Abstract
This work reports the establishment of a Chinese hamster ovary (CHO) cell line stably coexpressing the human αIIbβ3 integrin and the platelet-activating factor receptor (PAFR). These cells aggregate in response to PAF in a Ca++, αIIbβ3, and soluble fibrinogen (Fg)–dependent manner that is prevented by PAF antagonists or αIIbβ3 blockade. The aggregating response is accompanied by enhanced binding of fibrinogen and the activation-dependent IgM PAC1. This model has permitted us to identify, for the first time, intracellular signals distinctly associated with either αIIbβ3-mediated adhesion or aggregation. Nonreceptor activation of protein kinase C (PKC) by phorbol ester produced cellular adhesion and spreading onto immobilized Fg, but it was not a sufficient signal to provoke cellular aggregation. Moreover, inhibition of PKC impeded the PAF stimulation of cellular adhesion, whereas the aggregation was not prevented. The PAF-induced cellular aggregation was distinctly associated with signaling events arising from the liganded Fg receptor and the agonist-induced stimulation of a calcium/calmodulin-dependent signaling pathway. Sustained tyrosine phosphorylation of both mitogen-activated protein kinase (MAPK) and an approximately 100-kd protein was associated with the PAF-induced aggregation, whereas phosphorylation of focal adhesion kinase (FAK) was preferably associated with cellular adherence and spreading onto immobilized Fg.
Introduction
In addition to the role of platelet aggregation in maintaining a normal hemostasis, recent evidence suggests that a platelet dysfunction might be involved in the etiopathogenesis of certain thrombotic events.1,2 A prerequisite for platelet aggregation is the binding of soluble fibrinogen (Fg) to cell-surface receptors. The noncovalent calcium-dependent heterodimer formed by glycoproteins (GP) IIb and IIIa, integrin αIIbβ3, expressed only in platelets and in some tumor cells,3 is a receptor for Fg4 and other extracellular matrix adherent proteins. Under normal resting conditions the Fg is bound to the platelet αIIbβ3 with low affinity. The binding of platelets to exposed adherent proteins of the subendothelial matrix5,6 and stimulation of G-protein–coupled surface receptors by their agonists seem to bring out changes in the αIIbβ3 ligand avidity (receptor clustering) or affinity.7 It has been postulated that activation of cellulifugal (“in-out”) signaling pathway(s) would alter the conformation of αIIbβ3, increasing its ligand binding affinity.8 The activation of platelets is accompanied by a variety of cellular responses, like increased metabolic activity, perturbation of Ca++ and H+ homeostasis, increased adherence to extracellular matrix proteins as well as to other cells, and changes in size, shape, and motility related to association of the Fg receptor with the cytoskeletal structures. Activation of PKC as well as distinct patterns of tyrosine phosphorylation of intracellular protein have also been reported to be associated with platelet activation.9,10 The interrelationship of all the events taking place in activated platelets as well as their relevance to the increased ligand affinity of αIIbβ3 is not apparent. Thus, the elucidation of the mechanism(s) controlling platelet aggregation is hampered by the diversity of concomitant responses. Investigation of the eventual activation of human recombinant αIIbβ3 expressed in established cell lines has been attempted to circumvent this problem. However, to our knowledge, full activation of reconstituted αIIbβ3 receptors by physiologic signals, as indicated by soluble Fg-dependent aggregation, has not been yet demonstrated. Increased adherence to immobilized Fg has been observed in lymphoblastoid cells transfected with αIIbβ3 following postreceptor activation of PKC by phorbol 12-myristate 13-acetate (PMA) or by activation of a G-protein–coupled receptor by the agonist formyl Met-Leu-Phe.11 Based on these observations, it has been suggested that the intracellular signaling pathway involved in αIIbβ3 activation could be specific for hematopoietic cells. However, PMA-induced stimulation of adherence of Chinese hamster ovary (CHO) cells expressing αIIbβ3 to immobilized Fg has also been observed.12 A recent report indicates that the ligand occupancy of the GPIb-IX complex, a receptor for the von Willebrand factor (VWF), coexpressed in CHO cells along with the αIIbβ3 complex, enhanced cell adherence to immobilized Fg.13Because no cell aggregation was reported, it cannot be ascertained whether signaling through the VWF receptor was a sufficient signal to achieve full αIIbβ3 activation in transfected CHO cells. On these grounds, we hypothesized that a full αIIbβ3 receptor activation and, therefore, Fg-dependent aggregation of CHO cells, could be achieved if the proper signaling pathway was stimulated. In this regard, since CHO cells do not express any of the G-protein–coupled receptors known to mediate agonist activation of platelets, we consider it of interest to investigate the eventual agonist-induced activation of αIIbβ3 in CHO cells coexpressing a receptor for a physiologic agonist.
This work reports the development of a CHO cell model stably coexpressing the human platelet-activating factor (PAF) receptor (PAFR) and the Fg receptor (αIIbβ3). PAF elicits an αIIbβ3-mediated stimulation of adherence of these cells to immobilized Fg. Moreover, we observed agonist-initiated, soluble Fg, and αIIbβ3-dependent cellular aggregation, indicative of full Fg receptor activation. This model has allowed us to differentiate, for the first time, intracellular signaling pathways and patterns of tyrosine phosphorylation of proteins distinctly associated with agonist-induced stimulation of aggregation in a reconstituted αIIbβ3 system.
Materials and methods
Antibodies and reagents
A monoclonal antibody (mAb) specific for β3 was the gift of Drs J. González and M. V. Alvarez from the Institute Rocasolano (CSIC), Madrid, Spain. The anti-αIIb mAb 2bc1 was produced by standard techniques and purified by affinity chromatography with the recombinant extracellular fragment of αIIb. Phospho-specific p44/42 MAPK antibody was obtained from New England Biolabs (Beverly, MA). Polyclonal anti-FAK antibody and antiphosphotyrosine mAb PY99 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti–human PAFR was provided by Dr M. Rola-Pleszczynski (University of Sherbrooke, QC Canada). Antiphosphotyrosine mAb 4G10 agarose conjugate was from Upstate Biotechnology (Lake Placid, NY), fluorescein isothiocyanate (FITC)–labeled mAb PAC1 was from Becton Dickinson (San Jose, CA), and FITC-conjugated F(ab')2 fragment of rabbit anti–mouse Ig was from Dako A/S (Glostrup, Denmark). The horseradish peroxidase (HRP)–conjugated secondary goat anti–mouse Ig and goat anti–rabbit Ig were from Bio-Rad Laboratories (Hercules, CA) and Sigma-Aldrich (Madrid, Spain), respectively.
Platelet-activating factor-16 (PAF 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocoline) and PAF antagonist were obtained from Calbiochem (La Jolla, CA). Mitogen-activated protein kinase (MAPK) kinase inhibitor (PD98059), phosphatidylinositol 3-kinase (PI 3-K) inhibitor (LY294002), Arg-Gly-Asp-Ser (RGDS) and Arg-Gly-Glu-Ser (RGES) peptides, pertussis toxin (PTX), human fibrinogen (fraction I, type I), PMA, PKC inhibitors (H7 and staurosporine), Ca++-calmodulin pathway antagonist (W7), intracellular Ca++ chelator (BAPTA-AM), Na+/H+ exchanger inhibitor (EIPA), tyrosine protein kinase inhibitor (genistein), bisindolylmaleimide I (BIM-I), and dibutyryl cAMP (db-cAMP) were purchased from Sigma-Aldrich.
Establishment and characterization of CHO cell lines stably expressing αIIbβ3 integrin and/or the platelet-activating factor receptor
CHO cell lines stably expressing β3 (CHO-β3 cells) or αIIbβ3 complex (CHO-αIIbβ3 cells) were established as described previously.14 CHO, CHO-β3, and CHO-αIIbβ3 cells were transfected with 15 μg human PAFR cDNA subcloned in the expression vector pcDNA3 (Invitrogen, San Diego, CA), using the calcium phosphate precipitation procedure. The transfected cells were fed with medium containing 800 μg/mL G-418 every 3 to 4 days, and clones of cells stably expressing PAFR were selected by monitoring the effect of PAF on intracellular pH and calcium mobilization, as previously described.15,16 Expression of PAFR on stably transfected cells was also assessed by Western blot analysis.17
Adhesion of CHO cell lines to immobilized Fg
96-well flat-bottomed plates were coated with 10 μg/mL Fg in phosphate buffered saline (PBS) pH 7.2 (100 μL per well) for 2 hours at 37°C and then washed with PBS, blocked with 200 μL of 1% bovine serum albumin (BSA) for 1 hour at 37°C, and washed with PBS. CHO cell lines growing in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum were harvested by ethylenediaminetetraacetic acid (EDTA) treatment, collected by centrifugation, and resuspended in serum-free DMEM. Then, they were labeled with 10 μM calcein-AM (Molecular Probes, Leiden, The Netherlands) for 10 minutes, washed by centrifugation, and activated with 100 nM PAF for 2 minutes at room temperature. Then, they were plated onto dishes, in duplicate, at a density of 4 × 104 cells/well, in DMEM, and incubated for different periods of time at 37°C. Nonadherent cells were removed by carefully washing twice with 200 μL PBS. Cell adhesion was quantitated by cytofluorimetry. To calculate the percentage of attachment, basal adherence to BSA (cell binding to BSA-coated wells was always < 1%) was subtracted from attachment values. In some experiments, labeled cells were preincubated with inhibitors, which had no effect on cell viability. At the end of the assays, adherent cells were examined with a phase-contrast Nikon TMS microscope and micrographs were taken with a digital camera.
Soluble Fg-dependent aggregation of CHO cell lines
Cells growing in DMEM containing 10% fetal bovine serum were harvested by EDTA treatment, collected by centrifugation, and resuspended in serum-free DMEM. After 30 minutes at 37°C, they were incubated with 1 mg/mL Fg for 15 minutes at room temperature at a density of 2.5 × 106 cells/mL. Then, PAF was added and 250 μL cell suspension was plated onto wells of a 24-well culture dish precoated with 1 mg/mL BSA. In some experiments, cells were activated for 2 minutes with PAF prior to Fg addition. When indicated, cells were pretreated with inhibitors before Fg incubation. Approximately 10 to 15 minutes after plating, cell aggregates were examined by phase-contrast microscopy as above.
For flow cytometric assessment of aggregation, cells were fixed with 0.25% paraformaldehyde during 30 minutes at room temperature, and forward (FS) and side (SS) light scatter data from 10 000 events were collected in a linear mode. FS is related to the size of the analyzed particles and is an index of cell aggregate formation.
Flow cytometry analysis of PAC1 binding
CHO-αIIbβ3-PAFR cells (2.5 × 106 cells/mL) were resuspended in Tyrode buffer and incubated at 22°C for 15 minutes with FITC-PAC1, in the presence or absence of 100 nM PAF. Then, cells were fixed with 0.25% paraformaldehyde and binding was analyzed by flow cytometry.
Fibrinogen binding to CHO-αIIbβ3-PAFR cells
FITC-labeling of Fg was performed as described previously.18 A quantity of 8 × 105 cells were incubated with FITC-Fg (0 μM-7 μM) at 22°C for 15 minutes, in the presence or absence of 100 nM PAF, in a final volume of 150 μL. Then, cells were collected by centrifugation through a layer of 20% sucrose, and lysed in buffer containing 1% Triton X-100. The bound fluorescence was quantified in a microplate fluorescence reader (PolarStar Galaxy, BMG Lab Technologies, Offenburg, Germany). The effect of different inhibitors on the PAF-induced Fg binding was assayed at saturating Fg concentration. Nonspecific Fg binding was determined in parallel assays in the presence of 10 mM EDTA or 2 mM RGDS.
Antiphosphotyrosine immunoblot assay
Adherent or aggregating cells were washed with PBS supplemented with 30 mM sodium pyrophosphate, 50 mM sodium fluoride, and 100 μM sodium orthovanadate and then lysed in 50 μL to 100 μL ice-cold urea buffer (8 M urea, 4% CHAPS, 1% Triton X-100, 200 mM DTT, 1 mM sodium orthovanadate, 1 mM sodium fluoride, 1 mM sodium pyrophosphate, 2 mM EDTA, 40 mM Tris pH 7.5). Nonadherent cells were washed by centrifugation and lysed as before. Lysates were cleared by centrifugation (13 000g, 15 minutes), and proteins (50 μg) were separated on 6% to 16% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and electrotransferred onto polyvinylidenefluoride (PVDF) membranes. Membranes were incubated for 1 hour in blocking buffer (1% BSA in 10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20) and then in the same buffer containing a 1:500 dilution of the antiphosphotyrosine mAb PY99 for 1 hour. After washing, the membranes were incubated with HRP-conjugated anti–mouse Ig diluted 1:3000 for 1 hour, washed, and the bound antibody was visualized using the enhanced chemiluminescence (ECL) detection procedure.
Tyrosine phosphorylation of β3
At various times after PAF stimulation, cells were resuspended in PBS buffer, lysed using a Branson Sonifier 450 Sonicator (Branson, Danbury, CT), and pelleted by ultracentrifugation at 80 000g for 30 minutes. Pellets were extracted on ice with either radioimmunoprecipitation assay (RIPA) buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 158 mM NaCl, 1 mM EDTA, 10 mM Tris-HCl pH 7.2), or with a modified RIPA buffer (1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 50 mM Tris-HCl pH 7.4), supplemented with 25 μL/mL protease inhibitor cocktail (Sigma) and 4 mM phosphatase inhibitors (sodium orthovanadate, sodium fluoride, and sodium pyrophosphate). A quantity of 0.5 mg protein lysate was incubated with the anti-β3 mAb P37 followed by precipitation with protein A-sepharose beads. Beads were then washed 4 times with lysis buffer, and the precipitates recovered by boiling the beads in 40 μL SDS sample buffer (90 mM Tris-HCl pH 6.8, 3.2% SDS, 10% glycerol, 160 mM DTT, 0.4 mg/mL bromophenol blue). Immunoprecipitates were resolved by SDS-PAGE and transferred onto PVDF membranes. Tyrosine phosphorylation of immunoprecipitated β3 was analyzed by immunoblot with PY99 mAb as described above.
Tyrosine phosphorylation of β3 was also determined by using the agarose-conjugated antiphosphotyrosine mAb 4G10 to precipitate tyrosine-phosphorylated proteins, followed by immunoblot analysis of the immunoprecipitates with P37 mAb.
Immunoblot analysis of phosphorylated FAK and MAPK
Cells were lysed in modified RIPA buffer and immunoprecipitation of FAK was performed using a polyclonal anti-FAK antibody. Tyrosine phosphorylation of FAK was assessed by immunoblotting with antiphosphotyrosine mAb PY99 of either immunoprecipitates or total cell lysates, as described above. Identification of the phosphorylated MAPK in stimulated cells was assessed by immunoblot with a phospho-specific p44/42 MAPK antibody.
Results
PAF-induced stimulation of adherence of CHO-αIIbβ3-PAFR cells to immobilized fibrinogen
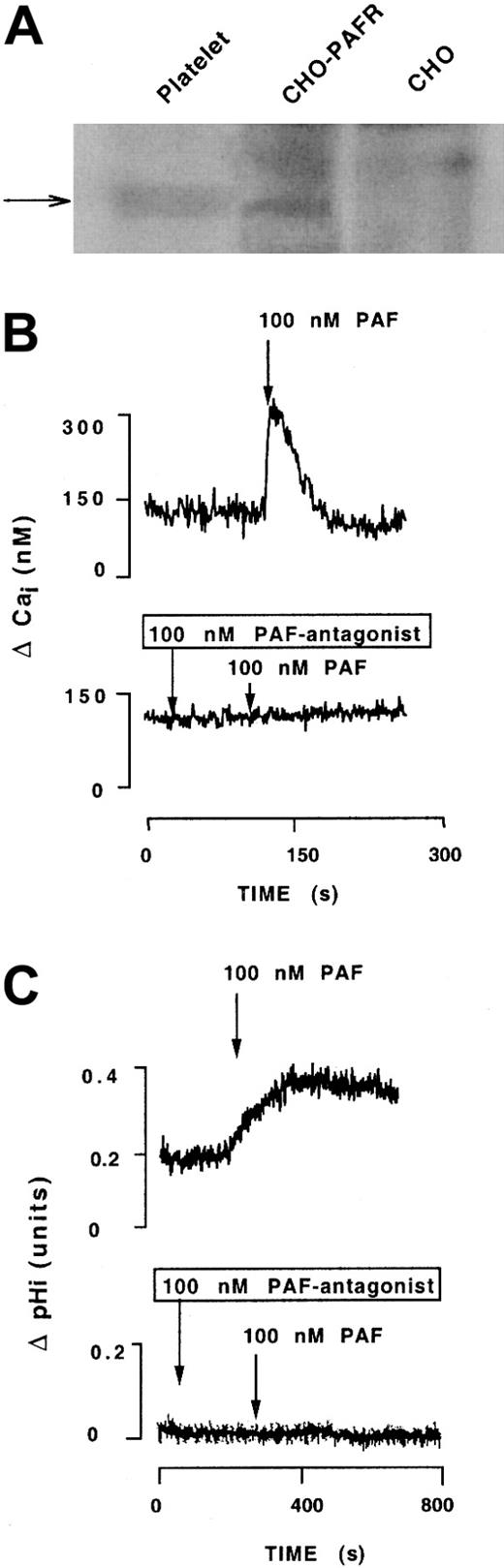
CHO cells or CHO cells stably expressing the human Fg receptor (CHO-αIIbβ3) or the β3 subunit (CHO-β3) were stably transfected with the PAF receptor. The features of the CHO-αIIbβ3 cells have been previously reported.14 The level of expression of PAFR was assessed by immunochemical and functional criteria. Western blotting with anti-PAFR revealed the presence of a band of the expected size in human platelets or CHO-αIIbβ3-PAFR cells but not in CHO-αIIbβ3 cells (Figure 1A). PAF induced an acute and transient elevation of cytosolic-free Ca++ and intracellular alkalization in CHO-PAFR or CHO-αIIbβ3-PAFR cells that were prevented by a PAF antagonist (Figure 1B-C). The ability of PAF to increase the level of cytosolic-free calcium in the presence of 5 mM EGTA suggests that this agonist mobilizes calcium primarily from intracellular stores.19 The CHO-αIIbβ3-PAFR cells express 3.1 × 106 αIIbβ3 receptors per cell as determined by the binding of the FITC-labeled anti-αIIb 2bc1.
Detection and characterization of PAFR stably transfected in CHO-αIIbβ3 cells.
(A) Cell extracts were subjected to Western blotting analysis using a rabbit polyclonal anti-PAFR. The arrow points to a band of the expected size of PAFR that is present in human platelets and CHO-αIIbβ3-PAFR cells, but absent in CHO-αIIbβ3 cells transfected with the void plasmid. (B) and (C) depict the effects of PAF in increasing the intracellular cytosolic levels of free calcium and intracellular pH, and the effect of a PAF antagonist in preventing both effects. Measurement of intracellular calcium and pH was carried out fluorimetrically as described in “Materials and methods.” The data are representative of at least 6 observations made in different cell preparations.
Detection and characterization of PAFR stably transfected in CHO-αIIbβ3 cells.
(A) Cell extracts were subjected to Western blotting analysis using a rabbit polyclonal anti-PAFR. The arrow points to a band of the expected size of PAFR that is present in human platelets and CHO-αIIbβ3-PAFR cells, but absent in CHO-αIIbβ3 cells transfected with the void plasmid. (B) and (C) depict the effects of PAF in increasing the intracellular cytosolic levels of free calcium and intracellular pH, and the effect of a PAF antagonist in preventing both effects. Measurement of intracellular calcium and pH was carried out fluorimetrically as described in “Materials and methods.” The data are representative of at least 6 observations made in different cell preparations.
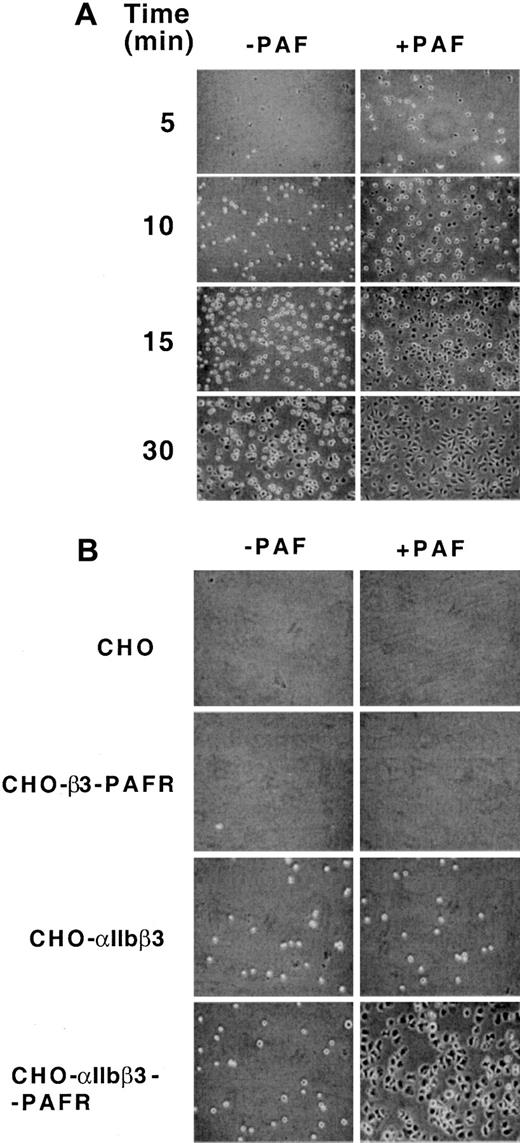
Figure 2A depicts the effect of PAF in enhancing the rate of adhesion and spreading of CHO-αIIbβ3-PAFR cells onto Fg-coated plates, an effect that was accompanied by the appearance of focal adhesion complexes and the formation of actin stress fibers (results not shown). This effect of PAF was clearly appreciated 5 to 10 minutes after seeding, whereas a similar degree of adhesion and spreading in the absence of agonist was observed at 30 minutes. Preincubation with increasing concentrations of soluble Fg produced a dose-dependent inhibition of the PAF stimulation of adherence, whereas it was ineffective in nonstimulated cells (results not shown). The effect of PAF in stimulating cell adherence was observed only in cells coexpressing PAF and Fg receptors (Figure 2B). On the other hand, nonstimulated CHO-αIIbβ3 cells adhered to Fg-coated plates, whereas CHO cells transfected with void plasmids or CHO cells coexpressing PAFR and the β3 chain of the Fg receptor (CHO-β3-PAFR cells) did not. These data suggest that PAF stimulates cellular adhesion and spreading through activation of αIIbβ3.
Effect of PAF on adhesion of CHO-αIIbβ3-PAFR cells to immobilized Fg.
(A) Time course of the effect of PAF on adhesion of CHO-αIIbβ3-PAFR cells to immobilized Fg. CHO cells (4 × 105) coexpressing αIIbβ3 and PAFR were added to the wells of microtiter plates coated with 10 μg/mL Fg, incubated for different periods of time at 37°C in the presence or absence of 100 nM PAF, and visualized by phase contrast microscopy. (B) Comparative effect of PAF on adhesion of CHO, CHO-β3-PAFR, CHO-αIIbβ3, and CHO-αIIbβ3-PAFR cell lines to immobilized Fg. Cells were allowed to adhere on Fg-coated wells for 5 minutes, in the absence or presence of 100 nM PAF.
Effect of PAF on adhesion of CHO-αIIbβ3-PAFR cells to immobilized Fg.
(A) Time course of the effect of PAF on adhesion of CHO-αIIbβ3-PAFR cells to immobilized Fg. CHO cells (4 × 105) coexpressing αIIbβ3 and PAFR were added to the wells of microtiter plates coated with 10 μg/mL Fg, incubated for different periods of time at 37°C in the presence or absence of 100 nM PAF, and visualized by phase contrast microscopy. (B) Comparative effect of PAF on adhesion of CHO, CHO-β3-PAFR, CHO-αIIbβ3, and CHO-αIIbβ3-PAFR cell lines to immobilized Fg. Cells were allowed to adhere on Fg-coated wells for 5 minutes, in the absence or presence of 100 nM PAF.
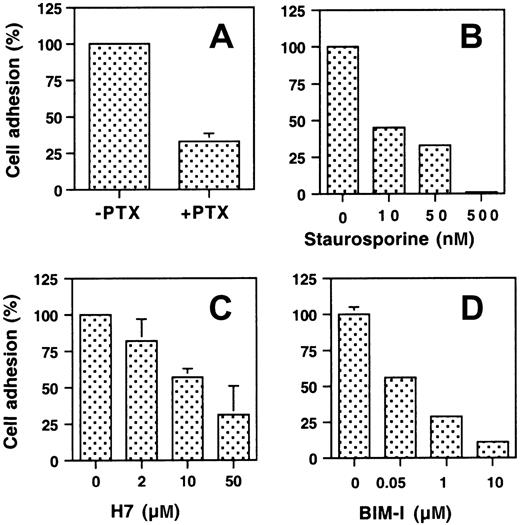
Because Gi proteins have been involved in the agonist-induced stimulation of αIIbβ3,20 we investigated the effect of PTX on the PAF-induced stimulation of cellular adherence. The stimulatory effect of PAF on cell adhesion was partially inhibited by PTX, suggesting that signaling through a Gi protein may be involved in this process. Receptors linked to G proteins stimulate PKC through elevation of cytosolic-free Ca++ and/or diacylglycerol21; therefore, we tested the effect of PKC inhibitors on the PAF-induced stimulation of cellular adherence. The 3 agents tested inhibited the effect of PAF, although staurosporine and the more specific inhibitor BIM-I produced a more complete inhibition than H7 (Figure 3).
Effect of PTX and PKC inhibitors on the effect of PAF in stimulating the adherence of CHO-αIIbβ3-PAFR cells to immobilized Fg.
(A) CHO-αIIbβ3-PAFR cells were incubated with 200 ng/mL PTX for 2 hours at 37°C and then stimulated with 100 nM PAF for 2 minutes. Cell adhesion to immobilized Fg at 10 minutes was measured as described in “Materials and methods.” To determine the effect of inhibiting PKC on the PAF-stimulated adhesion to immobilized Fg, cells were preincubated at 37°C with the indicated concentration of staurosporine (B) for 1 hour, H7 (C) for 45 minutes, or bisindolylmaleimide-I (BIM-I) for 30 minutes (D). The data are expressed relative to the adhesion of PAF-stimulated cells in the absence of inhibitor and are means ± SD of at least 3 experiments.
Effect of PTX and PKC inhibitors on the effect of PAF in stimulating the adherence of CHO-αIIbβ3-PAFR cells to immobilized Fg.
(A) CHO-αIIbβ3-PAFR cells were incubated with 200 ng/mL PTX for 2 hours at 37°C and then stimulated with 100 nM PAF for 2 minutes. Cell adhesion to immobilized Fg at 10 minutes was measured as described in “Materials and methods.” To determine the effect of inhibiting PKC on the PAF-stimulated adhesion to immobilized Fg, cells were preincubated at 37°C with the indicated concentration of staurosporine (B) for 1 hour, H7 (C) for 45 minutes, or bisindolylmaleimide-I (BIM-I) for 30 minutes (D). The data are expressed relative to the adhesion of PAF-stimulated cells in the absence of inhibitor and are means ± SD of at least 3 experiments.
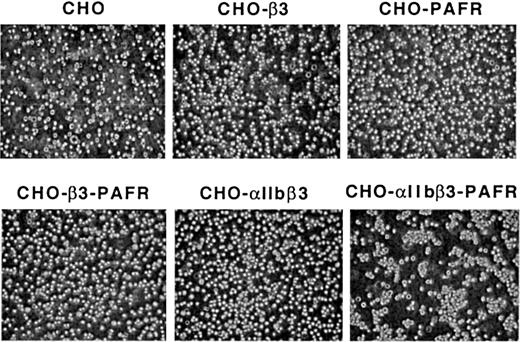
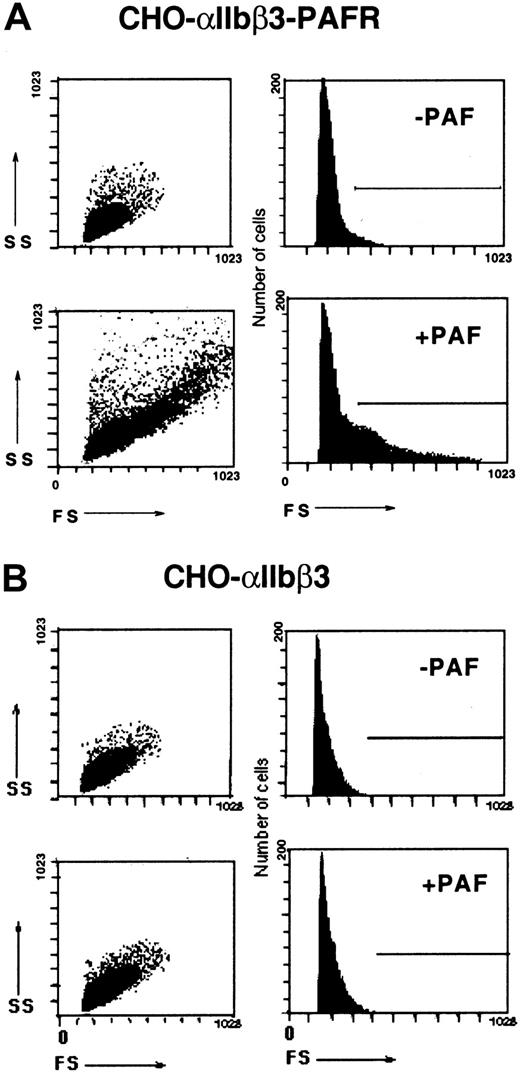
PAF-induced, soluble Fg-dependent aggregation of CHO-αIIbβ3-PAFR cells
A distinct feature of the CHO-αIIbβ3-PAFR cells in suspension is their ability to undergo soluble Fg-dependent aggregation upon stimulation with PAF. This effect of PAF was not observed in CHO cells expressing only PAF or Fg receptor (Figure4). The aggregating response was also demonstrable by flow cytometry (Figure 5) ruling out a subjective visual appreciation of the number of aggregates. These observations indicate that CHO cells possess the intracellular machinery needed to support a cross talk between PAF and αIIbβ3 recombinant receptors enabling the soluble fibrinogen-dependent cell aggregation. This assertion is further supported by the following observations: (1) the effect of PAF in enhancing cell adherence was selectively prevented by soluble Fg in a dose-dependent manner (results not shown); (2) the RGDS ligand mimetic peptide prevented the PAF-induced aggregation while the RGES did not (results not shown); and (3) antagonists of PAF, destabilization of the αIIbβ3 complex by EDTA, or a mAb directed against β3 all prevented the PAF-induced aggregation, while an irrelevant antibody did not (results not shown).
Soluble Fg-dependent aggregation of CHO-αIIbβ3-PAFR cells induced by PAF.
Cells (2.5 × 106/mL) were incubated with 1 mg/mL Fg for 15 minutes at room temperature in a 250 μL final volume and then stimulated with 100 nM PAF and plated onto BSA-precoated wells. Aggregate formation was examined by phase-contrast microscopy as described in “Materials and methods.” Original magnification, × 100.
Soluble Fg-dependent aggregation of CHO-αIIbβ3-PAFR cells induced by PAF.
Cells (2.5 × 106/mL) were incubated with 1 mg/mL Fg for 15 minutes at room temperature in a 250 μL final volume and then stimulated with 100 nM PAF and plated onto BSA-precoated wells. Aggregate formation was examined by phase-contrast microscopy as described in “Materials and methods.” Original magnification, × 100.
Flow cytometric assessment of the PAF-induced aggregation of CHO-αIIbβ3-PAFR cells.
Either CHO-αIIbβ3-PAFR (A) or CHO-αIIbβ3 cells (B) (2.5 × 106/mL) were incubated for 15 minutes with soluble Fg (0.5 mg/mL) in the presence or absence of 100 nM PAF and then fixed with 0.25% paraformaldehyde. Forward (FS) and side (SS) light scatter data of each sample were analyzed by flow cytometry as described in “Materials and methods.”
Flow cytometric assessment of the PAF-induced aggregation of CHO-αIIbβ3-PAFR cells.
Either CHO-αIIbβ3-PAFR (A) or CHO-αIIbβ3 cells (B) (2.5 × 106/mL) were incubated for 15 minutes with soluble Fg (0.5 mg/mL) in the presence or absence of 100 nM PAF and then fixed with 0.25% paraformaldehyde. Forward (FS) and side (SS) light scatter data of each sample were analyzed by flow cytometry as described in “Materials and methods.”
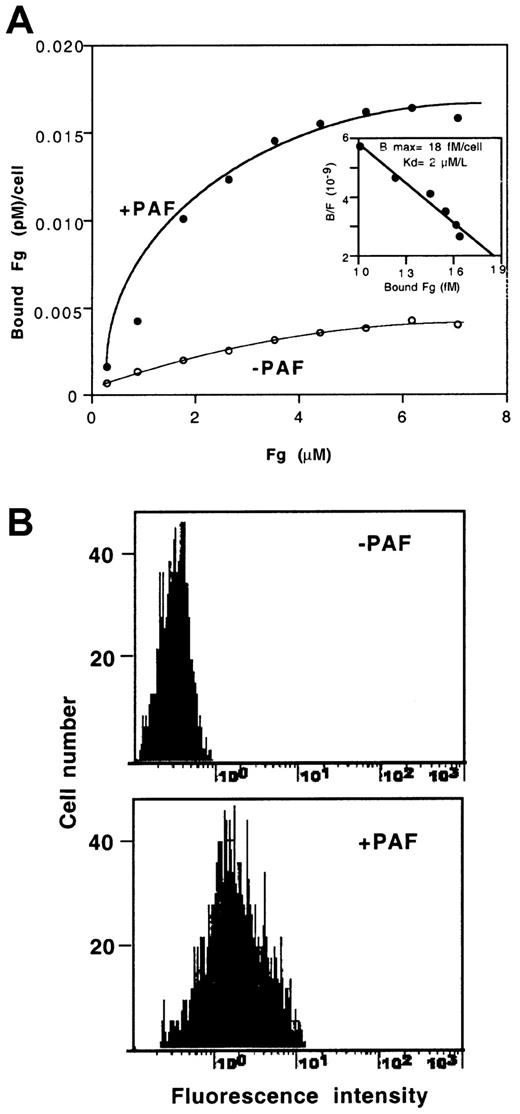
To assess the specificity of the PAF-induced aggregating response, we measured the binding of Fg and the activation-specific antibody PAC1 to CHO-αIIbβ3-PAFR cells. Figure 6A shows the FITC-Fg–binding isotherms in cells incubated with or without PAF. The αIIbβ3-dependent Fg binding was assessed by subtracting the fluorescence bound to cells treated with 10 mM EDTA. Exposure of cells to PAF led to a pronounced increase in the amount of Fg bound to the cells. Near saturation ligand binding was observed at approximately 4 μM Fg, which is within its physiologic range of plasma concentration. The dissociation constant (Kd) for Fg binding calculated by scatchard plot (Figure 6A, insert) was 2 μM. Taking into account that one molecule of Fg may bind 2 receptors, our results suggest that in PAF-stimulated cells all the αIIbβ3 receptor ligand sites would be occupied. The effect of PAF was also accompanied by a noticeable binding of the activation-dependent IgM PAC1 (Figure 6B).
Effect of PAF on binding of soluble Fg or PAC1 to CHO-αIIbβ3-PAFR cells.
(A) Binding of FITC-Fg to CHO-αIIbβ3-PAFR cells. Cells were incubated for 15 minutes with soluble FITC-Fg, in the presence or absence of 100 nM PAF, as described in “Materials and methods.” The data are means of 3 independent experiments performed in duplicate. The insert shows the Scatchard plot of data. (B) The histograms represent the expression of PAC1 binding sites in cells incubated with or without 100 nM PAF.
Effect of PAF on binding of soluble Fg or PAC1 to CHO-αIIbβ3-PAFR cells.
(A) Binding of FITC-Fg to CHO-αIIbβ3-PAFR cells. Cells were incubated for 15 minutes with soluble FITC-Fg, in the presence or absence of 100 nM PAF, as described in “Materials and methods.” The data are means of 3 independent experiments performed in duplicate. The insert shows the Scatchard plot of data. (B) The histograms represent the expression of PAC1 binding sites in cells incubated with or without 100 nM PAF.
The stimulation of PKC by PMA enhanced the adhesion of CHO-αIIbβ3-PAFR cells to immobilized Fg but it failed to induce cellular aggregation (results not shown). This observation implies that postreceptor activation of PKC is not a sufficient signal to promote cell aggregation. It also suggests that PAF-induced aggregation relies on a signaling event or events upstream of PKC activation. This assertion is supported by the lack of effect of PKC inhibitors, such as staurosporine, H7, or BIM-1, in preventing the PAF-induced cell aggregation and Fg binding to CHO-αIIbβ3-PAFR cells (Table1). The elevation of cytosolic-free Ca++ and activation of phospholipases are events distinctly associated with the activation of G-protein–linked surface receptors.21 To determine whether a perturbation of Ca++ homeostasis was involved in the PAF-induced cellular aggregation, we examined the effect of the calcium-calmodulin inhibitor W7 or the intracellular calcium chelator BAPTA. In both instances, the PAF-induced aggregation of CHO-αIIbβ3-PAFR cells was prevented and the Fg binding impeded (Table 1).
Differential effect of several treatments on the agonist-induced aggregation, adhesion, and fibrinogen binding to CHO cells coexpressing the human PAF and fibrinogen receptors
| . | Aggregation . | Adhesion . | Fg binding (%) . |
|---|---|---|---|
| PAF ant (200 nM) | − | − | ND |
| PTX (200 ng/mL) | + | − | ND |
| RGDS (1 mM) | − | − | 0 |
| H7 (25 μM) | + | − | 75 |
| Staurosporine (0.5 μM) | + | − | 73 |
| BIM-I (1 μM) | + | − | 91 |
| EDTA (5 mM) | − | − | 0 |
| W7 (20 μM) | − | + | 22 |
| BAPTA-AM (100 μM) | − | + | 0 |
| Genistein (100 μM) | + | + | 100 |
| PD98059 (50 μM) | + | + | ND |
| Ly294002 (20 μM) | + | + | ND |
| db-cAMP (500 μM) | + | + | ND |
| . | Aggregation . | Adhesion . | Fg binding (%) . |
|---|---|---|---|
| PAF ant (200 nM) | − | − | ND |
| PTX (200 ng/mL) | + | − | ND |
| RGDS (1 mM) | − | − | 0 |
| H7 (25 μM) | + | − | 75 |
| Staurosporine (0.5 μM) | + | − | 73 |
| BIM-I (1 μM) | + | − | 91 |
| EDTA (5 mM) | − | − | 0 |
| W7 (20 μM) | − | + | 22 |
| BAPTA-AM (100 μM) | − | + | 0 |
| Genistein (100 μM) | + | + | 100 |
| PD98059 (50 μM) | + | + | ND |
| Ly294002 (20 μM) | + | + | ND |
| db-cAMP (500 μM) | + | + | ND |
The effect of the different agents on the PAF-induced aggregation and adhesion of CHO-αIIbβ3-PAFR cells was assessed as described in “Materials and methods.” Positive (+) or negative (−) signs indicate the presence or absence of adhesion or aggregation. Fibrinogen binding is expressed as percent of the Fg bound to PAF-stimulated cells in the absence of antagonists.
CHO indicates Chinese hamster ovary; PAF, platelet-activating factor; PAF ant, PAF antagonist; PTX, pertussis toxin; RGDS, Arg-Gly-Asp-Ser; EDTA, ethylenediaminetetraacetic acid; ND, not determined.
Table 1 summarizes the effect of several agents on the PAF-induced stimulation of cell adhesion, aggregation, and Fg binding. A PAF antagonist prevented both adhesion and aggregation. Similar effects were observed by perturbing the state of association of the αIIbβ3 heterodimer by EDTA or by the presence of the ligand mimetic peptide RGDS. A parallel seems to exist between the Fg-binding and cell-aggregating responses. Data in Table 1 suggest that the PAF-induced aggregation is a process selectively regulated by the Ca++ signaling pathway, whereas the agonist-stimulation of cell adherence is controlled by the state of activation of PKC.
PAF-induced tyrosine phosphorylation of proteins
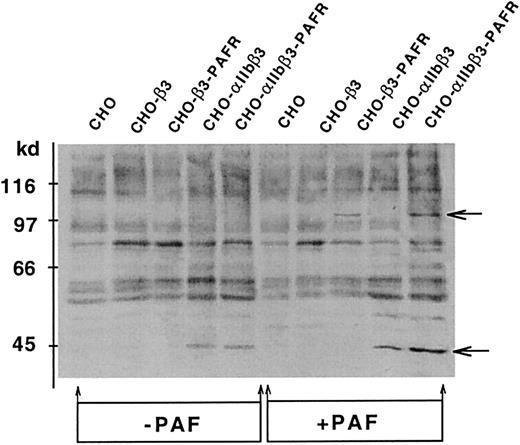
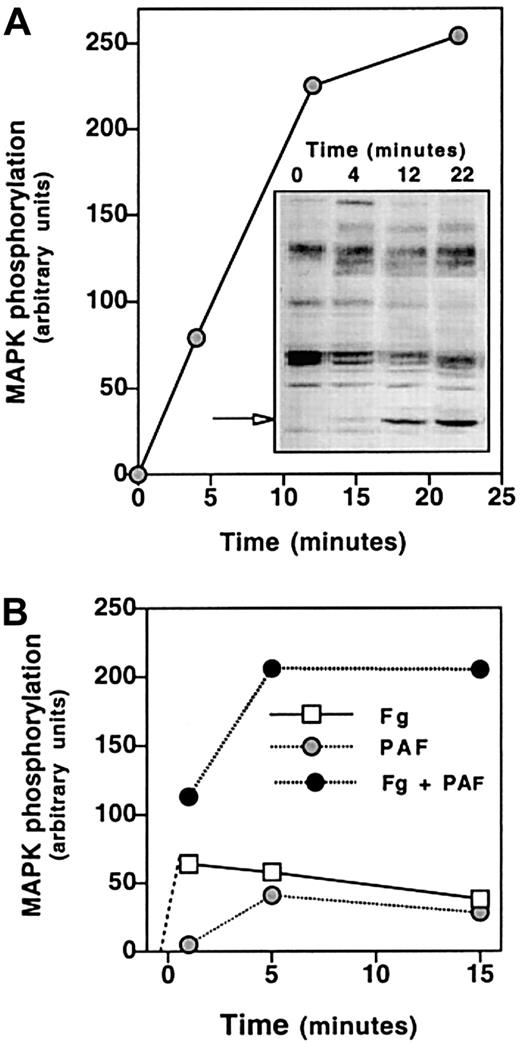
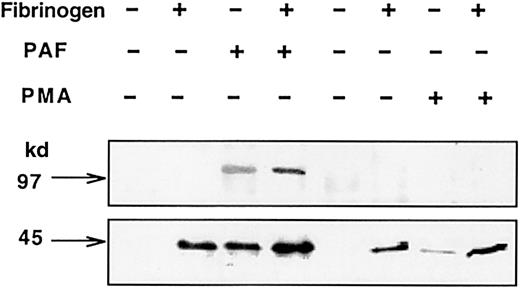
Signaling through αIIbβ3 is accompanied by tyrosine phosphorylation of proteins,9,10 and tyrosine kinase inhibitors prevent agonist-induced platelet aggregation.22Thus, we studied whether the PAF-induced activation of αIIbβ3 was accompanied by tyrosine phosphorylation of specific proteins. Data in Figure 7 show the antiphosphotyrosine reactivity of proteins from the indicated cell lines. PAF induced the phosphorylation of 2 proteins of approximately 100 kd and approximately 44 kd. The 100-kd protein is exclusively phosphorylated in cells expressing PAFR and, therefore, we will refer to it as the protein phosphorylated by agonist (p100-PPA). The PAF-induced phosphorylation of p100-PPA is more intense in CHO-αIIbβ3-PAFR than in CHO-β3-PAFR cells and is enhanced by the presence of Fg, suggesting an interaction between the PAF-stimulated signaling pathway and the Fg receptor. The 44-kd protein was identified as an isoform of the MAPK after immunoblotting with phospho-specific p44/42 MAPK antibody. Interaction with Fg phosphorylated MAPK in CHO-αIIbβ3 cells, whereas either PAF or Fg exerted a similar effect in CHO-αIIbβ3-PAFR. The effect of Fg builds up slowly and is maximal after at least 15 minutes of incubation (Figure8A). A half-maximal effect of Fg was observed at a concentration of at least 100 μg/mL. The effects of Fg and PAF in activating MAPK were synergic (Figure 8B). The PAF-induced phosphorylation of p100-PPA and MAPK was observed in either aggregating or adherent cells; however, in adherent cells the effect was transient and much less intense (results not shown).
Tyrosine phosphorylation induced by PAF in CHO cell lines.
Cells (2.5 × 106/mL) were incubated with 1 mg/mL Fg for 15 minutes at room temperature in a 250 μL final volume and then stimulated with 100 nM PAF and plated onto BSA-precoated wells. After 15 minutes, cells were lysed and proteins (50 μg) were separated by 6% to 16% SDS-PAGE, transferred to PVDF sheets and probed with antiphosphotyrosine mAb PY99, as described in “Materials and methods.” The arrows point to phosphorylated p100-PPA and MAPK proteins.
Tyrosine phosphorylation induced by PAF in CHO cell lines.
Cells (2.5 × 106/mL) were incubated with 1 mg/mL Fg for 15 minutes at room temperature in a 250 μL final volume and then stimulated with 100 nM PAF and plated onto BSA-precoated wells. After 15 minutes, cells were lysed and proteins (50 μg) were separated by 6% to 16% SDS-PAGE, transferred to PVDF sheets and probed with antiphosphotyrosine mAb PY99, as described in “Materials and methods.” The arrows point to phosphorylated p100-PPA and MAPK proteins.
Time course of Fg and/or PAF-induced tyrosine phosphorylation of proteins in CHO-αIIbβ3-PAFR cells.
(A) Cells (2.5 × 106/mL) were incubated with 100 nM PAF for 2 minutes at room temperature in a 250 μL final volume and then Fg was added at 1 mg/mL and cells were plated onto BSA-precoated wells. (B) Cells were incubated with 1 mg/mL Fg for 15 minutes at room temperature in a 250 μL final volume and then stimulated with 100 nM PAF and plated onto BSA-precoated wells. At the indicated times, cells were lysed and proteins (50 μg) were resolved by SDS-PAGE, transferred to PVDF membranes and probed with antiphosphotyrosine mAb PY99, as described in “Materials and methods.” The arrow points to the phosphorylated MAPK protein. The densitometric analysis was carried out using the public domain program NIH Image (http://rsb.info.nih.gov/nih-image/).
Time course of Fg and/or PAF-induced tyrosine phosphorylation of proteins in CHO-αIIbβ3-PAFR cells.
(A) Cells (2.5 × 106/mL) were incubated with 100 nM PAF for 2 minutes at room temperature in a 250 μL final volume and then Fg was added at 1 mg/mL and cells were plated onto BSA-precoated wells. (B) Cells were incubated with 1 mg/mL Fg for 15 minutes at room temperature in a 250 μL final volume and then stimulated with 100 nM PAF and plated onto BSA-precoated wells. At the indicated times, cells were lysed and proteins (50 μg) were resolved by SDS-PAGE, transferred to PVDF membranes and probed with antiphosphotyrosine mAb PY99, as described in “Materials and methods.” The arrow points to the phosphorylated MAPK protein. The densitometric analysis was carried out using the public domain program NIH Image (http://rsb.info.nih.gov/nih-image/).
Based on the differential effect of PMA on adhesion or aggregation, we studied its effects in phosphorylating p100-PPA and MAPK. Figure9 shows that PMA induced the phosphorylation of MAPK but not the phosphorylation of p100-PPA in CHO-αIIbβ3-PAFR cells, giving further support to the relationship between the activation of the PAF receptor and phosphorylation of p100-PPA. In the absence of Fg, PMA failed to induce a significant phosphorylation of MAPK. These observations seem to indicate that both PMA and PAF enhanced the Fg-mediated phosphorylation of MAPK. The ligand mimetic peptide RGDS neither stimulates nor potentiates the PAF-induced phosphorylation of MAPK. Neither genistein nor inhibitors of PKC alter the PAF-induced pattern of protein phosphorylation. In contrast, the calmodulin inhibitor W7 prevented the agonist-induced phosphorylation of both p100-PPA and MAPK and a similar but less intense effect was also produced by the intracellular calcium chelator BAPTA (data not shown). All other agents listed in Table 1 had no significant effects on the PAF-induced tyrosine phosphorylation of proteins.
Differential effects of PAF and PMA on tyrosine phosphorylation of p100-PPA and MAPK in CHO-αIIbβ3-PAFR cells.
Cells were incubated with either 100 nM PAF for 2 minutes or 100 nM PMA for 30 minutes. Then 1 mg/mL Fg was added and cells were plated onto BSA-precoated wells. After 15 minutes, cells were lysed and proteins (50 μg) were resolved by SDS-PAGE, transferred to PVDF membranes, and probed with antiphosphotyrosine mAb PY99, as described in “Materials and methods.”
Differential effects of PAF and PMA on tyrosine phosphorylation of p100-PPA and MAPK in CHO-αIIbβ3-PAFR cells.
Cells were incubated with either 100 nM PAF for 2 minutes or 100 nM PMA for 30 minutes. Then 1 mg/mL Fg was added and cells were plated onto BSA-precoated wells. After 15 minutes, cells were lysed and proteins (50 μg) were resolved by SDS-PAGE, transferred to PVDF membranes, and probed with antiphosphotyrosine mAb PY99, as described in “Materials and methods.”
Effect of PAF in inducing the phosphorylation of either the carboxyterminal end of β3 or FAK
It has been suggested that phosphorylation of the carboxyterminal end of β3 could play a role in determining the functional state of the αIIbβ3 complex.23 Immunoprecipitation with anti-β3 (P37) followed by blotting with antiphosphotyrosine or immunoprecipitation with antiphosphotyrosine followed by blotting with anti-β3 (P37) did not show differences in the phosphorylation state of β3 in CHO-αIIbβ3-PAFR cells with or without PAF stimulation. This observation agrees with the finding that mutations of tyrosine residues in the cytosolic end of β3 did not prevent the platelet aggregation in mice.24
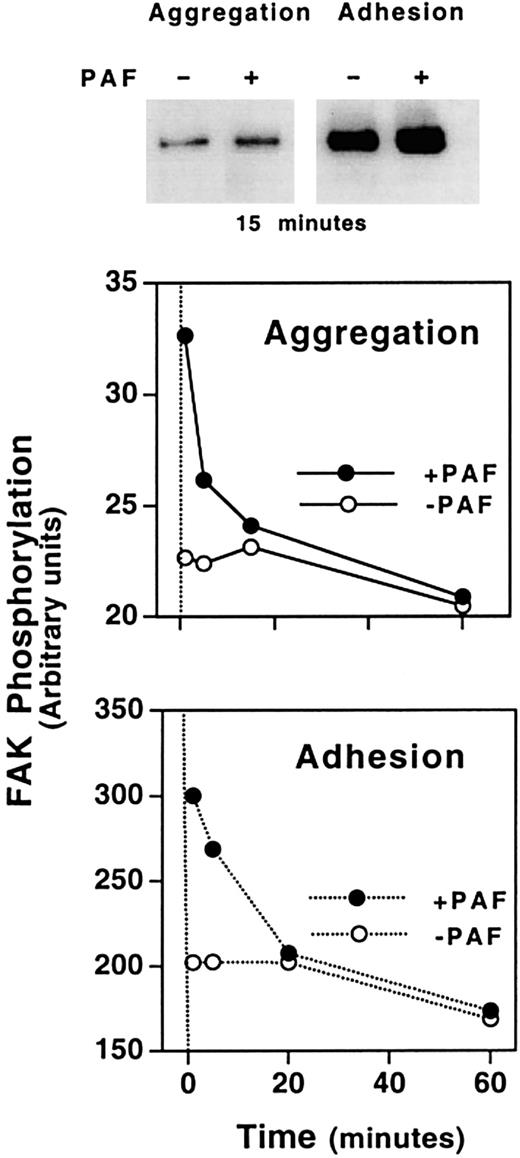
Focal adhesion kinase (FAK) is a 125-kd protein that becomes tyrosine phosphorylated in activated platelets. The phosphorylation of FAK was 4- to 5-fold higher in adherent than in aggregating CHO-αIIbβ3-PAFR cells (Figure 10, upper panel). In cell suspensions, PAF induced a phosphorylation of FAK within a minute of exposure to the agonist (Figure 10, middle panel). The intensity of this response was scarce and lasted less than 5 minutes. Adherent cells showed a rapid phosphorylation of FAK after cells seeding over an Fg-coated plate. The phosphorylation of FAK lasted for at least one hour and it was enhanced by PAF (Figure 10, lower panel). Thus, a correlation appears to exist between the phosphorylation of FAK and the PAF-induced adherence and spreading of cells.
Time course of PAF-induced tyrosine phosphorylation of FAK in CHO-αIIbβ3-PAFR cells in the presence of either soluble or solid-phase Fg.
In aggregation assays, cells were incubated with 1 mg/mL Fg for 15 minutes at room temperature and then were stimulated with 100 nM PAF and plated onto BSA-precoated wells. At the indicated times, cells were lysed and immunoprecipitation of FAK was performed using a polyclonal anti-FAK antibody. Immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine mAb PY99, as described in “Materials and methods.” In adhesion experiments, cells were added to Fg-coated wells and allowed to adhere for 15 minutes. Then 100 nM PAF was added and incubation continued until the indicate times. Cells were lysed and tyrosine phosphorylation of FAK was determined as above. The densitometric analysis was carried out using the public domain program NIH Image (http://rsb.info.nih.gov/nih-image/).
Time course of PAF-induced tyrosine phosphorylation of FAK in CHO-αIIbβ3-PAFR cells in the presence of either soluble or solid-phase Fg.
In aggregation assays, cells were incubated with 1 mg/mL Fg for 15 minutes at room temperature and then were stimulated with 100 nM PAF and plated onto BSA-precoated wells. At the indicated times, cells were lysed and immunoprecipitation of FAK was performed using a polyclonal anti-FAK antibody. Immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine mAb PY99, as described in “Materials and methods.” In adhesion experiments, cells were added to Fg-coated wells and allowed to adhere for 15 minutes. Then 100 nM PAF was added and incubation continued until the indicate times. Cells were lysed and tyrosine phosphorylation of FAK was determined as above. The densitometric analysis was carried out using the public domain program NIH Image (http://rsb.info.nih.gov/nih-image/).
Discussion
Agonist-induced activation of CHO cells expressing human recombinant αIIbβ3
As far as we know, attempts to reconstitute full functional human Fg receptors in established cell lines have not been totally successful. However, coexpression of αIIbβ3 with either the receptor for the agonist formyl Met-Leu-Phe (fMLP), or with the von Willebrand factor receptor, GPIb-IX complex, demonstrated that ligation of these receptors could influence the state of activation of αIIbβ3.11 13 The present study shows that the αIIbβ3 receptor can be fully activated by signals arising from liganded PAF receptors in CHO cells. The lack of effect of PAF on cell lines not expressing the PAF receptor (CHO or CHO-αIIbβ3 cells), and the inhibitory effects of PAF antagonists or αIIbβ3 blockade, support the specificity of the αIIbβ3 “activation.” To our knowledge, this is the first time that agonist-induced, soluble Fg-dependent aggregation of nonhematopoietic cells is demonstrated. The effect of PAF in activating αIIbβ3 was accompanied by enhanced binding of soluble fibrinogen and the activation-dependent IgM PAC1, suggesting that PAF may induce cell aggregation through conformational changes in αIIbβ3. Calculations based on the number of receptors and the kinetics of ligand binding indicate that PAF activation led to the occupancy of virtually all the Fg receptors in CHO-αIIbβ3-PAFR cells.
Our cell model has permitted the functional dissection of agonist-induced adhesive and aggregating responses and the identification of signaling events distinctly associated with each process. The PAF-induced cell aggregation was observed even though inhibitors of PKC prevented the stimulation of cell adherence to immobilized Fg. Conversely, the PAF-induced stimulation of cell adherence was not impeded when inhibitors of the calcium-calmodulin signaling pathway prevented the aggregating response. The physiologic significance of these findings is supported by the observation that inhibitors of Ca++-calmodulin prevented the PAF-induced stimulation of Fg binding to human platelets while the inhibitors of PKC did not (results not shown). A precedent of apparent dissociation between the agonist-induced stimulation of adherence and aggregation is implicit in the reported effect of thrombin in stimulating the adherence but not the aggregation of megakaryoblastic cells.25
Mechanism(s) of PAF-induced activation of αIIbβ3
Liganded PAFR are known to initiate Gi-sensitive and -insensitive signaling events. Platelets deficient in Gαq fail to aggregate in response to agonists26 and the thrombin receptor is coupled to Gαi.27 Thus, it seems plausible to assume that PAF may control the state of activation of αIIbβ3 through G-protein–coupled processes in CHO-αIIbβ3-PAFR cells. The herein reported results suggest that PAF may alter the state of activation of αIIbβ3 in a dual way: first, enhancing the adherence of cells to immobilized Fg in a PTX- and PKC-dependent manner; and second, in cell suspensions PAF enhances the binding of soluble Fg and PAC1, with the result of Ca++-calmodulin–dependent and PKC-independent cell aggregation. In this regard, the lack of effect of PTX on the PAF-induced aggregation is consistent with its failure to prevent the mobilization of Ca++ and phosphoinositides hydrolysis (results not shown).19,28 The PKC dependence of PAF-induced stimulation of cell adherence agrees with previous reports indicating that PKC regulates spreading of platelets29 and adhesion of cells to immobilized matrix proteins.29 30
Direct postreceptor activation of PKC by PMA was not a sufficient signal to elicit cell aggregation. Our data agree with the observation that PMA stimulates the integrin α5β1-dependent adhesion of CHO cells to immobilized fibronectin (Fn) with no change in Fn binding.31 Similarly, β3 mutations reduced αIIbβ3-dependent stabilization of cell adhesion to Fg without changing intrinsic Fg-binding affinity.32 Moreover, PMA stimulation of human megakaryoblastic cells expressing αIIbβ3 caused cells to adhere to immobilized Fg but did not support the binding of soluble Fg and aggregation.25 33
It is worth noting that CHO-αIIbβ3-PAFR cells adhere spontaneously to Fg-coated plates and PAF acts in enhancing the rate of this process. Unlike adherence, in the absence of PAF stimulation no aggregation was observed in cell suspensions in the presence of soluble Fg, suggesting that the limited amount of ligand bound under those conditions is not sufficient to support a significant rate of cell aggregation. The close relationship found between Fg-bound and cell-aggregating response supports the conclusion that PAF-induced aggregation is the result of enhanced binding of Fg to αIIbβ3.
Significance of PAF-induced tyrosine phosphorylation of proteins
The link between receptor-coupled G proteins and the activation of αIIbβ3 has not been yet elucidated. However, it is currently accepted that activation of nonreceptor protein tyrosine kinases (PTKs) and PI3-kinases could play an important role.22,34-36 In contrast to platelets and in agreement with a previous report,11 inhibition of PTKs by genistein did not prevent the agonist-induced activation of CHO-αIIbβ3-PAFR cells. Whether or not this is caused by a differential sensitivity of PTKs to genistein or by the specific inhibition of some phosphatases needs further investigation. Under our experimental conditions, we detected agonist- and/or fibrinogen-induced tyrosine phosphorylation of several proteins. The agonist-induced phosphorylation of FAK was prominent and persistent in cells adhered onto solid-phase Fg and almost absent in the presence of soluble Fg, in which case the agonist induced cell aggregation. The pattern of agonist-induced phosphorylation of FAK is consistent with its localization in focal adhesions and the postulate that ligation of αIIbβ3 is not a sufficient signal to elicit its phosphorylation.37,38 A similar differential effect of solid-phase or soluble Fg in triggering tyrosine phosphorylation of FAK has also been reported in the CMK megakaryoblastic cell line.33
A candidate to mediate the cross talk between PAF and αIIbβ3 receptors is the p100-PPA protein. The phosphorylation of p100-PPA was specifically associated with the engagement of the PAF receptor and enhanced by the simultaneous occupancy of αIIbβ3. A tyrosine-phosphorylated protein of similar apparent mass has been observed in human platelets and it was absent in a case of Glanzmann thombasthenia.39-41 Inhibition of the calcium-calmodulin signaling pathway impeded Fg binding, cell aggregation, and the phosphorylation of this protein, suggesting a relationship among these events.
Recombinant PAFR expressed in CHO cells has been reported to activate MAPK via PTX-sensitive and -insensitive G proteins in a manner that is not mediated by Ras.42 We observed a marked and sustained phosphorylation of MAPK distinctly associated with PAF-induced cell aggregation. Thus, a correlation exists between phosphorylation of p100-PPA and MAPK and the agonist-induced aggregation. Since the phosphorylation of these 2 proteins rely primarily on signals arising from the PAFR and αIIbβ3, it seems plausible to conclude that the PAF-induced aggregation is the result of coordinated signaling through both receptors.
We failed to detect changes in the phosphorylation of β3 associated with the activation of αIIbβ3. A similar finding has been reported in cells coexpressing αIIbβ3 and the fMLP receptor.11If these observations could be extrapolated to platelets they would indicate that phosphorylation of β3 is not needed to initiate platelet aggregation. This assertion is consistent with the fact that platelets expressing β3 in which the cytoplasmic tyrosine residues have been mutated show defective clot retraction but platelet aggregation was not prevented. Thus, phosphorylation of this subunit may act primarily in modulating the receptor responsiveness.
To conclude, a physiologic agonist like PAF can activate human αIIbβ3 receptor reconstituted in a rodent cell. In the presence of immobilized Fg, the PAF-mediated activation of αIIbβ3 leads to PKC-dependent enhancement of cell adherence and spreading accompanied by parallel changes in the phosphorylation of FAK. In the presence of soluble Fg, liganded PAFR enables the binding of PAC1 and enhances the binding of Fg to αIIbβ3, leading to Ca++-calmodulin–dependent cell aggregation and sustained phosphorylation of MAPK. The observation that Ca++-calmodulin inhibitors prevent the PAF-induced binding of Fg to human platelets suggests that our observations in CHO cells may be of physiologic significance. In any case, availability of cell lines coexpressing human αIIbβ3 and a surface receptor for a physiologic agonist offers a sound experimental model to further investigate the complex interactions between receptors, ligands, and signaling pathways controlling the state of activation of platelets.
We are grateful to Prof Craig Gerard (Harvard Medical School, Boston, MA) for the gift of the plasmid pBC12B1 containing the cDNA for the human platelet–activating factor receptor. mAbs directed against β3 were the gift of Drs Maria Victoria Alvarez and Jose Gonzalez, from the Rocasolano Institute of the Spanish Council of Research. A polyclonal antibody against PAF receptor was kindly provided by Dr Marek Rola-Pleszczynski, from the University of Sherbrooke, Canada.
Supported in part by grants from the Dirección General de Investigación Cientı́fica y Técnica (DGICYT PB97-1240, SAF 2000-0127 and DGICYT PM97-0016), Fondo de Investigaciones Sanitarias (96/2014), and Comunidad Autónoma de Madrid (08.4/0031/1998). L.S. was supported by a grant-in-aid from the Agencia Española de Cooperación Internacional (AECI).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Roberto Parrilla, Centro de Investigaciones Biológicas (CSIC), Velázquez 144, 28006-Madrid, Spain; e-mail: rparrilla@cib.csic.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal