Abstract
Self-renewal, pluripotency, and long-term reconstitution are defining characteristics of single hematopoietic stem cells.Pax5−/− precursor B cells apparently possess similar characteristics. Here, using serial transplantations, with in vitro recloning and growth of the bone marrow–homed donor cells occurring after all transplantations, we analyzed the extent of self-renewal and hematopoietic multipotency ofPax5−/− precursor B-cell clones. Moreover, telomere length and telomerase activity in these clones was analyzed at various time points. Thus far, 5 successive transplantations have been performed. Clones transplanted for the fifth time, which have proliferated for more than 150 cell divisions in vitro, still repopulate the bone marrow with precursor B cells and reconstitute these recipients with lymphoid and myeloid cells. During this extensive proliferation, Pax5−/− precursor B cells shorten their telomeres at 70 to 90 base pairs per division. Their telomerase activity remains at 3% of that of HEK293 cancer cells during all serial in vivo transplantations/in vitro expansions. Together, these data show thatPax5−/− precursor B-cell clones possess extensive in vivo self-renewal capacity, long-term reconstitution capacity, and hematopoietic multipotency, with their telomeres shortening at the normal rate.
Introduction
Mature cells of hematopoietic lineages have limited lifespans. Hence, to maintain their peripheral pools, they have to be generated throughout life. All cells of the hematopoietic system develop from pluripotent hematopoietic stem cells (HSCs). A single HSC can repopulate all blood cell lineages.1-3 Two of the 3 basic properties of HSCs, self-renewal and pluripotency, can be tested by in vivo transplantation into hematopoietically deficient, ie, lethally irradiated, hosts4 or by in vitro proliferation of the HSCs, followed by differentiation into distinct hematopoietic lineages under appropriate tissue-culture conditions.5 The third basic property, the capacity for long-term reconstitution, requires that, upon transplantation, HSCs home back to their appropriate sites in the bone marrow, from where they can be reisolated, recloned in vitro, and then retransplanted into secondary and subsequent hosts.
Experimentally, 2 types of HSC have been identified. The one, short-term HSC (ST-HSC), possesses limited self-renewal potential, pluripotency, and only short-term reconstitution capacity, while the other, long-term HSC (LT-HSC) has long-term self-renewal and hematopoietic reconstitution capacity.6 While HSCs have been found to persist throughout life in almost constant numbers,7,8 and while 3 to 5 consecutive transplantations of HSCs into lethally irradiated hosts have suggested that they might undergo 80 to 200 divisions,9-13 their in vitro capacity for self-renewal appears limited to about 20 cell divisions.14 Therefore, the self-renewal capacity of LT-HSCs appears to be strongly dependent on conditions, which can be provided by the host in vivo, but are insufficient with current in vitro culture conditions.
Normal somatic cells have a finite replicative lifespan.15Human fibroblasts have been found to undergo 50 ± 10 divisions, after which they lose the capacity to replicate and enter a state of senescence. One of the mechanisms by which this biological clock is thought to function is the shortening of telomeres with each cell division. Telomeric DNA capping the ends of chromosomes consists of TTAGGG repeats, which are thought to protect the chromosomes from illegitimate recombination, to localize the chromosomes within the nucleus, and to regulate the replication of chromosomes.16The normal rate of telomere loss has been measured as 50 to 100 base pairs (bp) per cell division,17 although accelerated losses have also been observed.18 Telomere loss can be counteracted by the enzyme telomerase, which synthesizes telomeric repeats and, hence, can lengthen telomeres.19Overexpression of telomere reverse transcriptase, the catalytic subunit of telomerase, in human somatic cells has been shown to result in elongation of telomeres and thereby extension of the lifespan of these cells.20 High telomerase activity has been detected in germ line cells, in some somatic cells, in cancer cells, and, more relevant to the present study, in LT-HSCs, while ST-HSCs have significantly less.21 By compensating for telomere losses, telomerase is thought to be able to maintain the long-term self-renewal capacity of LT-HSCs in vivo. However, extensive telomere shortening in HSCs upon serial transplantation has been reported.22
The chromosomes of different species have telomeres of different length. While human telomeres are around 6 kb long, mice have telomeres approximately 10 times longer; telomeres in mice can be 60 kb and longer in germ cells and primary HSCs.23 Hence, at normal rates of telomere losses and with insufficient telomerase activity, it would take human cells 60 divisions and mouse cells 600 divisions to lose their telomeres entirely.
Precursor B cells from Pax5-deficient mice are blocked in B-cell development at the stage of a DHJH-rearranged pre-B I cell.24These Pax5−/− pre-B I cells can be grown on stromal cells in the presence of interleukin-7 (IL-7),25are capable of in vitro differentiation to natural killer (NK) cells, dendritic cells (DCs), and myeloid cells26; of in vivo reconstitution of the pre-B I cell compartment in bone marrow; and of in vivo differentiation to thymocytes and T cells,27 as well as to NK cells, DCs, myeloid cells, and even erythrocytes.28 Hence,Pax5−/− pre-B I cells are flexible in their differentiation capabilities in a hematopoietically multipotent fashion.
In this paper, we investigate the replication capacity of clones of pre-B I cells from Pax5-deficient mice in vitro, and we measure their telomere length and telomerase activity and the relation of these to the numbers of cell divisions. Moreover, we analyze the ability of these clones to home back to the bone marrow upon intravenous tail injection and to repopulate the pre-B cell compartment as a measure of their long-term reconstitution capacity. Finally, we determine the in vivo potential of these clones to differentiate to thymocytes and mature T cells and, in some cases, also to NK cells, DCs, and other myeloid cells as a measure of their multipotent hematopoietic capacity during serial transplantations, with in vitro recloning of the bone marrow–homed donor cells occurring after all transplantations. We show here the results of 5 such repetitive in vivo transplantation/in vitro growth steps, which have expanded the originalPax5−/− pre-B I cell clones by at least 150 to 180 cell divisions. The results of our experiments document an impressive capacity of Pax5−/− pre-B I cells for self-renewal, long-term reconstitution, and multipotency, which are the properties of long-term repopulating, multipotent HSCs.
Materials and methods
Mice
RAG2−/− mice were originally provided by Dr F. Alt (Boston, MA). Balb/c RAG2−/− mice were generated at the Basel Institute for Immunology by backcrossing the RAG2 mutation into Balb/c mice for 15 generations. C57BL/6 RAG2/IL-2Rγ–chain double-deficient(RAG2γc−/−) mice were purchased from Taconic Farms (Germantown, NY).
Establishment of pre-B I cell clones/subclones
Primary pre-B I cell clones fromPax5−/− mice24 and wild-type mice, as well as Pax5−/−B220+c-Kit+ pre-B I cell subclones from the bone marrow of transplanted hosts were established as described.25,29 Primary Pax5−/−pre-B I cell clones were infected with a retrovirus encoding green fluorescent protein (GFP) as described.28
Transplantation of Pax5-deficient pre-B I cell clones
Balb/c RAG2−/− orRAG2γc−/− mice at 8 to 12 weeks of age were γ-irradiated with 400 rad, and 107 in vitro–grown Pax5−/− pre-B I cells were injected intravenously 6 to 8 hours after irradiation. Cell suspensions of various lymphoid organs were prepared by standard protocols as described.28
Carboxyfluorescein succinimidyl ester labeling
Carboxyfluorescein succinimidyl ester (CFSE) labeling of cells was performed as described.30 Briefly, cells were resuspended in phosphate-buffered saline at 5 × 107/mL. CFSE was added to a final concentration of 2.5 μM, and the cell suspension was incubated for 10 minutes at room temperature. At the end of the incubation period, the cells were washed 3 times.
Flow cytometry
Measurement of telomere length
The average length of telomere repeats in wild-type andPax5−/− pre-B I cell clones/subclones was determined by flow fluorescence in situ hybridization as described.31
Measurement of telomerase activity
Telomerase activity in individual wild-type andPax5−/− pre-B I cell clones/subclones was measured by photometric enzyme immunoassay with the use of the telomeric repeat amplification protocol as recommended by the manufacturer (Roche Moleular Biochemicals, Rotkreuz, Switzerland). Fluorescence intensity was measured as OD405nm-600nm.
Results
In vitro replication capacity of Pax5-deficient pre-B I cell clones
To test the capacity of a series of clones of pre-B I cells fromPax5−/− mice, CD19−B220+c-Kit+ cells from bone marrow were single cell–sorted by fluorescence-activated cell sorter (FACS) and grown on stromal cells in the presence of IL-7 to approximately 109to 1010 cells within 4 to 5 weeks by subculturing the expanding cells every 3 days on new stromal cells in fresh medium at a suitable concentration.29 Under these culture conditions, approximately 1 of 3 single ex vivo isolated cells establishes good clonal growth, comparable in plating efficiency to fetal liver–derived pre-B I cells with the same B220+c-Kit+phenotype from wild-type mice (Rolink et al27 and data not shown). Growth of Pax5-deficient pre-B I cell clones was monitored by cell counting, which allowed us to estimate the number of cell divisions each clone underwent (Figure1). We conclude that bone marrow–derivedPax5−/− pre-B I cell clones have plating efficiencies ex vivo and show proliferative capacity in vitro that are comparable to fetal liver–derived wildtype pre-B I cells.
In vitro proliferation capacity ofPax5−/− pre-B I cell clones.
Five randomly chosen Pax5−/− pre-B I cell clones were grown on stromal cells in the presence of IL-7 followed by intravenous injection into appropriate hosts. About 4 weeks later, thePax5−/− pre-B I cells were reisolated from the bone marrow of the recipient animals by single-cell FACS, regrown in vitro, and retransplanted. The numbers of in vitro cell divisions were estimated by cell counting during the in vitro expansion phases. The different symbols represent different clones.
In vitro proliferation capacity ofPax5−/− pre-B I cell clones.
Five randomly chosen Pax5−/− pre-B I cell clones were grown on stromal cells in the presence of IL-7 followed by intravenous injection into appropriate hosts. About 4 weeks later, thePax5−/− pre-B I cells were reisolated from the bone marrow of the recipient animals by single-cell FACS, regrown in vitro, and retransplanted. The numbers of in vitro cell divisions were estimated by cell counting during the in vitro expansion phases. The different symbols represent different clones.
In vivo repopulation of bone marrow by GFP-expressing Pax5−/− pre-B I cell clones, and recloning from the bone marrow of the transplanted host
We infected suitable numbers of Pax5−/−pre-B I cells from several clones with a GFP-expressing retrovirus. Previously, we had observed that GFP-expressingPax5−/− pre-B I cells repopulate the pre-B I cell compartment of B220+c-Kit+ cells in the bone marrow of RAG2−/− hosts, so that approximately 5% to 10% of the lymphoid cells appear GFP+.27 At 3 weeks after transplantation, this was also observed in the present study with all analyzed clones of GFP-expressing Pax5−/−pre-B I cells (data not shown). This repopulating capacity of thePax5−/− pre-B I cell clones remained upon secondary and subsequent transplantations (see below).
The Pax5−/− pre-B I cells were reisloated from bone marrow of transplanted hosts by single-cell FACS sorting and regrown in tissue culture on stromal cells in the presence of IL-7. After 30 to 35 divisions in vitro, the pre-B I cell subclones were retransplanted into new immunodeficient hosts and again reisolated ex vivo after 3 weeks as single B220+c-Kit+GFP+ pre-B I cells to be regrown in tissue culture. So far, this has been repeated 5 times. The proliferative capacity of Pax5−/− pre-B I cell clones in vitro (with 30 to 35 divisions in 4 to 5 weeks) remained nearly unchanged for all clones tested (Figure 1). Plating efficiency on stromal cells in the presence of IL-7 also remained high.
We conclude that the Pax5−/− pre-B I cell clones, which we established in tissue culture and upon consecutive in vivo transplantations, underwent at least 150 to 180 cell divisions in vitro without losing either their self-renewal or their long-term bone marrow reconstitution capacity. It should be noted here that this number of cell divisions is based only on the recovery of live cells after in vitro culture. For this reason, this number is a low estimate since cells also die in culture. Moreover, the divisions thatPax5−/− pre-B I cells might undergo in vivo are not included in this calculation (see below).
In vivo dividing, transplanted Pax5−/−pre-B I cell clones
Proliferative expansion of the Pax5−/−pre-B I cell clones is shown in Figure 1 with data obtained by cell counting in vitro. In Figure 1, it is assumed that these cell clones do not proliferate in vivo in the bone marrow of the host. However, we know that, 3 to 4 weeks after transplantation, between 10% and 15% of the GFP+Pax5−/− pre-B I cells in bone marrow are in S, G2, or M phases of the cell cycle (Rolink et al27 and data not shown). To estimate the proliferative capacity of the Pax5−/− pre-B I cell clones in vivo, in vitro–grown cell clones expressing the major histocompatibility complex allele H-2Kb were labeled with CFSE and transferred into Balb/c RAG2−/− hosts expressing the major histocompatibility complex allele H-2Kd. To compare the in vivo proliferation capacity of these cells to the in vitro capacity, an aliquot of the labeled cells was cultured on stromal cells in the presence of IL-7.
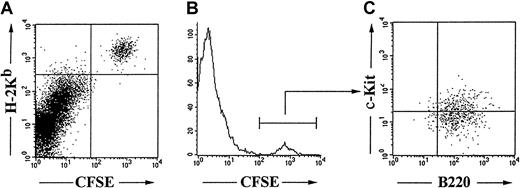
Dividing Pax5−/−-derived cells were found in the bone marrow. They were B220+c-Kit+ cells, hence phenotypically indistinguishable from the transplanted cell clones (Figure 2). Day-by-day analysis of the CFSE-labeled Pax5−/− pre-B I cells in bone marrow indicates that the clones of Pax5−/−pre-B I cells also divide in vivo, with cell-cycle times around 18 hours (Figure 3). Furthermore, it became evident that practically all CFSE-labeledPax5−/− pre-B I cells in vivo have proliferation kinetics that are similar to those in tissue culture at least for the time period for which CFSE-labeled cells could be followed, ie, for up to 5 days (data not shown).
Phenotype of in vivo proliferating Pax5−/−pre-B I cells.
CFSE-labeled H-2KbPax5−/− pre-B I cells were transplanted into H-2Kd Balb/c RAG2−/− hosts, and the bone marrow was analyzed 2 days after transplantation.
Phenotype of in vivo proliferating Pax5−/−pre-B I cells.
CFSE-labeled H-2KbPax5−/− pre-B I cells were transplanted into H-2Kd Balb/c RAG2−/− hosts, and the bone marrow was analyzed 2 days after transplantation.
Self-renewal capacity of Pax5−/− pre-B I cells in bone marrow.
CFSE-labeled Pax5−/− pre-B I cells were transplanted into RAG2−/− host, and the fluorescence intensity of the bone marrow–homed cells was determined after 3 days (red) and 4 days (brown). Fluorescence intensity after 1-day proliferation (black) in vitro is shown for comparison.
Self-renewal capacity of Pax5−/− pre-B I cells in bone marrow.
CFSE-labeled Pax5−/− pre-B I cells were transplanted into RAG2−/− host, and the fluorescence intensity of the bone marrow–homed cells was determined after 3 days (red) and 4 days (brown). Fluorescence intensity after 1-day proliferation (black) in vitro is shown for comparison.
We conclude that when we include the in vivo analyses, the proliferation capacity of Pax5−/− pre-B I cell clones is possibly much higher than described above and shown in Figure1. If these Pax5−/− pre-B I cell clones continued to divide for the 3 weeks in vivo as we observed them to do for the first 5 days, then by the 40th week in their life, they could have divided as many as 300 to 400 times.
Reduction of telomere length in Pax5−/− pre-B I cell clones during in vitro/in vivo proliferation
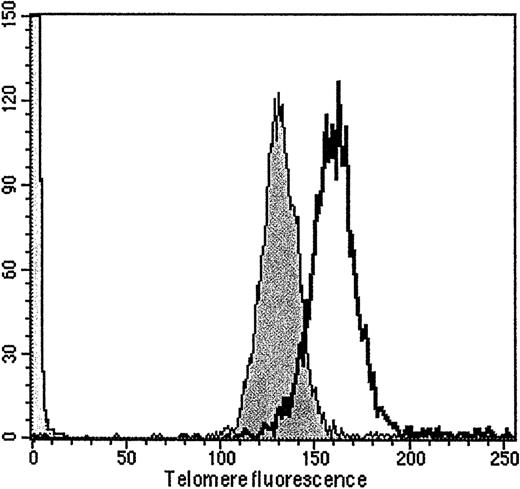
Shortening of telomeres is thought to be a determining factor for the finite replicative lifespan of normal somatic cells.32,33 It has been suggested that mechanisms counteracting telomere shortening operate in HSCs.21 SincePax5−/− pre-B I cells, like HSCs, possess long-term self-renewal capacity, we determined their telomere length with increasing time of proliferation in vitro and in vivo. Data obtained by flow fluorescence in situ hybridization clearly demonstrate that in Pax5−/− pre-B I cells, telomere lengths decrease with time of proliferation (Figure4). On the basis of the in vitro cell proliferation only (Figure 1), the Pax5−/−pre-B I cell clones reduced their telomere length by 70 to 90 bp per cell division. If telomere length were also reduced by cell divisions in vivo and if the cells continued to divide throughout the entire time that they reside in bone marrow and at the same rate as in vitro, then the rate of telomere reduction could be as little as half, ie, 35 to 45 bp per division. Normal somatic cells shorten their telomeres by 50 to 100 bp per division.17 Therefore, mechanisms counteracting telomere shortening seem not to be strong inPax5−/− pre-B I cells.
Telomere length of one representative serially transplanted Pax5−/− pre-B I cell clone.
Telomere length after 35 (61.5 kb, open) and after 192 (51.1 kb, shaded) enumerated in vitro cell divisions.
Telomere length of one representative serially transplanted Pax5−/− pre-B I cell clone.
Telomere length after 35 (61.5 kb, open) and after 192 (51.1 kb, shaded) enumerated in vitro cell divisions.
Telomerase activity in Pax5−/− pre-B I cell clones
Telomerase activity has been proposed to counteract telomere shortening.21 Therefore, we tested telomerase activity in wild-type and Pax5−/− pre-B I cell clones using HEK293 cancer cells as high telomerase–expressing control cells. The results of our analyses indicate that telomerase activity in Pax5−/− pre-B I cells is about 30-fold lower than in HEK293 cells (Figure 5). Furthermore, telomerase activity in wild-type and Pax5−/− pre-B I cell clones is not significantly different and does not change with in vitro or in vivo proliferation (Figure 5, and data not shown). We conclude that this low telomerase activity is apparently unable to avert telomere shortening at normal or even slightly elevated levels in Pax5−/− pre-B I cells.
Telomerase activity in one serially transplantedPax5−/− pre-B I cell clone.
Photometric enzyme immunoassay with telomeric repeat amplification was used to determine telomerase activity in (●) the primaryPax5−/− pre-B I cell clone after 35 divisions; (⧫) hexagonary Pax5−/− pre-B I cell clone after 192 divisions in vitro; and (Δ) HEK293 cells (positive control) or (■) heat-inactivated HEK293 cells (negative control).
Telomerase activity in one serially transplantedPax5−/− pre-B I cell clone.
Photometric enzyme immunoassay with telomeric repeat amplification was used to determine telomerase activity in (●) the primaryPax5−/− pre-B I cell clone after 35 divisions; (⧫) hexagonary Pax5−/− pre-B I cell clone after 192 divisions in vitro; and (Δ) HEK293 cells (positive control) or (■) heat-inactivated HEK293 cells (negative control).
Stability and changes in multipotency of hematopoietic differentiation capacity of Pax5−/− pre-B I cell clones upon serial in vivo transplantation and in vitro replication
During serial in vivo transplantation and in vitro growth ofPax5−/− pre-B I cell clones, which were recloned ex vivo after each in vivo transplantation step, hematopoietic differentiation capacity was measured by in vivo differentiation to T-cell receptor (TCR)αβ– and TCRγδ–expressing thymocytes, to NK T cells, and, in some cases, to NK cells, DCs, and even myeloid cells or, rarely, erythrocytes.
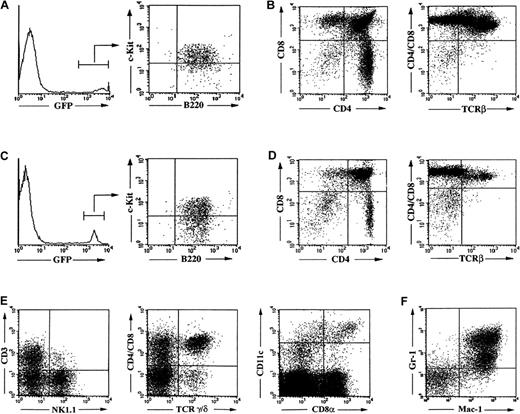
In all serial transplantations, the B220+c-Kit+pre-B I cell compartment in bone marrow is repopulated by the transplanted GFP-expressing Pax5−/− pre-B I cells (Figure 6A,C). However, with each subsequent transplantation, we observed a gradual reduction in the cellularity of the thymus from a total of 49.2 × 106thymocytes after the first transplantation into RAG2γc−/− hosts to a total of 2.7 × 106 cells after the fourth transplantation (Figure6B,D). Most of the losses appeared to be accountable for losses in the TCRαβ–expressing T-cell compartments. Throughout all serial transplantations, ie, even in the fifth consecutive hosts, TCRγδ–expressing thymocytes, NK-T cells, and NK cells, DCs, and myeloid cells were detectable in apparently similar numbers.
Reconstitution and hematopoietic differentiation capacity of Pax5−/− pre-B I cells upon serial in vivo transplantation and in vitro expansion.
Flow cytometric analysis of bone marrow (panel A) and thymus (panel B) 3 weeks after reconstitution with a primary GFP+Pax5−/− pre-B I cell clone. Flow cytometric analysis of bone marrow (panel C) and thymus (panel D) 3 weeks after transplantation of a quarternary GFP+Pax5−/− pre-B I cell clone. Flow cytometric analysis of reconstitution of the γδ TCR-expressing thymocyte, the NK T cell, NK cell, and CD8− and CD8+ DC compartments in the thymus (panel E) and of the myeloid compartment (gated on GFP-expressing cells) in the spleen of a fifth serial GFP+Pax5−/− clone-transplant recipient that lacks αβ TCR-expressing thymocytes (panel F).
Reconstitution and hematopoietic differentiation capacity of Pax5−/− pre-B I cells upon serial in vivo transplantation and in vitro expansion.
Flow cytometric analysis of bone marrow (panel A) and thymus (panel B) 3 weeks after reconstitution with a primary GFP+Pax5−/− pre-B I cell clone. Flow cytometric analysis of bone marrow (panel C) and thymus (panel D) 3 weeks after transplantation of a quarternary GFP+Pax5−/− pre-B I cell clone. Flow cytometric analysis of reconstitution of the γδ TCR-expressing thymocyte, the NK T cell, NK cell, and CD8− and CD8+ DC compartments in the thymus (panel E) and of the myeloid compartment (gated on GFP-expressing cells) in the spleen of a fifth serial GFP+Pax5−/− clone-transplant recipient that lacks αβ TCR-expressing thymocytes (panel F).
Previously, we have shown that 4 to 6 months after transplantation of GFP-expressing Pax5−/− pre-B I cells, few donor-derived GFP+Ter119+ erythrocytes (0.15% to 3% of all erythroid cells in the blood) could be observed in fewer than 15% of the recipient animals.28 The same low numbers (and low frequency) of GFP+Ter119+ erythrocytes were also present in the few serially transplanted mice tested as late as 4 to 6 months after receiving the transplant (data not shown).
Of the Pax5−/− pre-B I cell subclones established from the fourth host, only 2 of 5 clones could reconstitute all tested lineages while the other 3 clones no longer developed TCRαβ–expressing T cells in the fifth consecutive host. In the same TCRαβ–expressing T cell–deficient hosts, we could still find TCRγ/δ–expressing thymocytes, NK T cells, NK cells, DCs (Figure6E), and even myeloid cells (Figure 6F).
We conclude that the self-renewing long-term reconstitutingPax5−/− pre-B I cell clones also remain multipotent for these long proliferative phases of their life with the exception of their capacity to develop the TCRαβ–expressing compartments. This selective loss of TCRαβ–expressing thymocyte development remains to be investigated on a molecular genetic basis.
In summary, our experiments show that Pax5−/−pre-B I cell clones retain self-renewal and long-term reconstitution capacity for several weeks and months of in vitro and in vivo proliferation in which they lose more than half of their telomere lengths. During the same time, they retain a large part of their hematopoietic multipotency.
Discussion
This study has investigated the self-renewal capacity, long-term bone-marrow reconstitution capacity, and in vivo multipotency of 5 randomly chosen Pax5−/− pre-B I cell clones, established from the CD19−B220+c-Kit+ cell population of bone marrow of these mutant mice by single-cell cloning. We have shown that all clones display the same remarkable capacity to proliferate for at least 150 to 180 cell divisions in vitro on stromal cells in the presence of IL-7. All clones are perfectly capable of homing back to the pre-B I cell compartment in bone marrow following transplantation intravenously, of remaining reclonable in vitro, of dividing again, and of surviving the in vitro growth/in vivo homing procedure for at least 5 times. Since we have established these clones by random selection from ex vivo explantedPax5−/− pre-B I cells at high plating efficiency, it is very likely that the vast majority of allPax5−/− pre-B I cells have these properties.
The efficiency of cloning of the CD19−B220+c-Kit+ pre-B I cells isolated from bone marrow of Pax5−/− mice remains about 30% for the 5 in vitro expansion/in vivo homing series.Pax5−/− pre-B I cell clones proliferate for at least 150 to 180 divisions in vitro and keep their phenotype during this proliferative expansion. The high efficiency of cloning and the prolonged capacity of proliferating in vitro is shared byPax5−/− pre-B I cells isolated from bone marrow and wild-type pre-B I cells isolated from fetal liver but not from bone marrow.34 It should be remembered thatPax5−/− fetal liver does not contain any detectable clonable pre-B I cells.25
While Pax5−/− pre-B I cells efficiently home to the bone marrow upon transplantation, neither fetal liver– nor bone marrow–derived wild-type pre-B I cells can do so. Thus wild-type andPax5−/− pre-B I cells share extensive self-renewal capacity, but only Pax5−/− pre-B I cells have, and retain, long-term bone marrow reconstitution capacity.
Wild-type and Pax5−/− pre-B I cells differ in their hematopoietic differentiation capacity. Wild-type cells have the overwhelming, apparently singular, capacity to differentiate to B-lineage cells when they are induced by removal of IL-7 in vitro or when they are transplanted into immunodeficient hosts.34By contrast, Pax5−/− pre-B I cells are blocked in their capacity to differentiate to more mature B-lineage cells in vitro or in vivo. Instead, they can give rise in vitro to myeloid cells, such as macrophages, granulocytes, and osteoclasts; to DCs; and to NK cells.26 In vivo they can differentiate to all these cell types and also to erythrocytes and to thymocytes and mature T cells.27,28 While all RAG2−/−hosts transplanted with primary Pax5−/− pre-B I cell clones develop T-lineage cells, only approximately half of all recipients develop myeloid cells and fewer than 15% show erythroid development.28 From these experiments, it has previously been concluded that Pax5−/− pre-B I cells also have the third capacity of HSCs: that of hematopoietic multipotency.
Are these properties of HSCs retained or lost upon repeated in vitro proliferation/in vivo homing steps? The first property, self-renewal, appears to be retained at constant rates of proliferation with a stable phenotype and with essentially unchanged high cloning efficiencies. This also appears to be the case for the long-term reconstitution capacity in the bone marrow pre-B I cell compartment, with even a suggestion that the strength of the Pax5−/−pre-B I cells is superior to that of the wild-type compartments in the hosts. Hematopoietic multipotency, however, appears not to be as stable. Thus, the cellularity of the thymus populated byPax5−/− pre-B I cell–derived cells in the host decreases with increasing numbers of repetitive transplantations and is even lost after the fifth consecutive transplantation. This apparent loss of thymopoiesis, ie, the capacity to produce TCRαβ–expressing T cells, by the dividing and homingPax5−/− pre-B I cells is currently not understood, and experiments are underway to identify defects on the molecular and cellular levels that contribute to this evolving deficiency, including the possible involvement of increasing telomere shortening. On the other hand, other differentiation capacities appear not to change or decrease dramatically, since after the fifth consecutive transplantation, the reisolatedPax5−/− pre-B I cells from all clones tested are still perfectly capable of differentiating to cells of the myeloid lineage, to DCs, and to NK cells in vitro (data not shown).
Overall, Pax5−/− pre-B I cell clones appear to have all the properties of HSCs, ie, the capacity of self-renewal, long-term reconstitution, and multipotency. In contrast to HSCs, which have a limited capacity to proliferate under similar in vitro culture conditions,14Pax5−/− pre-B I cell clones can be grown on stromal cells in the presence of IL-7 for extended periods, retaining their reconstitution and multipotent potential.
In a normal steady-state condition, about 8% of LT-HSCs enter cell cycle each day and approximately 5% of LT-HSCs are in S, G2, or M phases of the cell cycle.35 However, the number of cycling HSCs is increased to approximately 10% upon transplantation and remains elevated for several months.22Our observation that 10% to 15% of Pax5−/−pre-B I cells are in cell cycle 3 to 4 weeks after transplantation is in absolute accordance with their finding. However, in contrast to HSCs, which apparently lose telomere length at an increasing rate with each successive transplantation,22 transplantedPax5−/− pre-B I cell clones apparently lose telomeres at indistinguishable rates during the series of 5 in vivo transplantations/in vitro expansions, ie, between 35 and 90 bp per division, depending on how many divisions the cells may undergo in vivo. This is considered a normal rate of telomere loss in somatic cells, which cannot restore the shortening by de novo telomere synthesis with telomerase.16 Our results indicate that Pax5−/− pre-B I cells might differ from normal LT-HSCs in the rate of telomere loss during serial transplantations.16 We should mention that we are as yet unable to attribute the rate of telomere loss to either in vitro proliferation, in vivo transplantation, or, more likely, a combination of both. Hence, it is not yet possible to fairly compare thePax5−/− pre-B I cell clones with the HSCs during serial transplantation without interspersed in vitro expansion.22 This problem requires a more detailed, more quantitative analysis of telomere loss inPax5−/− pre-B I cell clones in vivo and in vitro; this is currently being undertaken.
We have recently shown that the Pax5−/− pre-B I cells can also give rise to erythropoiesis, although at a low frequency.28 If this efficiency for erythropoiesis, platelet development, and myeloid cell development can be improved by further changes, these cells may, in fact, become convenient sources for cells that can reconstitute a lethally irradiated host in bone marrow transplantation setups. If such cells could also be developed for human hematopoiesis, they might eventually be usable in the repair of human hematopoietic deficiencies, in gene therapy protocols (with the help of gene transfer), and in restoration after irradiation and chemotherapy in malignancies and autoimmune diseases.
The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche, Basel, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christoph Schaniel, Department of Molecular Biology, Lewis Thomas Laboratory, Rm 216, Princeton University, Princeton, NJ 08544; e-mail: schaniel@molbio.princeton.edu; or Antonius G. Rolink, Institut für Immunologie, Universität Basel, Zentrale Dienste, Klingelbergstrasse 70, CH-4056 Basel, Switzerland; e-mail: antonius.rolink@unibas.ch.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal