Abstract
Hemophilia B is an X-linked coagulopathy caused by absence of functional coagulation factor IX (FIX). Using adeno-associated virus (AAV)–mediated, liver-directed gene therapy, we achieved long-term (> 17 months) substantial correction of canine hemophilia B in 3 of 4 animals, including 2 dogs with an FIX null mutation. This was accomplished with a comparatively low dose of 1 × 1012 vector genomes/kg. Canine FIX (cFIX) levels rose to 5% to 12% of normal, high enough to result in nearly complete phenotypic correction of the disease. Activated clotting times and whole blood clotting times were normalized, activated partial thromboplastin times were substantially reduced, and anti-cFIX was not detected. The fourth animal, also a null mutation dog, showed transient expression (4 weeks), but subsequently developed neutralizing anti-cFIX (inhibitor). Previous work in the canine null mutation model has invariably resulted in inhibitor formation following treatment by either gene or protein replacement therapies. This study demonstrates that hepatic AAV gene transfer can result in sustained therapeutic expression in a large animal model characterized by increased risk of a neutralizing anti-FIX response.
Introduction
Hemophilia B is a sex-linked bleeding disorder caused by a deficiency of functional coagulation factor IX (FIX). Current replacement therapy consists of intravenous infusion of protein concentrate. However, this treatment is costly and inconvenient and carries with it the risk of blood-borne disease transmission. Furthermore, bleeds are often treated only after they have occurred, rather than prophylactically, so that chronic joint damage occurs and the risk of a fatal bleed is always present. Hemophilia is an ideal model for gene therapy because precise regulation and tissue-specific transgene expression are not required.1,2 A number of animal models are available including knockout mice and well-described hemophilic dog colonies with phenotypes corresponding to the human disease.3-5 Clinical end points for treatment are well defined. An increase of factor levels to more than 1% will improve the phenotype of the disease from severe to moderate, with reduced frequency of spontaneous bleeds, and a further increase to more than 5% will result in a mild phenotype; that is, patients would likely require factor infusion only after severe injury or during surgery. Currently the most serious complication of treatment is the formation of inhibitory antibodies to the deficient protein, which occurs with a frequency of 3% to 4% in patients with hemophilia B.6,7Inhibitor formation is observed mostly in those patients with extensive loss of FIX coding information.6 8
Sustained expression of canine FIX (cFIX) in dogs with a missense mutation has been observed following administration of an adeno-associated virus (AAV) vector into the portal vein for hepatic gene transfer or into skeletal muscle.9-11 The latter approach is currently being tested in a phase 1 clinical trial.12 AAV vectors can be produced in a helper virus-free system, are devoid of any viral gene products, and often fail to activate antigen-specific cytotoxic T lymphocytes.13 However, inhibitor formation is still a frequent complication following intramuscular administration of AAV vector in hemophilia B mice (with a large F9 gene deletion) and dogs with a FIX null mutation.14,15In these animal models, muscle-directed gene therapy was successful only when combined with transient immunosuppression. Inhibitor formation in the null mutation dogs has also been described in the context of lentiviral transfer of a cF9 gene to the liver.16 These data underscore a potentially serious immunologic complication for all gene replacement strategies for treatment of genetic disease, that is, a harmful immune response to the transgene product in a recipient who is not tolerant to the therapeutic protein encoded by the donated gene.
In this study, we show sustained correction of canine hemophilia B following AAV-mediated, liver-directed gene transfer in the context of a FIX null mutation that is usually associated with a high risk of inhibitor formation in protein or gene therapy.15 16 These data are encouraging for gene-based treatment and are directly relevant to a recently initiated clinical trial of AAV-mediated F9gene transfer to patients with severe hemophilia B.
Materials and methods
AAV vector construction
Vector AAV-(ApoE)4/hAAT-cFIX was constructed by replacing the cytomegalovirus (CMV) enhancer/promoter in the previously described expression cassette with a liver-specific ApoE/hAAT enhancer/promoter combination.9 This 1.1-kb sequence is comprised of the human α1-antitrypsin promoter and 4 copies of the ApoE enhancer as described by Ponder and colleagues.17 The expression cassette also contains a chimeric β-globin/CMV intron, the cFIX complementary DNA (cDNA), and the human growth hormone polyadenylation (hGH poly A) signal as described.9 AAV2 vector was produced by triple transfection of HEK-293 cells in a helper virus-free system, which uses 2 helper plasmids to supply adenoviral gene functions (E2A, E4, and VA) and the AAV2 rep/cap genes.18 Plasmids were grown in Escherichia coli DH5α cells and purified using the Qiagen (Santa Clarita, CA) Giga kit for preparation of endotoxin-free DNA. The AAV helper plasmid has been engineered to increase cap expression and to decrease generation of wild-type AAV to undetectable levels (< 1 in 109 vector particles) in a replication center assay.19 AAV vector was purified from cell lysates by repeated rounds of CsCl density gradient centrifugation as described.9,15 18 Vector was osmotically stabilized in Hepes-buffered saline, pH. 7.8, filter-sterilized, and stored at −80°C prior use. Vector titers were determined by quantitative slot blot hybridization. The Limulus amoebocyte lysate assay (Sigma, St Louis, MO) was performed to confirm absence of detectable endotoxin in vector preparations.
Experimental animals
The experimental animals used in this study were Lhasa Apso-Basenji cross dogs from the hemophilia B colony housed at the Scott-Ritchey Research Center at Auburn University. These dogs were males with severe hemophilia B caused by a 5–base pair (bp) deletion and a C>T transition in the F9 gene that results in an early stop codon and unstable FIX transcript.5 One of the dogs treated with the AAV vector also had pyruvate kinase (PK) deficiency, an erythrocyte metabolism disorder resulting in a low hematocrit of 20% as compared to normal levels of about 40%.20 Additionally, a female hemophilia B dog with an FIX missense mutation of the University of North Carolina (UNC)–Chapel Hill colony was treated.3 All animals were housed in US Department of Agriculture–approved facilities and the experimental protocol was approved by the institutional Animal Care and Concern Committees of Auburn University and UNC-Chapel Hill.
Vector administration
The animals were premedicated with diazepam (5 mg) or butorphanol (5 mg) or both and atropine (0.6 mg) before anesthetic induction with isoflurane. A midline laparotomy was performed; a mesenteric vein was then isolated and a 20-gauge catheter inserted and tied off with stay sutures. The AAV-(ApoE)4/hAAT-cFIX vector (in a 10-mL volume) was administered by slow bolus infusion (1-2 minutes) and the catheter flushed with 5 to 10 mL heparinized saline before removal and ligation of the mesenteric vein (mesenteric vein administration results in subsequent delivery of the vector to the portal vein for hepatic gene transfer). The abdomen was closed using standard surgical procedures. Butorphanol was administered as needed to provide postoperative analgesia. The dogs were prophylactically administered 90 mL plasma immediately before surgery and 45 mL 8 to 12 hours later. Abnormal reactions or toxicity were not noted following vector administration based on clinical examination and routine clinical pathology tests. Portal vein infusion of vector in hemophilia B dog E34 following midline laparotomy was preformed as described previously.10 11 Vector administration by this method was well tolerated in this animal as well.
FIX, coagulation, and antibody assays
Blood samples were drawn from hemophilia B dogs as described.15 The whole blood clotting time (WBCT), activated clotting time (ACT), activated partial thromboplastin time (aPTT) of plasma samples, and FIX activity levels were measured as previously reported.9,15 The cFIX antigen levels in plasma samples were determined by enzyme-linked immunosorbent assay (ELISA).9,15 Anti-cFIX was demonstrated by immunocapture assay specific to canine IgG1, IgG2, IgM, and IgA, by Western blot, or by Bethesda assay as described previously.14,15 One Bethesda unit (BU) represents inhibition of normal FIX activity by 50%. The cFIX protein used in these assays was a purified plasma-derived preparation from Enzyme Research Laboratories (South Bend, IN), and all antibodies were purchased from Bethyl Laboratories (Montgomery, TX).15 Antiphospholipid was detected by dilute Russell viper venom time (RVVT). Neutralizing antibodies (NABs) against AAV2 vector particles were measured by inhibition of in vitro LacZ transduction as described.15 The treated animals did not have a pre-existing NAB titer, but all developed NABs to AAV2 after vector administration.
DNA analysis
Total genomic DNA was isolated from canine liver or spleen tissue using the Easy DNA kit from Invitrogen (Carlsbad, CA). Vector-specific sequences were detected by Southern blot hybridization using a 0.9-kb probe specific to the human α1-antitrypsin promoter and intron sequences in the AAV vector.
Results
Administration of liver-specific AAV vector results in sustained high-level FIX expression in 3 hemophilia B dogs and transient expression in 1 dog
AAV-(ApoE)4/hAAT-cFIX vector was infused into the hepatic circulation of 4 hemophilia B dogs (via mesenteric or portal vein) for hepatocyte-specific expression of cFIX. The vector uses a strong liver-specific promoter/enhancer combination.17 Two animals from the Auburn dog colony (Brad and Semillon) and 1 animal from the UNC-Chapel Hill colony (E34) received vector at a dose of ∼1 × 1012 vector genomes (vg)/kg (Table1). Before treatment none of these animals had detectable circulating cFIX antigen or cFIX activity owing to a FIX null mutation (5-bp deletion plus a C>T transition in theF9 gene resulting in an early stop codon at amino acid residue 146 before the activation peptide of FIX, Auburn dogs) or a FIX missense mutation (G>A resulting in a single amino acid substitution of glutamic acid for Gly379 in the catalytic domain of FIX, UNC dog).3 5
Summary of dogs with hemophilia B treated by intramesenteric vein or portal vein (E34) administration of AAV-(ApoE)4/hAAT-cFIX
| Animal (colony) . | Age* (mo) . | Weight* (kg) . | Total dose (vg) . | Dose/kg (vg/kg) . | PK deficiency . | WBCT (min) . | APTT (s) . | cFIX (ng/mL) . | cFIX activity (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Brad† | 9 | 10.2 | 1.25 × 1013 | 1.2 × 1012 | No | 12 ± 2.5 | 29.5 ± 3.5 | 590 ± 150 | 8.5 ± 2 |
| (Auburn) | |||||||||
| Semillon† | 5.5 | 6.0 | 9.7 × 1012 | 1.6 × 1012 | No | 13.5 ± 4 | 35.5 ± 2 | 220 ± 65 | 5 ± 2.5 |
| (Auburn) | |||||||||
| Beech† | 12 | 10.5 | 3.6 × 1013 | 3.4 × 1012 | Yes | ≥ 10 | ≥ 36.2 | ≤ 2560 | ≤ 3 |
| (Auburn) | |||||||||
| E34‡ | 5 | 12.3 | 9.6 × 1012 | 8 × 1011 | No | 11 ± 2.5 | 32 ± 4.5 | 262 ± 92 | 5 ± 2.5 |
| (UNC) |
| Animal (colony) . | Age* (mo) . | Weight* (kg) . | Total dose (vg) . | Dose/kg (vg/kg) . | PK deficiency . | WBCT (min) . | APTT (s) . | cFIX (ng/mL) . | cFIX activity (%) . |
|---|---|---|---|---|---|---|---|---|---|
| Brad† | 9 | 10.2 | 1.25 × 1013 | 1.2 × 1012 | No | 12 ± 2.5 | 29.5 ± 3.5 | 590 ± 150 | 8.5 ± 2 |
| (Auburn) | |||||||||
| Semillon† | 5.5 | 6.0 | 9.7 × 1012 | 1.6 × 1012 | No | 13.5 ± 4 | 35.5 ± 2 | 220 ± 65 | 5 ± 2.5 |
| (Auburn) | |||||||||
| Beech† | 12 | 10.5 | 3.6 × 1013 | 3.4 × 1012 | Yes | ≥ 10 | ≥ 36.2 | ≤ 2560 | ≤ 3 |
| (Auburn) | |||||||||
| E34‡ | 5 | 12.3 | 9.6 × 1012 | 8 × 1011 | No | 11 ± 2.5 | 32 ± 4.5 | 262 ± 92 | 5 ± 2.5 |
| (UNC) |
Results for WBCT, aPTT, cFIX plasma levels as determined by ELISA, and cFIX activity as percentage of activity in normal dog plasma are average values (± 1 SD) for weeks 3 to 86 (Brad), weeks 3 to 42 (Semillon), or weeks 3 to 48 (E34) after vector administration. Values for Beech represent peak levels before inhibitor development. The ranges for coagulation times in normal dogs are 6 to 10 minutes (WBCT), 1 to 2 minutes (ACT), and 18 to 20 seconds (aPTT). In hemophilia B dogs they are more than 60 minutes (WBCT), more than 4 minutes (ACT), and more than 60 seconds (aPTT).
Age and weight at time of vector administration.
Male hemophilia B dogs of Auburn colony with FIX null mutation.
Female hemophilia B dog of UNC-Chapel Hill colony with FIX missense mutation.
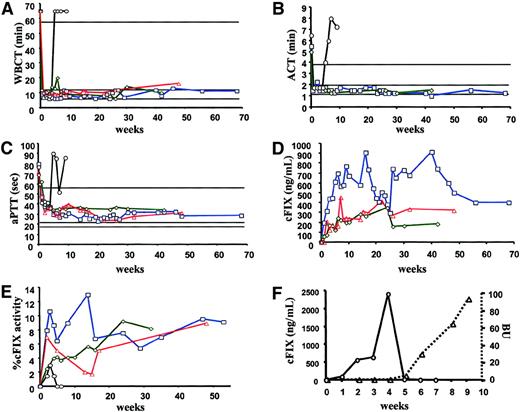
Brad, the first dog treated, also received a total of 180 mL plasma on day 0 before, during, and after surgical laparotomy and vector administration and 45 mL daily for the next 4 days. (All other animals received only ∼135 mL plasma before and just after surgery.) By day 14, 10 days after the last plasma infusion, the ACT in Brad was 1.5 minutes (normal range, 1-2 minutes) as compared to 5.5 minutes the day before vector administration. The ACT has remained in the normal range for more than 17 months following vector administration (Figure1B). During the same period, the WBCT was within the normal range (12.1 ± 2.6 minutes versus > 60 minutes before treatment), and aPTT values (29.4 ± 3.6 seconds) were significantly shortened from pretreatment times of 79.9 seconds (Figure1A,C). The cFIX antigen was undetectable before vector administration but had increased to 317 ng/mL by week 2 and peaked at 907 ng/mL on week 16 (Figure 1D). Antigen levels of 590 ± 150 ng/mL have persisted for the duration of the study. Likewise, cFIX activity of 8.6% ± 2.1% of a canine plasma pool has also persisted for the more than 17-month observation period (Figure 1E and Table 1). The dog also had a normal cuticle bleed time after treatment (data nor shown). The other 2 dogs (E34 and Semillon) also showed sustained complete or nearly complete correction of the WBCT and ACT (not measured in E34) and substantial correction of the aPTT from more than 60 seconds before treatment to about 32 to 35 seconds (Table 1 and Figure 1A-C). The cFIX antigen levels averaged 220 ± 65 ng/mL for Semillon and 262 ± 92 ng/mL for E34 (Figure 1D). FIX activity averaged 4.9% ± 2.6% of normal canine plasma for Semillon and 5% ± 2.5% for E34 (Figure 1E and Table 1). Expression was sustained in both animals for more than 6 months in Semillon and more than 7 months in E34 (experiment ongoing).
Coagulation parameters after vector administration.
WBCT (A), ACT (B), aPTT (C), cFIX antigen levels in plasma (D), and cFIX activity levels (E; percent activity of pooled normal canine plasma) as a function of time after administration of AAV-ApoE-hAAT vector in hemophilia B dogs Brad (1 × 1012vg/kg, □, blue line), Semillion (1 × 1012 vg/kg, ⋄, green line), E34 (▵, red line, ACT was not measured in this dog), and Beech (3 × 1012 vg/kg, ○, black line, data for Beech are omitted in graph D). Vector was administered into the mesenteric (Brad, Semillon, Beech) or portal vein (E34) for liver-directed gene transfer. The ranges for coagulation times in normal, healthy dogs are 6 to 10 minutes (WBCT), 1 to 2 minutes (ACT), and 18 to 20 seconds (aPTT). In hemophilia B dogs they are more than 60 minutes (WBCT), more than 4 minutes (ACT), and more than 60 seconds (aPTT). (F) The cFIX antigen levels (○) and formation of inhibitory anti-cFIX (in BU, ▵) in Beech after vector administration.
Coagulation parameters after vector administration.
WBCT (A), ACT (B), aPTT (C), cFIX antigen levels in plasma (D), and cFIX activity levels (E; percent activity of pooled normal canine plasma) as a function of time after administration of AAV-ApoE-hAAT vector in hemophilia B dogs Brad (1 × 1012vg/kg, □, blue line), Semillion (1 × 1012 vg/kg, ⋄, green line), E34 (▵, red line, ACT was not measured in this dog), and Beech (3 × 1012 vg/kg, ○, black line, data for Beech are omitted in graph D). Vector was administered into the mesenteric (Brad, Semillon, Beech) or portal vein (E34) for liver-directed gene transfer. The ranges for coagulation times in normal, healthy dogs are 6 to 10 minutes (WBCT), 1 to 2 minutes (ACT), and 18 to 20 seconds (aPTT). In hemophilia B dogs they are more than 60 minutes (WBCT), more than 4 minutes (ACT), and more than 60 seconds (aPTT). (F) The cFIX antigen levels (○) and formation of inhibitory anti-cFIX (in BU, ▵) in Beech after vector administration.
None of the 3 successfully treated hemophilia B dogs described above had evidence for bleeds following vector administration (a total of 4.3 years of observation for all 3 animals, the UNC dog E34 has been followed for more than 1 year since gene transfer) or liver biopsy (see below). Although bleeding episodes are rarely observed in the Auburn dogs,15 dogs of the UNC-Chapel Hill colony animals receive plasma infusion in response to a bleed on average 6 to 7 times per year, albeit with considerable variation among individual animals.9
A third null mutation dog, Beech, was injected with 3.4 × 1012 vg/kg (∼3 times higher vector dose, Table1). WBCT and ACT values were within the normal range after gene transfer (weeks 2-4), but returned to baseline by week 5 (Figure 1A,B). The aPTT results were consistent with these observations, showing decreasing values through week 4 (without ever achieving a normal value), but returning to a greater than pretreatment value of 90.4 seconds by week 5 (Figure 1C). The cFIX antigen level rose to more than 2 μg/mL by week 4 but had dropped to 13 ng/mL by week 5, and was undetectable by week 6 (Figure 1F). FIX activity showed a similar pattern, rising from 0% to 1.3% by week 2, peaking at 3.0% on week 3, and returning to 0% by week 5 (Figure 1E). As shown below, loss of systemic cFIX expression was due to formation of an inhibitory anti-cFIX that first emerged at week 5. The discrepancy between cFIX antigen levels measured by ELISA and cFIX activity levels in Beech are likely due to the presence of an antiphospholipid antibody in this animal (vide infra) as determined by RVVT assay as described before for a different animal of this colony.15 At 11 weeks after vector administration, Beech developed a fatal intra-abdominal bleed, which, due to a lack of canine bypass reagents such as factor VIIa, could not be treated.
Antibody responses against cFIX
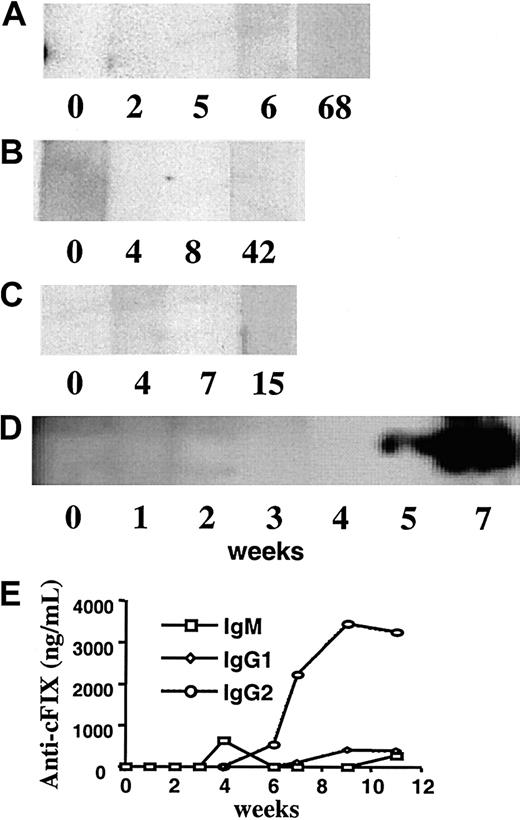
Beech, the dog with transient FIX expression, developed FIX-specific antibodies concomitant with the loss of FIX antigen and activity. The Bethesda titer increased from 0 (before treatment through week 4) to 4.0 BU at week 5 with a subsequently rising titer (Figure 1F). Anti-cFIX IgG was undetectable in serum from week 0 through 4, but was demonstrated in week 5 and subsequently by Western blot (Figure 2D). Immunocapture assay showed synthesis of IgM at week 4 followed by high titer IgG2 anti-cFIX at week 5 and low titer IgG1 at week 9 (Figure 2E). Brad, Semillon, and E34, the dogs with sustained FIX expression, had no evidence for anti-cFIX by Western blot, immunocapture assay, or Bethesda assay at any time point tested (Figure 2A-C and data not shown). IgA anti-cFIX was not detected in any of the treated animals (data not shown), and no animals had anti-cFIX before treatment.
Western blot analysis after infusion of AAV vector.
Western blot analysis demonstrating presence or absence of anti-cFIX IgG in hemophilia B dogs Brad (A), Semillon (B), E34 (C), and Beech (D) as a function of time after AAV vector was infused. Numbers indicate weeks after vector administration. (E) Serum levels of anti-cFIX immunoglobulins in Beech as a function of time after vector administration. Note that no anti-cFIX was detected in Brad, Semillon, or E34.
Western blot analysis after infusion of AAV vector.
Western blot analysis demonstrating presence or absence of anti-cFIX IgG in hemophilia B dogs Brad (A), Semillon (B), E34 (C), and Beech (D) as a function of time after AAV vector was infused. Numbers indicate weeks after vector administration. (E) Serum levels of anti-cFIX immunoglobulins in Beech as a function of time after vector administration. Note that no anti-cFIX was detected in Brad, Semillon, or E34.
Lack of vector-related toxicity
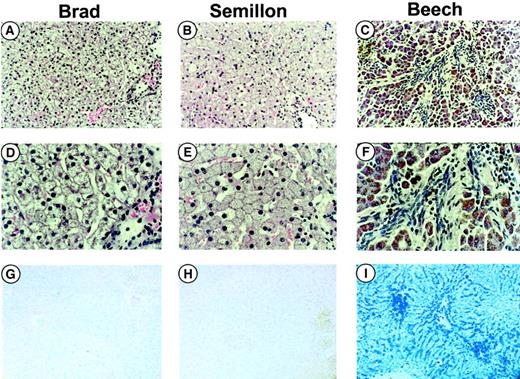
Serum chemistry panels showed no changes in liver (alanine aminotransferase [ALT]) enzyme tests for dogs Semillon, Beech, and E34 following vector administration (1-3 × 1012 vg/kg, data not shown). None of these animals experienced surgical complications and apparently tolerated vector infusion well. Brad had elevated ALT levels (4 times upper level of normal) during the first 3 to 5 days after vector administration as a result of a surgical complication. The dog had a hematoma at its right hind leg that was present before surgery. The resulting blood loss caused cardiac arrest during the procedure (before vector infusion), which required cardiopulmonary resuscitation including injection of atropine, epinephrine, and sodium bicarbonate to restore cardiac activity and normal circulation. Subsequently, vector was successfully infused without incident. Liver biopsies performed in Brad and Semillon 27 and 32 weeks after vector administration showed normal hepatic tissue without evidence of inflammation or other pathologic changes (Figure3A-B). Brad and Semillon received a small amount of plasma (∼50 mL) prior to liver biopsy and had no subsequent bleeds or other indication for requirement of additional FIX infusion. Hepatic tissue of Beech (necropsy performed 11 weeks after vector administration), the animal with PK deficiency, showed evidence for fibrosis (Figure 3C,F), early cirrhosis, and iron overload (Figure 3I, blue stain) due to chronic hemolytic anemia.
Histologic examinations.
Histology of formalin-fixed liver sections from hemophilia B dogs (with FIX null mutation) Brad (A, D, G), Semillon (B, E, H), and Beech (C, F, I) treated with the AAV-(ApoE)4/hAAT-cFIX vector. The tissues were stained with hematoxylin and eosin (H&E, A-F) or Prussian blue (PB), a stain for detection of iron (G-I). Tissues from Brad and Semillon were taken by biopsy at week 27 and 32, respectively, and tissue from Beech was taken by necropsy at week 11. Beech had PK deficiency, a disease characterized by iron deposition in the liver as evident by the blue stain in panel I. Fibrosis (note fibrotic changes in panels C and F) is present in panels C and F but not in panels A, B, D, and E. Original magnification × 100 (A-C and G-I) or × 400 (D-F).
Histologic examinations.
Histology of formalin-fixed liver sections from hemophilia B dogs (with FIX null mutation) Brad (A, D, G), Semillon (B, E, H), and Beech (C, F, I) treated with the AAV-(ApoE)4/hAAT-cFIX vector. The tissues were stained with hematoxylin and eosin (H&E, A-F) or Prussian blue (PB), a stain for detection of iron (G-I). Tissues from Brad and Semillon were taken by biopsy at week 27 and 32, respectively, and tissue from Beech was taken by necropsy at week 11. Beech had PK deficiency, a disease characterized by iron deposition in the liver as evident by the blue stain in panel I. Fibrosis (note fibrotic changes in panels C and F) is present in panels C and F but not in panels A, B, D, and E. Original magnification × 100 (A-C and G-I) or × 400 (D-F).
DNA analysis of vector sequences
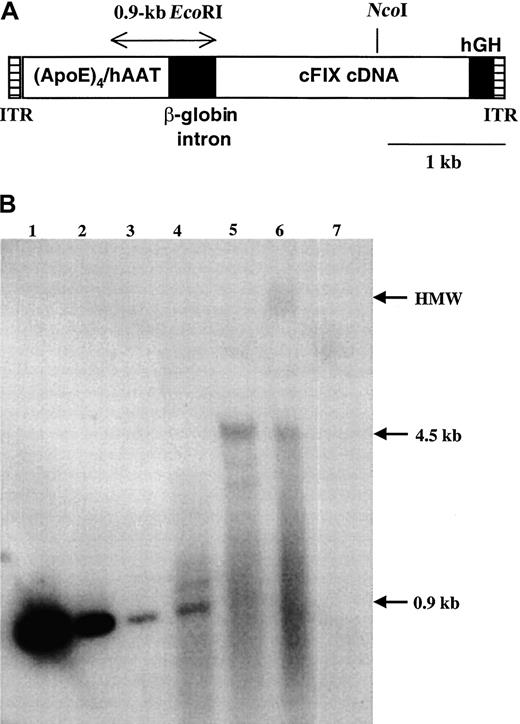
Total genomic DNA from liver and spleen tissue obtained from Beech at necropsy was analyzed by Southern blot hybridization. Using a restriction enzyme (EcoRI) that releases a defined, vector-specific fragment of 0.9 kb, we estimated a gene copy number of 0.1 copies per diploid genome in the liver after comparison of signal strength with plasmid standards (Figure4B, lanes 1-4). No signal was obtained from splenic DNA (Figure 4B, lane 7). A small amount of episomal double-stranded vector (monomer) was observed in uncut liver DNA (lane 6). Most of the vector was present as a high-molecular-weight species, at least in part arranged as head-to-tail concatemers (digest with enzyme NcoI that cuts once within the vector, thereby releasing a fragment of the size of the unit length of the vector, lane 5). These results are consistent with previous observations for AAV-mediated gene transfer to liver and skeletal muscle.21 22
DNA analysis of vector sequences.
(A) Diagram of AAV-(ApoE)4/hAAT-cFIX vector. Shown are AAV2 inverted terminal repeats (ITRs), a 1.1-kb enhancer/promoter sequence containing 4 repeats of the human ApoE enhancer linked to the human α1-antitrypsin promoter, a chimeric β-globin/CMV intron, the cFIX cDNA, and hGH poly A signal. A 0.9-kb EcoRI fragment used as a probe for Southern hybridization and a unique NcoI restriction site are also indicated. (B) Southern blot hybridization. Lanes 1-3: plasmid pAAV-(ApoE)4/hAAT-cFIX encoding the vector,EcoRI digested, 1 ng, 100 pg, and 10 pg, respectively. Lanes 4-6: genomic DNA isolated from liver of hemophilia B dog Beech, 40 μg/lane. Lane 4, EcoRI digest; lane 5, NcoI digest; lane 6, uncut DNA. Lane 7: genomic DNA isolated from spleen, 40 μg, cut with EcoRI. Indicated on the right margin are high-molecular-weight DNA (HMW), the 4.5-kb fragment representing the unit length of the double-stranded vector, and the 0.9-kbEcoRI fragment used for estimation of gene copy number.
DNA analysis of vector sequences.
(A) Diagram of AAV-(ApoE)4/hAAT-cFIX vector. Shown are AAV2 inverted terminal repeats (ITRs), a 1.1-kb enhancer/promoter sequence containing 4 repeats of the human ApoE enhancer linked to the human α1-antitrypsin promoter, a chimeric β-globin/CMV intron, the cFIX cDNA, and hGH poly A signal. A 0.9-kb EcoRI fragment used as a probe for Southern hybridization and a unique NcoI restriction site are also indicated. (B) Southern blot hybridization. Lanes 1-3: plasmid pAAV-(ApoE)4/hAAT-cFIX encoding the vector,EcoRI digested, 1 ng, 100 pg, and 10 pg, respectively. Lanes 4-6: genomic DNA isolated from liver of hemophilia B dog Beech, 40 μg/lane. Lane 4, EcoRI digest; lane 5, NcoI digest; lane 6, uncut DNA. Lane 7: genomic DNA isolated from spleen, 40 μg, cut with EcoRI. Indicated on the right margin are high-molecular-weight DNA (HMW), the 4.5-kb fragment representing the unit length of the double-stranded vector, and the 0.9-kbEcoRI fragment used for estimation of gene copy number.
Discussion
The FIX-deficient dogs in the Auburn colony have been previously shown to rapidly produce high-titer inhibitors to cFIX (within 9-14 days) following IV infusion of purified cFIX or transduction of skeletal muscle with a cFIX-encoded AAV vector (1 × 1012vg/kg) indicating that the animals are at high risk for inhibitor formation.15 Similarly, inhibitor formation has been reported in other liver-directed gene transfer strategies in these dogs.16 This strain of hemophilia B dogs may therefore be an excellent large animal model to mimic the 3% to 4% of hemophilia B patients who develop inhibitors in conventional protein replacement therapy. As is the case in many FIX-deficient patients with inhibitors, these animals are not tolerant to FIX because of the severity of their mutation, which causes lack of synthesis of cFIX antigen in the liver.5 Consequently, a neutralizing anti-cFIX response blocked systemic expression in dogs of this colony after muscle-directed gene therapy at vector doses that conferred sustained expression of cFIX in hemophilia B dogs with a FIX missense mutation (inhibitor formation in 2 of 2 null mutation dogs and 0 of 4 missense mutation dogs at dose ≤ 3 × 1012vg/kg).9 15 To our surprise, we were able to achieve sustained expression of cFIX even in hemophilia B dogs of the Auburn colony (at levels that result in substantial correction of the bleeding disorder) using hepatic gene transfer.
Efficacy of hepatic gene transfer and expression
In treatment of hemophilia, quantitative differences in factor levels translate into qualitative differences in improvement of the disease phenotype. Previous studies in the missense mutation model of canine hemophilia B have demonstrated sustained expression of up to about 1% of normal levels in muscle- or liver-directed gene therapy with AAV vectors, whereas other gene transfer strategies had yielded only subtherapeutic or transient cFIX expression.23,24 In another recent study on liver-directed gene transfer with AAV, vector doses of 5 × 1012 vg/kg resulted in expression of 0.5% to 4% of normal levels in the missense mutation dogs.11Compared to the latter, expression levels per delivered vector particle were 5- to 15-fold higher in the studies presented here and 10- to 50-fold higher than in our previous studies on muscle-directed gene transfer in canine hemophilia B. These results are encouraging for clinical application in humans, because high levels of expression are achievable with relatively low vector doses, and the scale-up from the dog model to humans is minimal as opposed to data from mouse studies. Expression levels reported here should be adequate for substantial or nearly complete correction of the bleeding disorder in patients.
Lack of inhibitor formation after hepatic gene transfer
Use of species-specific transgenes allows us to define the risk of a neutralizing antibody response against expressed FIX antigen. For muscle-directed gene therapy, we have identified the choice of vector, vector dose, and the underlying FIX mutation as important factors that influence the risk of immune responses (Herzog et al9; Fields et al14; Herzog et al15; Fields et al25; and R.W.H., P. A. Fields, T.C.N., K.A.H., unpublished data, May 2000). In this study, we show that an alternative route of administration and choice for target tissue of transgene expression, namely liver, can allow systemic expression of FIX antigen in the context of an unfavorable mutation. Sustained expression of human FIX has been documented in hemophilia B mice with a large F9 gene deletion resulting in absence of endogenous FIX.10 However, hemophilic mice were bred on a C57BL/6 background and have also been successfully treated by systemic administration of a highly immunogenic first-generation adenoviral vector, a result that is not reproducible in other strains of mice.26,27 Nonetheless, route of administration is an important determinant of the risk of a humoral immune response to a secreted transgene product, although the immunologic mechanisms are not understood at this point.26,28 29
The results from the Auburn dogs raise the question of whether liver-directed gene transfer may have the potential to induce tolerance to the expressed FIX antigen even in the absence of immune modulation. Induction of immune tolerance by gene transfer will be crucial for treatment of hemophilic patients who had not been extensively treated with coagulation factor antigen, if gene therapy were ever to be used for treatment of children with hemophilia. Based on our results on muscle-directed gene transfer (rapid induction of a T-helper cell-dependent antibody response15,25), one would expect activation of T-cell responses against the transgene product to follow a substantially different mechanism in the context of hepatic gene transfer. This likely reflects differences in the population of antigen-presenting cells in these tissues. For example, liver sinusoidal endothelial cells have been shown to confer antigen-specific CD8+ T-cell tolerance and have also been implemented in CD4+ T-cell tolerance, although the latter is less clear.30,31 Antigen presentation in oral tolerance has been documented to result in immune deviation causing synthesis of IgA instead of IgG and consequently inefficient clearance of the antigen.32 However, we found no evidence for IgA anti-cFIX in serum samples from our treated dogs. Interestingly, both Brad and Semillon have been subjected to liver biopsy, and neither dog has shown an anti-cFIX response indicating that immunologic unresponsiveness was maintained even after an invasive procedure. The liver has been shown to confer antigen-specific tolerance in several experimental models including portal vein tolerance, oral tolerance, and liver allotransplants across incompatible major histocompatibility complex barriers (which cannot easily be achieved for other organs), and may emerge as a preferred target for maintaining tolerance to a systemic transgene product as well.32-35 The AAV vector with reduced potential for inflammation and increased capacity for sustained expression may be ideal for this purpose. It is likely that every combination of target tissue and vector is characterized by a distinct set of immunologic signals.
In one dog (Beech), inhibitor formation occurred, although much delayed compared with our results from muscle-directed gene transfer. Beech, unlike the other 2 dogs, also had PK deficiency, which causes chronic hemolytic anemia and an iron overload syndrome. Following necropsy, some level of fibrosis and early stages of cirrhosis were observed in liver tissue as expected in a PK-deficient animal. There was no obvious toxicity/liver pathology that may be attributed to vector treatment. It is unclear whether the PK deficiency, which may be associated with antibodies against red blood cells or antiphospholipid in this particular breed of dogs, poses an additional risk factor for inhibitor formation.15 20 Alternatively, liver pathology secondary to iron overload (and thus altered local cytokine milieu), perhaps in combination with increase in vector dose, may have contributed to inhibitor formation in an animal already inhibitor prone because of the null mutation. Interestingly, elevated liver enzyme levels and other toxicity caused by surgical complications in hemophilia B dog Brad (likely including significant changes in cytokine expression that may provide strong activation or “danger” signals to the immune system) did not predispose to inhibitor formation. This suggests that transient liver toxicity caused by cardiopulmonary arrest, and chronic liver pathology caused by PK deficiency, are not comparable events from an immunologic viewpoint.
In conclusion, the data presented here are encouraging for treatment of patients with hemophilia B by in vivo hepatic gene transfer with an AAV vector. Treatment was successful in 3 of 4 large animals with severe disease including 2 of 3 animals of a strain that is prone to inhibitor formation due to a FIX null mutation. Moreover, expression levels were not only therapeutic, but near the curative range at low vector doses.
The authors thank Avigen, a company in which K.A.H. holds equity, for supplying helper plasmids for AAV vector production and assistance with the NAB assay, and D. Ni for technical assistance.
Supported by National Institutes of Health grant R01 HL61921 to K.A.H. R.W.H is supported by a Career Development Award from the National Hemophilia Foundation.
J.D.M. and R.W.H. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Katherine A. High, The Children's Hospital of Philadelphia, Abramson Research Center, Rm 310, 34th St and Civic Center Blvd, Philadelphia, PA 19104; e-mail: high@emailchop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal