Abstract
The development of plasmacytoid dendritic cells (pDC2) from human CD34+ stem cells in vivo was studied in RAG-2−/− interleukin (IL)-2Rγ−/− mice that lack functional T and B cells and natural killer cells. CD34+ cells isolated from fetal liver or thymus were labeled with 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) and were injected into a human thymus grafted subcutaneously in the RAG-2−/− IL-2Rγ−/− mice. One to 4 weeks later the CFSE label was found not only in T cells but also in CD123+/high CD4+CD45RA+ pDC2, indicating that the CD34+ cells can develop into pDC2 within a thymus. In addition to pDC2, CFSE-labeled dendritic cells with a mature phenotype, determined by the cell surface markers CD11c, CD83, and CD80, were found in the injected human thymus graft. pDC2 was not found in the periphery of mice carrying a human thymic graft, indicating that the intrathymic pDC2 failed to emigrate from the thymus. We also demonstrate that pDC2 can develop outside the thymus because relatively high percentages of pDC2 were found in the periphery after the intravenous injection of CD34+CD38−fetal liver cells in RAG-2−/− IL-2Rγ−/−mice without a human thymus graft. These data indicate that the thymus and the peripheral pDC2 develop independently of each other.
Introduction
Immature dendritic cell (DC) subsets have been identified in peripheral blood, lymphoid organs, and thymus. Among the immature DCs, interferon (IFN)-producing type 2 DC precursors or plasmacytoid DCs (referred to here as pDC2) represent a recently defined cell type that seems to link innate and adaptive immunity. These cells are present in T-cell areas of the lymph nodes1 and peripheral blood and have the capacity to produce high levels of type 1 IFN on confrontation with viruses such as herpes simplex and influenza virus.2,3 On activation with IL-3 and CD40L or viruses, pDC2 differentiate into mature DCs capable of stimulating CD4+ naive T cells to proliferate and differentiate into T helper 2 (Th2)4 or T cells that produce high levels of IFN-γ and interleukin (IL)-10,5 6respectively.
We and others have identified a cell type similar to pDC2 in the medulla of the human thymus.7-11 Like the peripheral pDC2, these cells have the capacity to develop into mature DC2, but some differences were observed in the expression of certain cell surface antigens.8 Notably, though peripheral pDC2 do not express CD7 and are heterogeneous with respect to the expression of CD2 and CD5, thymus DC2 express these antigens.8,9,11 Moreover, at variance with peripheral pDC2, thymus pDC2 respond to granulocyte macrophage–colony-stimulating factor and produce less IFN-α on infection with virus.9 These differences may imply either that the thymus and the peripheral pDC2 are different subsets or that the thymus microenvironment induces alterations such as the expression of CD2, CD5, and CD7.
In recent studies, the developmental origin of pDC2 was addressed. The results of these investigations strongly suggest that pDC2 belong to the lymphoid lineage. Thymus and peripheral pDC2 express high levels of transcripts for pre–T-cell receptor alpha (pTα),8 and λ5,11 suggesting a developmental relationship between these cells and T and B cells. More recently, we demonstrated that the overexpression of Id2 or Id3, each of which inhibits the activity of transcription factors that belong to the basic helix–loop–helix protein family, inhibited the development of CD34+progenitors into pDC2 without blocking development into CD14+ pDC1.12 These observations, combined with our findings that Id overexpression also inhibited T- and B-cell development,13-15 provide strong evidence for a common developmental origin of pDC2, T, and B cells. Blom et al16recently identified a precursor of pDC2 (pro-DC2) that expressed high levels of IL-3Rα (CD123), CD4, and CD34. The pro-DC2 develops from a CD34+CD45RA+ late progenitor. The cytokine Flt-3 (FL) seems to be important for pDC2 development given that primitive CD34+ stem cells develop into pDC2 under the influence of this cytokine.16
Anatomic sites of pDC2 development have yet to be determined. The presence of these cells in the medulla suggests that they can develop within the thymus. Consistent with this is our observation that CD34+CD1− thymus precursors are able to develop into pDC2 in vitro.12 However, it is unknown whether pDC2 also develop within a thymus in vivo. In addition, it has not been determined whether thymus pDC2 can emigrate from the thymus and populate the periphery or that peripheral pDC2 and thymus pDC2 have distinct developmental origins. We have used a human–severe combined immunodeficiency (SCID) mouse model to address these points. Instead of the commonly used C.B-17 scid/scid (SCID) or the nonobese diabetic (NOD)/LtSz-scid (NOD/SCID) mice, we used H-2d RAG2−/−IL-2Rγ (gamma common)−/− (RAG2−/−γc−/− ) mice.17 18 Fetal thymus and liver can be successfully transplanted under the skin of these mice. After 3 months the thymus is easily palpable and accessible for injection of tagged precursor cells. Biopsy specimens can be taken from these transplants, allowing analysis of the kinetics of tagged precursor cells in the same transplant. Moreover, primitive CD34+CD38− fetal liver cells injected intravenously in RAG2−/− γc−/− mice without a human thymus transplant can develop into human lymphocytes. Using these models we demonstrate here that thymus and peripheral pDC2 develop independently.
Materials and methods
Reagents and monoclonal antibodies
Monoclonal antibodies (mAbs) to CD3, CD4, CD5, CD8, CD13, CD14, CD33, CD38, CD45RA, CD123, and CD80 conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE), CD45-PerCP or CD45-APC, and unconjugated CD1a and CD4 were obtained from Becton Dickinson Immunocytometry Systems (BDIS, San Jose, CA). CD1-PE, CD54-PE, CD83-PE, and unconjugated CD83 were obtained from Coulter/Immunotech (Luminy, France). Unconjugated CD11c, CD4-Tricolor (TC), CD8-TC, CD34-TC, and CD45RA-TC were obtained from DAKO A/S (Glostrup, Denmark), CD11c-PE were obtained from Biosource International (Nivelle, Belgium). BDCA-2–FITC and PE were obtained from Miltenyi Biotec (Bergisch Gladbach, Germany). Unconjugated CD45RA (G1-15) was obtained from Bristol Meyers Squibb (Seattle, WA). Unconjugated CD80 (B7-24) was kindly provided by Dr M. de Boer (Tanox, Amsterdam, The Netherlands). CFSE 5- and 6-carboxyfluorescein diacetate and succinimidyl ester (56-CFDA, SE) mixed isomers were obtained from Molecular Probes (Eugene, OR). CFSE was used at a final concentration of 5 to 25 μM per 0.05 to 2.5 × 107 cells. IL-7 and stem cell factor (SCF) were obtained from R&D (Abingdon, United Kingdom).
Isolation of CD34+ cells from fetal liver and fetal thymus
Human fetal tissues were obtained from elective abortions. Gestational age was determined by ultrasonic measurement of the diameter of the skull and ranged from 14 to 20 weeks. The use of this tissue was approved by the Medical Ethical Committee of the Netherlands Cancer Institute and was contingent on informed consent. Human fetal liver cells were isolated by gentle disruption of the tissue by mechanical means, followed by density gradient centrifugation over Ficoll-Hypaque (Lymphoprep; Nycomed Pharma, Oslo, Norway). CD34+ cells were isolated from these samples by immunomagnetic cell sorting using a CD34 separation kit (varioMACS; Miltenyi Biotec), were stained with anti-CD34 and anti-CD38 mAbs, and were further purified into CD34+CD38− cells by sorting with a FACStar plus (Becton Dickinson, San Jose, CA). Purity of the CD34+CD38− cells used in this study was greater than 99%. Single-cell suspensions were made from fetal and postnatal thymus by mincing tissues and pressing them through a stainless steel mesh. Large aggregates were removed, and the cells were washed once before subpopulations were separated.
CFSE labeling of CD34+ cells
CD34+ cells obtained from fetal liver or thymus were labeled with CFSE before injection into the RAG2−/−γc−/− mice. Purified cells were transferred to polypropylene tubes, washed, and resuspended in 1 mL phosphate-buffered saline (PBS) (Ca and Mg free). CFSE was added, and the cells were incubated for 10 minutes at 37°C. Thereafter, 1 mL per tube PBS–10% bovine serum albumin (BSA) was added to bind the remaining CFSE. Cells were washed twice with 10 mL per tube cold PBS–1% BSA and were resuspended in 1 mL medium. Cells were counted, and the concentration was adjusted for injection into the RAG2−/−γc−/− mice. CFSE was used at a final concentration of 5 to 25 μM per 0.05 to 2.5 × 107 cells.19CFSE is a dye that is easily detectable by flow cytometry and fluorescence microscopy in combination with other cell surface markers and is maintained for several weeks, even in dividing cells, and is not toxic in vitro or in vivo.20
Retrovirus- and lentivirus-mediated gene transfer
The retroviral vector LZRS harboring internal ribosomal entry site-enhanced green fluorescence protein (GFP) was described previously.21,22 Helper virus–free recombinant retroviruses (titer 106/mL, as determined by transduction of mouse 3T3 fibroblast cells) were produced after transfection of the retroviral constructs into the 293T-based ΦNX-A amphotropic packaging cell line and selection on the selectable marker puromycin.23 Transduction of CD34+CD38− fetal liver cells was performed as described previously.22 Briefly, the progenitor cells were cultured overnight in the presence of 10 ng/mL IL-7 (R&D Systems) and 10 ng/mL SCF (R&D) followed by incubation for 7 to 8 hours or overnight with virus supernatant in plates coated with fibronectin (30 μg/mL; Takara Biomedicals, Otsu, Shiga, Japan).
A third-generation, self-inactivating lentiviral vector system was the basis of our lentiviral vector construct and was used as described.24,25 To obtain a fragment from hepatitis B virus (HBV) containing the enhancers I, II, and PRE, a 1237-bp HBV fragment was amplified by polymerase chain reaction with the primers GACGGAAATTGCACCTGTA and CATGGTGCTGGTGCGCAGA. The fragment was cloned as a SacI–SalI fragment containing 1142-bp HBV sequence (HBV subtype AYW accession J02203, bases 682-1818) in the sense orientation after the GFP open-reading frame into the lentiviral vector pRRLpgkgfpsin (PGK).24 GFP expression in the lentiviral vector pRRLpgkgfpsin is driven by a phosphoglycerate kinase promoter.
Incorporation of the HIV central poly purine tract (PPT) was essentially as described.26,27 A 180-bp fragment of the infectious HIV-1 clone LAI-1 containing the PPT, nucleotides 4302 to 4482 of GenBank accession #NC_001802, was amplified using primers CAGTATCGATAAGCTTACAAATGGCAGTATTCATCC and CCTTATCGATTCCAAAGTGGATCTCTGCTGTCC. HIV PPT was cloned asXhoI/blunt fragment in the sense orientation upstream of the PGK promoter. Lentivirus was generated by cotransfection of 293T cells and titrated as described.28 Transduction of CD34+CD38− fetal liver cells with HIV-GFP was performed using fibronectin as described above for LZRS-IRES-GFP, except that the CD34+CD38− cells were precultured overnight in the absence of cytokines.
Generation of RAG-2−/− IL-2Rγ (γc)−/− mice with human lymphocytes
H-2d RAG-2−/− mice were obtained from Drs Anton Rolink and Shunichi Takeda (Basel Institute for Immunology, Switzerland) and were crossed with γc−/− mice to obtain H-2d RAG-2−/− γc−/−mice.17 18 These double knockout mice have no functional T and B cells and no natural killer (NK) cells. Mice were bred and maintained in isolators and were fed autoclaved food and water, and all manipulations were performed under laminar flow. Mice were used when they were 6 to 10 weeks old.
RAG-2−/− γc−/− mice were provided with a human thymus by grafting small (1-2 mm) human fetal thymus and liver fragments subcutaneously in 300 μL Matrigel (Collaborative Biomedical Products, Bedford, MA) diluted 1:4 in RPMI 1640 (Life Technologies, Paisley, Scotland). CD34+ cells purified from fetal liver and thymus were labeled with CFSE before injection in the RAG-2−/− γc−/− mice with an established thymus graft. CFSE-labeled cells (5 × 104-3 × 106) were injected in each animal 16 to 35 weeks after grafting fetal thymus and liver either in the transplant or intravenously into the tail vein. Biopsy samples were taken of the human transplants 1 to 6 weeks after injection, and phenotype was determined in cells obtained from the samples. At the end point, the mice were killed and the thymus graft was removed for immunohistochemistry and flow cytometry. RAG-2−/−γc−/− mice with human lymphocytes in the periphery were generated by injecting 0.5 to 1 × 106 CD34+precursor cells intravenously into the tail veins of mice irradiated with 3.5 Gy.
Immunofluorescence staining and flow cytometry
Surface immunophenotyping of thymocytes with directly conjugated antibodies was performed as previously described.29 FACScalibur (BDIS) was used in these experiments, and 10 000 to 1 000 000 events were acquired on each sample. Multiparameter data acquisition and analysis were performed with Cell Quest software (BDIS). The immunophenotype of CFSE+ donor cells was determined by placing an electronic gate on the CFSE+ cells.
Histology and immunohistochemical analysis
Tissue samples were taken from the thymus transplant. Material was fixed in 10% neutral formol-saline, processed to paraffin sections, and stained with hematoxylin. Triple staining of tissue sections of the thymus transplants was performed as described previously, with some modifications.8 Briefly, cryostat fragments of thymus tissue were cut into 4-μm sections, air-dried overnight, and fixed in acetone for 10 minutes at room temperature. Slides were first incubated with 5% (vol/vol) normal goat serum (Central Laboratory, Blood Transfusion Service of the Red Cross, Amsterdam, The Netherlands), then with optimal dilutions of primary mouse mAb in PBS containing1% (wt/vol) BSA (PBS/BSA)) for 30 minutes at room temperature, followed by incubation with biotinylated goat antimouse IgG (DAKO) and Cy5-conjugated streptavidin (Jackson Immunoresearch Laboratories, Palo Alto, CA). Upon blocking with normal mouse serum, sections were incubated with a second PE-labeled mouse mAb, rabbit anti-PE (Biogenesis, Poole, England), and Cy3-conjugated goat antirabbit IgG (Jackson Immunoresearch Laboratories). For each fluorochrome label, isotype control antibodies were included.
Confocal laser scanning microscope analysis
Confocal laser scanning microscope (CLSM) images were obtained on a Leica TCS SP (Leica Microsystems, Heidelberg, Germany) confocal system, equipped with an Ar/Kr/HeNe laser combination. Images were taken using a 40 × 1.25 NA objective. Possible cross-talk between CFSE, Cy3, and Cy5 signals, which could give rise to false-positive colocalization of different signals, was avoided by careful selection of the imaging conditions.
Results
Human fetal thymus and fetal liver transplants form a complete thymus under the skin of Rag2−/−γc−/− mice
It is well established that human fetal liver and thymus can be successfully transplanted under the kidney capsule of SCID mice. SCID mice have been used in the pioneering studies of McCune et al.30 More recently, NOD/SCID mice were shown to be better recipients because more T cells could be observed in the periphery of transplanted NOD/SCID than in traditional SCID mice. It has also been shown that the progenitor cells injected in such thymus transplants can develop into T cells. A major drawback of injection into thymus transplants growing under the kidney capsule is that the mice must undergo anesthesia and surgery for marked progenitor cells to be injected. Therefore, we established a model in which the transplant is placed under the skin. RAG2−/− γc−/− mice were used rather than NOD/SCID mice. These mice also lack NK cells, as do the NOD/SCID mice,31 but, more important, they do not develop the thymomas present in most NOD/SCID mice beyond 5 months of age,31 a complication that interferes with long-lasting experiments. Human fetal thymus and liver fragments were grafted subcutaneously. Most of the transplanted mice developed a well-differentiated thymus that was already clearly palpable 8 weeks after transplantation and that continued to grow for, on average, 4 additional months. The thymus transplant was still clearly detectable in most mice 12 weeks to 1 year after transplantation. Histologic staining of sections of thymus of these mice revealed a histology similar to that of normal thymus, including cortex, medulla, and Hassal corpuscles (Figure 1). Human thymus transplants have normal proportions of all T-cell subsets and also contain pDC2 along with mature CD83+ DCs in the medulla and at the corticomedullary junction (data not shown), exactly as has been observed in a postnatal thymus.8
Histology of thymus–liver transplant in Rag2−/−/γc−/− mice.
Fetal thymus and liver fragments were grafted subcutaneously. The thymus graft was removed 9 months after transplantation. Magnification, × 250. M indicates medulla; C, cortex; H, Hassal corpuscles.
Histology of thymus–liver transplant in Rag2−/−/γc−/− mice.
Fetal thymus and liver fragments were grafted subcutaneously. The thymus graft was removed 9 months after transplantation. Magnification, × 250. M indicates medulla; C, cortex; H, Hassal corpuscles.
CFSE-labeled CD34+ fetal liver cells and fetal thymocytes develop into pDC2 in the human thymus grafted in Rag2−/− γc−/− mice
The presence of pDC2 in the thymus transplant of Rag2−/− γc−/− mice may mean that these cells developed from precursor cells within the thymus because they did not show the development of hematopoietic cells outside the thymus. Alternatively, these pDC2 could already have been present in the fetal liver or thymus fragments at the time of transplantation and might not have been derived from CD34+ cells developing in the thymus. To distinguish between these possibilities, CD34+cells were MACS purified or FACS sorted from the human fetal liver (99% pure), labeled with CFSE, and injected into the human thymus implant of the Rag2−/− γc−/− mice. Six weeks after injection, the thymus implant was removed for analysis. Flow cytometric (Figure 2) and CLSM (Figure 3) analyses revealed the presence of CFSE+ thymocytes (CD4+CD8+cells) and CFSE+CD123+/high, cells indicating that CD34+CD38− fetal liver cells developed into T cells and pDC2. As expected, the CFSE+CD4+CD8+ cells expressed CD1a and varying levels of CD3 (data not shown), confirming that these cells represented immature T cells. Note that the percentages of CD123+/highCD45RA+ cells in the CFSE-positive gate were much higher than in the CFSE-negative gate (Tables 1,2). This is presumably because differentiation of the precursor cells to pDC2 does not involve many cell divisions. Development of CD34+ into T cells is accompanied by multiple rounds of cell division, leading to a loss of the CFSE label. CFSE+CD123+/high cells (which were also CD45RA+; not shown) are located mainly in the medulla and at the corticomedullary junction (Figure 3), where they are also found in the normal postnatal thymus.8 Thus, thymus precursors have the capacity to develop into pDC2 in a thymus microenvironment in vivo. To provide support for the notion that CD34+precursors migrate to the thymus, where they develop not only to T cells but also to pDC2, we injected CFSE-labeled CD34+fetal liver cells intravenously into Rag2−/−γc−/− mice that carried a thymus graft. CFSE+CD123+/highCD45+ and CFSE+CD4+CD8+ cells (Tables1, 2) could be detected in the thymus transplant of these animals by flow cytometric analysis, indicating that CD34+ cells migrated to the human transplant after intravenous injection. Time-course experiments were conducted to determine immunophenotypic changes after intrathymic and intravenous injections of CFSE-labeled, MACS-purified CD34+ fetal liver cells. This was done by sequential analyses of biopsy specimens taken from the thymus graft. These experiments showed that CFSE+CD123+/highCD45RA+ cells could be detected as early as 1 week after intrathymic injection (data not shown). Of significance, CFSE-labeled CD34+ thymocytes injected intravenously or intrathymically developed into pDC2 (Table3). These data confirmed the results of our in vitro experiments, which demonstrated that CD34+CD1a− postnatal thymocytes have the capacity to develop into pDC2 on coculture with the mouse stromal cell line S17.12
CD34+CD38− fetal liver cells develop to thymocytes and pDC2 in vivo after intrathymic injection in RAG2−/− γc−/− mice with a human thymus transplant.
Sorted CD34+CD38− fetal liver cells were labeled with CFSE and injected into the thymus graft. Six weeks after injection, the thymus graft was removed and the cells were labeled with PE-labeled CD4 and CD123 antibodies and TC-labeled CD4, CD8, and CD45RA antibodies. Isotype control antibodies were used to set the cursors.
CD34+CD38− fetal liver cells develop to thymocytes and pDC2 in vivo after intrathymic injection in RAG2−/− γc−/− mice with a human thymus transplant.
Sorted CD34+CD38− fetal liver cells were labeled with CFSE and injected into the thymus graft. Six weeks after injection, the thymus graft was removed and the cells were labeled with PE-labeled CD4 and CD123 antibodies and TC-labeled CD4, CD8, and CD45RA antibodies. Isotype control antibodies were used to set the cursors.
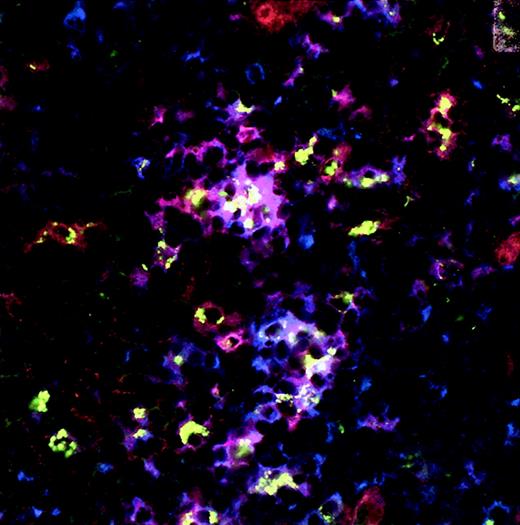
Development of immature and mature DCs after intrathymic injection of CD34+ cells in mice.
Immunohistochemistry and confocal microscopy were performed 6 weeks after intrathymic injection of CFSE-labeled sorted CD34+CD38− fetal liver cells (A) and 4 weeks after the injection of MACS-purified CD34+ fetal liver cells (B) using CD123-PE and unconjugated CD83 mAbs. CFSE-labeled cells show green dots. CD123-PE–positive cells are red (visualized by rabbit anti-PE and Cy3-conjugated goat antirabbit) and CD83+ cells, visualized by goat antimouse biotin, and streptavidin-Cy5 cells are blue. CFSE-labeled cells that express CD123 alone (green dotted red cells), CD83 alone (green dotted blue cells), and CD123 and CD83 (green dotted purple cells) are visible. Isotype controls were negative. Original magnification × 800 (A) and × 400 (B).
Development of immature and mature DCs after intrathymic injection of CD34+ cells in mice.
Immunohistochemistry and confocal microscopy were performed 6 weeks after intrathymic injection of CFSE-labeled sorted CD34+CD38− fetal liver cells (A) and 4 weeks after the injection of MACS-purified CD34+ fetal liver cells (B) using CD123-PE and unconjugated CD83 mAbs. CFSE-labeled cells show green dots. CD123-PE–positive cells are red (visualized by rabbit anti-PE and Cy3-conjugated goat antirabbit) and CD83+ cells, visualized by goat antimouse biotin, and streptavidin-Cy5 cells are blue. CFSE-labeled cells that express CD123 alone (green dotted red cells), CD83 alone (green dotted blue cells), and CD123 and CD83 (green dotted purple cells) are visible. Isotype controls were negative. Original magnification × 800 (A) and × 400 (B).
Development of CFSE-labeled CD34+ fetal liver cells after injection into the human thymus transplanted in RAG2−/− γc−/− mice
| . | CD34+38− it . | |
|---|---|---|
| CFSE− (%) . | CFSE+ (%) . | |
| CD4+CD8+ | 85.0 | 87.0 |
| CD1−CD34+ | 0.3 | 0.2 |
| CD1+CD34+ | 0.5 | 2.0 |
| CD123+CD45RA+ | 0.05 | 8.0 |
| . | CD34+38− it . | |
|---|---|---|
| CFSE− (%) . | CFSE+ (%) . | |
| CD4+CD8+ | 85.0 | 87.0 |
| CD1−CD34+ | 0.3 | 0.2 |
| CD1+CD34+ | 0.5 | 2.0 |
| CD123+CD45RA+ | 0.05 | 8.0 |
CD34+ fetal liver cells were obtained by FACS sorting (CD34+CD38−, 99% pure, experiment 1), labeled with CFSE and injected intrathymically (it) into RAG2−/−γc−/− transplanted with a human fetal thymus liver graft. The expression of cell surface antigens was determined in the thymus graft 6 weeks after injection using PE-labeled antibodies CD4, CD123, CD1a, and the TC-labeled antibodies CD8, CD34, and CD45RA. Isotype control antibodies were used to set the cursors. Electronic gates were placed on CFSE+ and CFSE− cells, and the percentages of different subpopulations were determined in each gate and are indicated in the table.
Development of CFSE-labeled CD34+ fetal liver cells after injection into transplanted RAG2−/−γc−/− mice
| . | CD34+it . | CD34+ iv . | ||
|---|---|---|---|---|
| CFSE−(%) . | CFSE+ (%) . | CFSE−(%) . | CFSE+ (%) . | |
| CD4+CD8+ | 79.0 | 80 | 86.0 | 80 |
| CD1−CD34+ | 0.05 | 0 | 0.15 | 0 |
| CD1+CD34+ | 0.17 | 1 | 0.38 | 2 |
| CD123+CD45RA+ | 0.03 | 6 | 0.02 | 6 |
| . | CD34+it . | CD34+ iv . | ||
|---|---|---|---|---|
| CFSE−(%) . | CFSE+ (%) . | CFSE−(%) . | CFSE+ (%) . | |
| CD4+CD8+ | 79.0 | 80 | 86.0 | 80 |
| CD1−CD34+ | 0.05 | 0 | 0.15 | 0 |
| CD1+CD34+ | 0.17 | 1 | 0.38 | 2 |
| CD123+CD45RA+ | 0.03 | 6 | 0.02 | 6 |
CD34+ fetal liver cells were obtained by MACS sorting only (experiment 2), labeled with CFSE and injected intrathymically (it) or intravenously (iv) into RAG2−/−γc−/− transplanted with a human fetal thymus liver graft. The expression of cell surface antigens was determined in the thymus graft 4 weeks after injection using PE-labeled antibodies CD4, CD123, CD1a, and the TC-labeled antibodies CD8, CD34, and CD45RA. Isotype control antibodies were used to set the cursors. Electronic gates were placed on CFSE+ and CFSE− cells, and the percentages of different subpopulations were determined in each gate and are indicated in the table.
Intrathymic development of CFSE-labeled CD34+thymocytes
| . | CD34+ thymocytes (it) . | CD34+ thymocytes (iv) . | ||
|---|---|---|---|---|
| CFSE− (%) . | CFSE+(%) . | CFSE− (%) . | CFSE+ (%) . | |
| CD4+CD8+ | 90.0 | 90.0 | 87.0 | 82.0 |
| CD1−CD34+ | 1.5 | 1.0 | ND | ND |
| CD1+CD34+ | 0.4 | 0.06 | ND | ND |
| CD123+CD45RA+ | 0.02 | 18.0 | 0.03 | 16.0 |
| . | CD34+ thymocytes (it) . | CD34+ thymocytes (iv) . | ||
|---|---|---|---|---|
| CFSE− (%) . | CFSE+(%) . | CFSE− (%) . | CFSE+ (%) . | |
| CD4+CD8+ | 90.0 | 90.0 | 87.0 | 82.0 |
| CD1−CD34+ | 1.5 | 1.0 | ND | ND |
| CD1+CD34+ | 0.4 | 0.06 | ND | ND |
| CD123+CD45RA+ | 0.02 | 18.0 | 0.03 | 16.0 |
CD34+ fetal thymocytes were labeled with CFSE and injected intravenously (iv) or intrathymically (it) into RAG2−/− γc−/− transplanted with a human fetal thymus liver graft. The expression of cell surface antigens was determined in the thymus graft 4 weeks after injection, as described in the Table 1 footnote. ND indicates not done.
CFSE-labeled CD34+ fetal liver cells develop into different types of DCs on injection in the thymus transplant of Rag2−/− γc−/− mice
Recently it was documented that the human thymus contains different populations of DCs.10 11 It was suggested that thymus DCs form a heterogeneous population consisting of myeloid- and lymphoid-derived cells. Mature DCs are hardly detectable in thymic suspensions prepared without enzymes as collagenase. To determine whether CD34+ cells can differentiate to distinct types of DCs within the thymus environment in vivo, we chose to perform CLSM analysis on the thymus implants 4 to 6 weeks after injection rather than to analyze cell suspensions with flow cytometry. Using 3-color CLSM, we not only observed CFSE+ cells with the phenotype of immature pDC2 (CD123+/highCD45RA+, CD123+/highCD4+, CD123+/highHLA-DR+), we also observed CFSE+ cells with intermediate levels of CD123 expression that showed the simultaneous expression of CD80. We also detected a small population of CD123+/highCD83+ cells on injection of CFSE+CD34+ fetal liver cells, suggesting the maturation of pDC2 to a more mature phenotype (Figure 3A,B). In addition, as shown in Figure4, CFSE-labeled CD34+CD38− cells developed into immature CD123+/highCD11c− and more mature CD123+/highCD11c+ DCs. Thus, these data may suggest that the full maturation of CD123+/highlymphoid-derived pDC2 can occur in the thymus after intrathymic injection of CD34+ FL cells. CD83+ cells expressing the myeloid marker CD13 were also found, suggesting that these CD83+ cells are DCs of myeloid origin (data not shown). Together these data indicate that different populations of DCs develop from CD34+ precursors injected into the thymus. Whether CFSE-labeled mature DC populations represent the full spectrum of DCs that can develop from intrathymic precursors is not determined because some DCs might have undergone multiple cell divisions, resulting in loss of the CFSE label. Experiments analyzing DC subpopulations developed from genetically labeled CD34+cells should solve this.
Development of immature and mature lymphoid-derived DCs (DC2) after intrathymic injection of CD34+ cells in RAG2−/− γc−/−mice with a human thymus transplant.
Immunohistochemistry and confocal microscopy were performed 6 weeks after intrathymic injection of CFSE-labeled sorted CD34+CD38− fetal liver cells using CD123-PE and unconjugated CD11c mAbs. CFSE-labeled cells show green dots. CD123-PE–positive cells are red (visualized by rabbit anti-PE and Cy3-conjugated goat antirabbit), and CD11c+ cells visualized by goat antimouse biotin and streptavidin-Cy5 are blue. CFSE-labeled cells that express CD123 alone (green dotted red cells), CD11c alone (green dotted blue cells), and CD123 and CD11c (green dotted purple cells) are visible. Isotype controls were negative. Original magnification × 400.
Development of immature and mature lymphoid-derived DCs (DC2) after intrathymic injection of CD34+ cells in RAG2−/− γc−/−mice with a human thymus transplant.
Immunohistochemistry and confocal microscopy were performed 6 weeks after intrathymic injection of CFSE-labeled sorted CD34+CD38− fetal liver cells using CD123-PE and unconjugated CD11c mAbs. CFSE-labeled cells show green dots. CD123-PE–positive cells are red (visualized by rabbit anti-PE and Cy3-conjugated goat antirabbit), and CD11c+ cells visualized by goat antimouse biotin and streptavidin-Cy5 are blue. CFSE-labeled cells that express CD123 alone (green dotted red cells), CD11c alone (green dotted blue cells), and CD123 and CD11c (green dotted purple cells) are visible. Isotype controls were negative. Original magnification × 400.
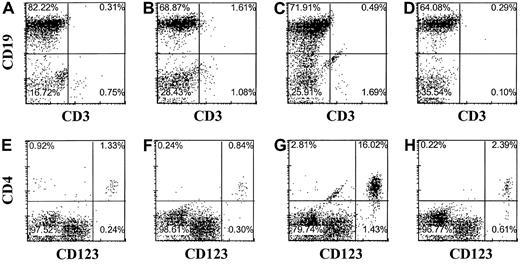
pDC2 and B cells develop in Rag2−/−γc−/− mice injected with CD34+CD38− fetal liver cells
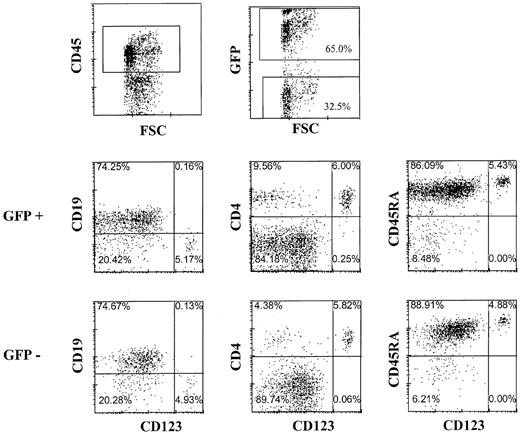
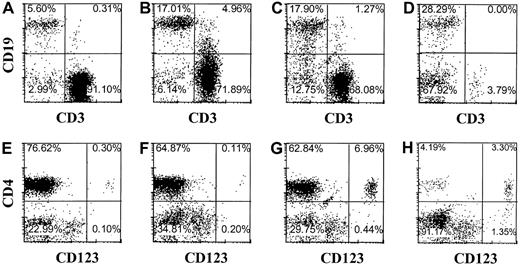
The observation that pDC2 can develop within the thymus raises the question whether peripheral pDC2 originate from the thymus. Although often high percentages of T cells were readily detectable in the peripheral blood and various organs of Rag2−/−γc−/− mice with transplanted thymi, we consistently failed to detect any CD123+/high pDC2 in the peripheral blood, lung, spleen, or bone marrow, even at time points up to 5 months after transplantation of liver and thymus (results not shown). One small explanation for these observations is that the pDC2 do not survive in the periphery of these mice. To investigate this we injected purified CD34+CD38− fetal liver cells intravenously in mice without a human thymus transplant. Peripheral blood, spleen, liver, and bone marrow of these mice were analyzed after 16 weeks for the presence of pDC2 and other human cells. Abundant CD45+ human leukocytes could be found in these mice (Figure5), consistent with earlier observations in NOD/SCID mice.32,33 In addition to finding a majority of B cells (Figure 5), we found significant numbers of CD123+/highCD4+ pDC2 (Figure 5) in the periphery of these mice. Although not shown, pDC2 purified from the liver of the injected mice produced IFN-α on infection with UV-inactivated herpes simplex virus, similar to the action of pDC2 in healthy mice.2 These cells co-expressed CD45RA and the pDC2 marker BDCA2 (results not shown), confirming their pDC2 identity.34 Relatively high numbers of pDC2 were found in the liver and in the bone marrow (Figure 5). These data strongly suggest that B and pDC2 cells developed from the CD34+ SCID repopulating cell (SRC) defined by La Rochelle et al.35 To confirm this we made use of the documented fact that the SRC is a resting cell that can be efficiently transduced with a lentiviral but not a retroviral vector.35,36 Therefore, we examined whether CD34+CD38− fetal liver cells transduced with a lentiviral (HIV) vector or a retroviral vector harboring GFP develop into pDC2 and B cells. CD34+CD38− cells were isolated by FACS sorting and were cultured overnight either with medium or with the cytokines SCF and IL-7. CD34+ cells cultured in medium were transduced with GFP in the HIV vector, and the cells cultured in cytokines were transduced with GFP in the LZRS vector and then injected intravenously into RAG2−/− γc−/− mice. Part of the transduced cells was cultured further with cytokines and inspected for GFP expression 4 days later. This analysis demonstrated that 75% of the HIV-GFP–transduced cells and 40% of the GFP-LZRS–transduced cells expressed GFP (results not shown). Figure6 shows the analysis of the pDC2 and B cells in the liver of the mice injected with HIV-GFP–transduced cells. Sixty-five percent of the cells expressing human CD45 were GFP positive. Percentages of pDC2 and B cells in the GFP-positive and -negative gate were similar. The same percentages of pDC2 and B cells were found in the RAG2−/− γc−/− mice injected with LZRS-GFP− transduced CD34+cells, but these cells did not express any GFP (results not shown), consistent with the findings of Dick et al.36 These results confirm that B and pDC2 cells develop from CD34+SRC in the periphery of the injected RAG2−/−γc−/− mice. More important, the data indicate that the absence of pDC2 in the periphery of RAG2−/−γc−/− mice with a thymus graft did not result from an inability of these cells to survive in the periphery. To exclude that the thymus graft somehow interferes with the survival of pDC2 in the periphery, we injected CD34+ FL cells in mice with an already established graft. Figure 7demonstrates that these mice not only have T cells but that they have pDC2 and B cells in peripheral blood and various organs.
pDC2 and B cells develop from CD34+ cells independent of a human thymus graft in Rag2−/− γc−/− mice.
Murine peripheral blood (A,E), spleen (B,F), liver (C,G), and bone marrow (D,H) were examined by flow cytometry for the presence of human cells (CD45+) expressing CD3, CD4, CD19, and CD123 16 weeks after intravenous injection of CD34+ fetal liver cells. Immunophenotype is shown of cells expressing human CD45. Isotype control antibodies were used to set the cursors.
pDC2 and B cells develop from CD34+ cells independent of a human thymus graft in Rag2−/− γc−/− mice.
Murine peripheral blood (A,E), spleen (B,F), liver (C,G), and bone marrow (D,H) were examined by flow cytometry for the presence of human cells (CD45+) expressing CD3, CD4, CD19, and CD123 16 weeks after intravenous injection of CD34+ fetal liver cells. Immunophenotype is shown of cells expressing human CD45. Isotype control antibodies were used to set the cursors.
pDC2 and B cells develop from CD34+ cells transduced with human immunodeficiency virus vector in Rag2−/− γc−/− mice.
Murine liver was examined by flow cytometry for the presence of GFP-transduced cells expressing the indicated antigens 20 weeks after injection of CD34+ fetal liver cells transduced with the GFP-HIV vector. Isotype control antibodies were used to set the cursors.
pDC2 and B cells develop from CD34+ cells transduced with human immunodeficiency virus vector in Rag2−/− γc−/− mice.
Murine liver was examined by flow cytometry for the presence of GFP-transduced cells expressing the indicated antigens 20 weeks after injection of CD34+ fetal liver cells transduced with the GFP-HIV vector. Isotype control antibodies were used to set the cursors.
The presence of a thymus graft does not affect pDC2 development from intravenous-injected CD34+ fetal liver cells.
Mice were transplanted with thymus and liver fragments under the skin and 8 weeks later were injected with autologous CD34+ fetal liver cells. Immunophenotype is shown of cells expressing human CD45 in blood (A,E), spleen (B,F), liver (C,G), and bone marrow (D,H).
The presence of a thymus graft does not affect pDC2 development from intravenous-injected CD34+ fetal liver cells.
Mice were transplanted with thymus and liver fragments under the skin and 8 weeks later were injected with autologous CD34+ fetal liver cells. Immunophenotype is shown of cells expressing human CD45 in blood (A,E), spleen (B,F), liver (C,G), and bone marrow (D,H).
Discussion
Distinct DC subsets of different immunophenotypes, such as immature and mature DCs and plasmacytoid DCs or pDC2, are present in the human thymus.8,10,11 Thymus pDC2 are similar to pDC2 found in lymph nodes and peripheral blood. CD123+/high pDC2 are of lymphoid origin and can develop in vitro from CD34+CD1− thymocytes, CD34+CD38− fetal liver stem cells, and CD34+ cord blood stem cells.12,16 We addressed 2 questions: one was whether pDC2 developed in the thymus in situ, and the other was whether the peripheral pDC2 originated in vivo from thymus pDC2. To examine the first question, we used a human SCID mouse model in which we grafted RAG2−/− γc−/−mice with human fetal liver and thymus under the skin. We demonstrate here that CFSE-labeled CD34+ cells, either of fetal liver or fetal thymus origin, developed into pDC2 after injection into the thymus graft, indicating that the thymus microenvironment is permissive for the development of these lymphoid-derived cells. Moreover, we show that intravenously injected CD34+ cells isolated from fetal liver or thymus migrate to the thymus graft and develop into thymocytes and pDC2 within the graft. These observations support the notion that thymus pDC2 originate from CD34+ cells that have migrated in the thymus. Kinetics experiments revealed that pDC2 are present as early as 1 week after injection in the graft (results not shown), indicating that the development of CD34+ cells into pDC2 occurs rapidly. This is consistent with our in vitro observations that pDC2 develop from CD34+CD1a− thymus precursors in 4 days.12 Furthermore, it is of note that 4 to 6 weeks after the intrathymic injection of CFSE-labeled CD34+ fetal liver cells, the proportion of pDC2 of the total CFSE+population was much higher than that of the CFSE−population, indicating that the development of pDC2 requires only limited cell division as compared to that of thymocytes, consistent with pDC2 development in vitro.12 Our findings are in agreement with earlier observations in the mouse. It has been documented that in the thymus T cells and DCs develop in parallel, probably from the same cohort of precursor cells.37 38 The frequency of the developing thymus DCs was 1000-fold lower that that of the T cells.
The observation that CD34+ cells develop in the thymus into pDC2 raised the question whether the thymus is a primary organ for development of these cells as it is for T cells. This seems unlikely. A thymus graft contains between 0.5 and 2 × 106 pDC2; however, virtually no pDC2 could be detected in these mice outside the thymus graft at any time point in RAG2−/−γc−/− mice grafted with a human thymus, though emigrating T cells are easily detectable in the peripheral blood and various organs of these mice. We could exclude the explanation that pDC2 are unable to survive in the periphery of RAG2−/−γc−/− mice because these cells are readily detectable after intravenous injection of CD34+ stem cells. Moreover, peripheral pDC2 are also clearly detectable in thymus graft-bearing mice intravenously injected with CD34+ stem cells, indicating that the presence of a thymus graft does not affect the development and survival of peripheral pDC2. Although our experiments do not completely rule out that some pDC2 migrate out of the thymus, we conclude that the thymus is not the prime source of peripheral pDC2. This implies that peripheral pDC2 and thymus pDC2 develop through 2 independent pathways. In preliminary experiments, we failed to detect in the thymus pro-DC2 with the phenotype CD34+/dimCD45RA+ (results not shown), which have been found in cord blood,16 suggesting that thymus pro-DC2 may have a different phenotype than peripheral blood pro-DC2. This would be consistent with different developmental pathways for thymus and peripheral pDC2.
Recently it was shown that the human thymus contains different subsets of immature and mature DCs. Vandenabeele10demonstrated the presence of CD11b+ and CD11b−mature DCs and speculated that the CD11b− population was derived from pDC2. Bendriss-Vermare et al11 reported the presence of pDC2 but also of CD11c+CD13+immature, presumably myeloid DCs. Moreover, these authors documented that the mature interdigitating DC compartment contained cells that expressed the lymphoid-specific transcription factor SpiB, which is also highly expressed in pDC2. Because this factor is not expressed in myeloid DCs, Bendriss-Vermare et al11 concluded that a proportion of the mature thymus DCs is derived from pDC2. Our data extend these findings because we show here that different subsets of immature and mature DCs can develop from CD34+ cells within the thymus. In addition to the immature CD123+/high pDC2, we also observed CD83+ cells with and without CD123. It is tempting to speculate that CD83+CD123+ cells represent the mature offspring of the pDC2, but firm proof for that notion is lacking. This would require the demonstration that CFSE-labeled highly purified CD123+/highCD45RA+thymocytes injected into the thymus develop into mature DCs. We failed so far to achieve this, presumably because purified CD123+/high cells are fragile and may not survive CFSE labeling and injection. We also found some CFSE-labeled CD83+ cells that expressed high levels of the myeloid marker CD13, suggesting that they are of myeloid origin. No mature lymphoid or myeloid-derived DCs were detectable in RAG2−/− γc−/− mice carrying a thymus transplant only, indicating that the DC compartment in the thymus is derived from CD34+ cells and not from immigrating mature DCs that developed elsewhere.
Here we have shown the usefulness of the RAG2−/−γc−/− mice for studies to the in vivo development of human lymphocytes. Like the commonly used SCID, the RAG2−/− γc−/− mice lack T and B cells, but, in contrast to SCID mice, they lack NK cells as well. NOD/SCID mice have also an NK cell deficiency,31 but this is not as strong as it is in RAG2−/− γc−/− mice. The most important advantage of RAG2−/−γc−/− mice compared with NOD/SCID mice is that the RAG2−/− γc−/− mice do not develop thymomas. The fact that a considerable proportion of NOD/SCID mice develop these tumors starting at 5 months of age31 limits their general usefulness, particularly in long-term experiments. In contrast to the results reported here, Mazurier et al39 failed to achieve the engraftment of CD34+stem cells without the co-injection of IL-3, GM-CSF, and erythropoietin. The reasons for these discrepancies are unknown but may be attributed to the different genetic backgrounds of the mouse strains.
We thank the staff at the Bloemenhove Kliniek (Heemstede, The Netherlands) for providing fetal tissues, E. Noteboom and Anita Pfauth for their help with cell sorting, and M. de Boer, J. Schmitz, and J. Kirberg for their gifts of antibodies and mice.
Supported by grants HD29341 and HD37597 from the National Institutes of Health (C.H.U.).
K.W. and C.H.U. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hergen Spits, Division of Immunology, Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX, Amsterdam, Netherlands; e-mail: hspits@nki.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal