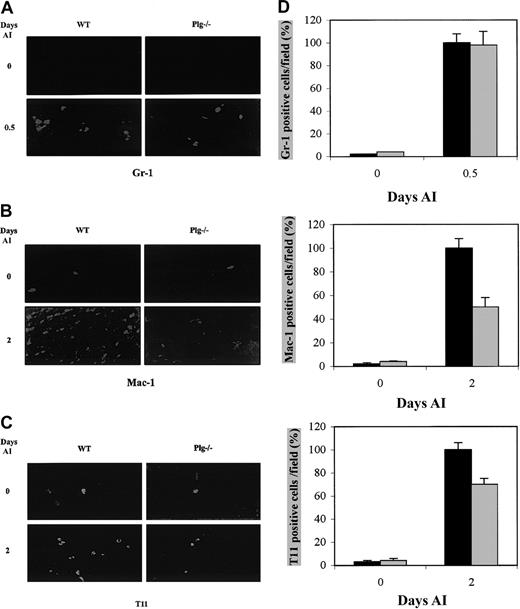
Circulating inflammatory cells are recruited after injury to skeletal muscle during the inflammatory phase.3 While neutrophils have been detected in the early hours following muscle injury, maximal levels of macrophages and T lymphocytes appear later during the muscle injury response. It is widely held that Plg system plays a role in inflammation through plasmin-mediated directional cell migration. To directly characterize plasmin's involvement in the inflammatory response following muscle injury, the presence of neutrophils, macrophages, and T lymphocytes in normal and in regenerating muscle of WT and Plg-deficient mice was analyzed by immunohistochemistry using antibodies against Gr-1, Mac-1, and T11, well-characterized neutrophil, macrophage, and T-lymphocyte markers, respectively. No Gr-1+ cells were detected in noninjured muscle sections of WT or Plg-deficient mice (Figure6A, 0 days AI). Twelve hours after injury, there was a similar increase in Gr-1–expressing cells at the sites of injury in both types of animals (Figure 6A, 0.5 days AI,P < .05). Only a few resident Mac-1+ cells were detected in the intact muscle sections of WT and Plg−/− mice (Figure 6B, 0 days AI). Two days AI, the number of Mac-1–expressing cells augmented at the injury site of both types of animals; however, the number of Mac-1+ cells in Plg-deficient mice was reduced to almost 50% with respect to WT mice (Figure 6B, 2 days AI, P < .05). Similarly, 2-days AI the number of T11-expressing cells increased with respect to resting muscle in both WT and Plg-deficient mice; however, the number of T11-expressing cells at the injury site in muscles of Plg-deficient mice was 35% lower than in WT mice (Figure 6C, 2 days AI,P < .05). Quantification of these data are represented in Figure 6D. These results suggested that the recruitment of both macrophages and T lymphocytes to the injured muscle was reduced in the absence of plasmin.
Immunohistochemical demonstration of the presence of inflammatory cells in muscles of WT and Plg-deficient mice after glycerol-induced injury.
Detection of neutrophils (Gr-1+) (A), macrophages (Mac-1+) (B), and T cells (T11+) (C) in transverse sections of control and injured skeletal muscles of WT and Plg-deficient mice at the indicated times AI. (D) Quantitative analysis of Gr-1+, Mac-1+, and T11+ cells in regenerating muscles of WT and Plg-deficient mice. Gr-1+, Mac-1+, and T11+ cells were counted in 10 random fields per section, and data from 12 sections per muscle were pooled. The mean values for both limbs were to provide a mean value for each animal (3 animals were studied at each time point). Histograms show the percentage of reduction of Mac-1+ and T11+ cells (50% and 30%, respectively) in Plg−/− mice (gray bars) with respect to WT mice (black bars); statistical significant (P < .05) is denoted. Gr-1–expressing cells were maximal 12 hours AI in both WT and Plg-deficient mice. Mac-1– and T11-expressing cells increased 2 days AI in muscle of WT and Plg-deficient mice; however, the number of Mac-1+ and T11+ cells was lower in Plg−/− mouse muscle. Original magnifications × 400.
Immunohistochemical demonstration of the presence of inflammatory cells in muscles of WT and Plg-deficient mice after glycerol-induced injury.
Detection of neutrophils (Gr-1+) (A), macrophages (Mac-1+) (B), and T cells (T11+) (C) in transverse sections of control and injured skeletal muscles of WT and Plg-deficient mice at the indicated times AI. (D) Quantitative analysis of Gr-1+, Mac-1+, and T11+ cells in regenerating muscles of WT and Plg-deficient mice. Gr-1+, Mac-1+, and T11+ cells were counted in 10 random fields per section, and data from 12 sections per muscle were pooled. The mean values for both limbs were to provide a mean value for each animal (3 animals were studied at each time point). Histograms show the percentage of reduction of Mac-1+ and T11+ cells (50% and 30%, respectively) in Plg−/− mice (gray bars) with respect to WT mice (black bars); statistical significant (P < .05) is denoted. Gr-1–expressing cells were maximal 12 hours AI in both WT and Plg-deficient mice. Mac-1– and T11-expressing cells increased 2 days AI in muscle of WT and Plg-deficient mice; however, the number of Mac-1+ and T11+ cells was lower in Plg−/− mouse muscle. Original magnifications × 400.
Systemic fibrinogen depletion restores muscle regeneration in Plg-deficient mice
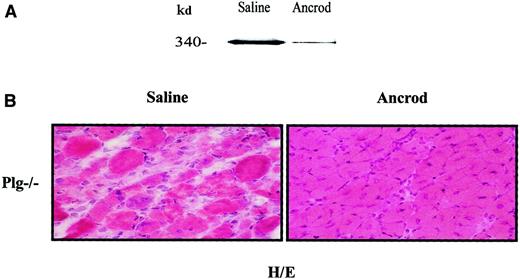
The classical role of plasmin is the degradation of fibrin deposits or fibrinolysis (reviewed by Lijnen and Collen23). In a previous study, we demonstrated an accumulation of fibrin following muscle injury in Plg-deficient mice, but not in WT mice, correlating with the persistence of muscular degeneration features in Plg−/− mice after muscular injury.15 Depletion of circulating fibrinogen can be achieved by administration of the viper venom ancrod.15 24Ancrod- or saline-delivering osmotic pumps (3 U/d) were implanted in Plg−/− mice during 3 days before muscle injury and then throughout the experimental period (up to 10 days AI). Western blot analysis using an antifibrinogen antibody demonstrated the reduction of plasma fibrinogen upon ancrod administration, without any effect on survival (Figure 7A, compare ancrod and saline). Furthermore, signs of improved muscular regeneration were detectable in ancrod-treated Plg-deficient mice 10 days after injury, with centrally located nuclei inside the regenerated fibers. In contrast, histologic features of muscle degeneration persisted in saline-treated Plg-deficient mice at the same stage AI, including high numbers of degenerated myotubes and fibrosis in the intercellular space throughout the damaged muscle (Figure 7B). The extent of muscle regeneration of ancrod-treated Plg-deficient mice 10 days AI resembled the regeneration status of a WT mouse at the same time AI. This observation supports the idea of a potential pathogenic role of fibrin(ogen) accumulation during muscle regeneration in Plg-deficient mice.
Effect of systemic fibrinogen depletion on muscle regeneration of Plg-deficient mice.
Plg-deficient mice were implanted 3 days before glycerol-induced muscle degeneration with ancrod-filled mini-osmotic pumps (3U/d over 13 days) (ancrod) or with buffered-filled pumps (saline). (A) Ancrod treatment reduces fibrinogen in blood of Plg-deficient mice. Forty micrograms of sodium citrate–treated blood obtained from saline- or ancrod-infused mice was analyzed in a 6% polyacrylamide gel by Western blotting using a polyclonal antimouse fibrinogen antibody (1:1000). The 340-kd approximate molecular mass species indicated by an arrow corresponds to murine fibrinogen and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane. (B) Fibrinogen depletion restores muscle regeneration in Plg-deficient mice. Cryosections from saline- and ancrod-treated Plg-deficient mice were stained with H/E 10 days AI. While well-advanced regeneration is visible in ancrod-treated Plg−/− mice, a regeneration defect is still observed in saline-treated mice 10 days after injury. Original magnification × 400.
Effect of systemic fibrinogen depletion on muscle regeneration of Plg-deficient mice.
Plg-deficient mice were implanted 3 days before glycerol-induced muscle degeneration with ancrod-filled mini-osmotic pumps (3U/d over 13 days) (ancrod) or with buffered-filled pumps (saline). (A) Ancrod treatment reduces fibrinogen in blood of Plg-deficient mice. Forty micrograms of sodium citrate–treated blood obtained from saline- or ancrod-infused mice was analyzed in a 6% polyacrylamide gel by Western blotting using a polyclonal antimouse fibrinogen antibody (1:1000). The 340-kd approximate molecular mass species indicated by an arrow corresponds to murine fibrinogen and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane. (B) Fibrinogen depletion restores muscle regeneration in Plg-deficient mice. Cryosections from saline- and ancrod-treated Plg-deficient mice were stained with H/E 10 days AI. While well-advanced regeneration is visible in ancrod-treated Plg−/− mice, a regeneration defect is still observed in saline-treated mice 10 days after injury. Original magnification × 400.
Discussion
The aim of our work was to investigate the involvement of the broad-spectrum serine protease plasmin in cellular and tissular remodeling occurring during skeletal myogenesis in vitro and skeletal muscle regeneration in vivo. Our results clearly show that inhibition of plasmin activity by α2-antiplasmin reduced the extent of myoblast fusion and differentiation in vitro. Moreover, mice lacking Plg showed a pronounced regeneration defect after experimentally induced injury of muscle, suggesting a protective role for Plg in the muscular regeneration process. We recently demonstrated persistent muscle degeneration in uPA-deficient but not in tPA-deficient mice.15 Altogether, this indicates that between the 2 pathways of Plg activation (uPA- or tPA-mediated), uPA-mediated Plg activation is the major one in the muscle regeneration process. This conclusion is supported by zymographic analysis of regenerating muscles of WT mice, which only showed uPA activity. Interestingly, in C2C12 murine myoblasts, uPA activity was also predominant. Finally, the similar phenotypes of uPA- and Plg-deficient mice indicates, in this experimental muscle regeneration model, the predominance of uPA-dependent plasmin effects.
Extravascular fibrin deposition is a key feature in pathologies characterized by inflammation and tissue repair, including impaired skin wound healing25 and glomerulonephritis.26In these 2 situations, failure to remove plasmin was attributable to reduced uPA- or tPA-mediated fibrinolysis, respectively. In experimentally induced muscle degeneration, we also found that uPA deficiency led to increased levels of muscular fibrin, indicating the importance of uPA in extravascular fibrin clearance.15 In the present study, using Plg-deficient mice we have demonstrated the importance of plasmin for fibrin clearance following muscle injury. Fibrin accumulation in the extracellular basal membrane may have deleterious effects, such as the impediment of normal nutrition to the muscle tissue. The hypothesis that increased fibrin levels may contribute to impaired muscular regeneration was further explored by systemic fibrinogen depletion of Plg-deficient mice. Administration of ancrod reduced plasma fibrinogen levels in Plg-deficient mice, resulting in an almost complete restoration of the normal muscle regeneration process. These results clearly show that fibrin(ogen) accumulation has a pathogenic role in sustaining muscle degeneration.
Our finding that loss of plasmin activity impedes muscle regeneration raises the question of how this proteolytic activity is involved in tissue repair. Inflammation is a process frequently associated with tissue repair, because degenerating tissues are invaded by inflammatory cells. Our study showed that in response to glycerol-induced muscle injury, neutrophils, macrophages, and T lymphocytes accumulated near the injury site in the first days AI. Similar results in macrophage recruitment were reported in a study of regeneration following bupivacaine-induced muscle injury in the rat27 and following crush injury in mouse.3 We have observed that mice with a specific deficit in Plg show a reduced staining for Mac-1+ and T11+ cells 48 hours AI, indicating that the number of macrophages and T lymphocytes reaching the injury site is reduced in the absence of Plg. Recently, deficient macrophage recruitment was also reported by our group in regenerating muscle of uPA-deficient mice.15 This suggests that uPA/plasmin activity may have a profound effect on inflammation and inflammation-related muscular disease. Previous studies with uPA-deficient mice demonstrated that uPA is required for the pulmonary inflammatory response to Cryptococcus neoformans, because a lack of uPA resulted in inadequate macrophage recruitment, uncontrolled infection, and death.28 Similarly, monocyte and lymphocyte recruitment was significantly diminished in Plg-deficient mice after thioglycollate-induced acute peritoneal inflammation.29Additionally, components of the Plg system may regulate the expression or/and activity of cytokines involved in inflammatory processes. For example, endogenously produced uPA can amplify tumor necrosis factor-α neosynthesis by mononuclear phagocytes, representing a novel mechanism by which a phagocyte-derived protease contributes to generating proinflammatory signals.30 In addition, plasmin has been shown to release macrophage-derived interleukin-1 and to activate transforming growth factor-β.31,32 It is therefore tempting to speculate that uPA/plasmin might be playing a similar role in the inflammation caused by muscle injury. Thus, the reduced presence of macrophages and T lymphocytes in the injured muscles of Plg-deficient mice might be due either to a reduction in the migration capacity of inflammatory cells devoid of plasmin activity or to a reduced potential of these cells to traverse fibrin-rich matrices. Furthermore, it has been shown that activated macrophages (equivalent to those that accumulate at the site of muscle damage) produce soluble factors (fibroblast growth factor and platelet-derived growth factor), which are highly chemoattractant and also mitogenic for muscle precursor cells.33 Thus, the activated macrophages that accumulate in response to muscle damage will not only phagocytose necrotic tissue but also facilitate the repair of damaged myofibers. These processes will be altered if macrophages do not accumulate at the injury site, providing a potential explanation for the persistent muscle degeneration observed in Plg-deficient mice.
The hypothesis that, in general, Plg activation facilitates cellular penetration of fibrin-containing matrices fits with a putative scenario occurring in normal skeletal muscle regeneration, with local conversion of Plg to plasmin and subsequent fibrin degradation. Plasmin is probably one member of a team of carefully regulated and specialized matrix degrading enzymes, including serineproteases, metalloproteases, and other classes of proteases, which together serve in matrix remodeling and cellular reorganization of wound fields. Our data suggest that plasmin is particularly useful in fibrin solubilization and, without it, cellular reorganization of fibrin reach matrices is severely impeded. We conclude that the uPA/Plg system is required for normal regeneration and resolution of muscle damage in mice. The reduced presence of macrophages at the injury site suggests that a key factor slowing muscle repair in Plg-deficient animals may be the diminished ability of responding macrophages to proteolytically dissect their way through the extracellular matrix to the injury site. In this model, one major matrix component within damaged areas that may represent a particular impediment to inflammatory cell migration in the absence of Plg is fibrin. However, based on the existing data, we cannot exclude that the plasmin deficiency may impede cell migration because of the lost contribution of plasmin to degrade other matrix components or the lack of other key matrix proteinases or of growth factors. Our results have shown that plasmin is required for myogenesis in vitro, because inhibition of plasmin activity with α2-antiplasmin abrogated fusion and differentiation of C2C12 cells (Figures 1 and 2). Although the exact mechanism of this effect needs further examination, it seems likely that plasmin may be required for myoblast migration and alignment prior to myoblast fusion. Metalloproteinases such as MMP-2 and MMP-9, meltrin-α, and cathepsin B are also required for myotube formation in vitro,5-8 and MMP-2 and MMP-9 expression has been reported in a cardiotoxin-induced degeneration-regeneration process of myofibers in vivo.9 Moreover, we have also found induction of both MMP-2 and MMP-9 following glycerol-induced skeletal muscle regeneration in WT mice (R.L.-A. and P.M.-C, unpublished results, 2001). Because most MMPs then can be directly activated by cleavage to a lower molecular weight protein by plasmin,10 it is tempting to speculate that activation of MMP-2 and MMP-9 in regenerating skeletal muscle might also be mediated by plasmin proteolysis. Apart from its effects on extracellular matrix metalloproteases, uPA/plasmin can cleave and activate latent forms of growth/angiogenic factors such as transforming growth factor-β and hepatocyte growth factor/scatter factor.34,35 Both factors are expressed within injured muscle and are believed to have important muscle regeneration effects by promoting the activation of quiescent satellite cells after injury in vivo.36,37 Therefore, modulation of plasmin proteolytic activity may indirectly influence cell recruitment and the growth and differentiation of cellular constituents in regenerating muscle, although this role remains to be demonstrated both in vitro and in vivo. In line with this, a defect in the differentiation of satellite cells of MyoD-deficient mice has been described38 and, as a consequence, the skeletal muscle regeneration capacity of these mice is compromised. Interestingly, the phenotypes of Plg-deficient mice (this study) and MyoD-deficient mice appear to overlap partially.
In conclusion, our results demonstrate that plasmin fulfills a beneficial role in skeletal muscle regeneration, mainly through uPA-mediated fibrinolytic activity. Compounds aimed at decreasing fibrin levels in muscle may be clinically useful in dystrophic muscular therapies. Future experiments will be directed toward further definition of the benefit of defibrinogenating, anticoagulant, or fibrinolytic agents in dystrophinopathies. This report constitutes the first demonstration for a role of plasmin in skeletal myogenesis in vitro and muscle regeneration in vivo. Rescue experiments will show whether exogenously supplied plasmin can compensate for the mutation. If this is the case, PAIs might prove to be useful for the treatment of muscle injuries or muscle dystrophies, particularly if uPA/plasmin evokes the response preferentially in muscle.
We are very grateful to Dr Keld Dano for the antifibrinogen antibody and Dr R. Lijnen for α2-antiplasmin. The helpful assistance of E. Ramı́rez is acknowledged.
Supported by grants from the European Union 1999/C361/06 (E.S., G.A., M.S.), DGES PM97-008, Fundació La Marató-TV3 (F.L., M.P.), and FIS 01/1474. M.S. was also supported by the Catalan government (Reincorporació de Doctors).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pura Muñoz-Cánoves, Centre d'Oncologia Molecular, Institut de Recerca Oncològica, Aut Castelldefels, km 2.7, 08907 L'Hospitalet de Ll, Barcelona, Spain; e-mail: pmunoz@iro.es.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal