Graft-versus-host disease (GVHD), a major complication after allogeneic transplantation, can be abrogated by the Campath (anti-CD52) monoclonal antibody. The induction of acute GVHD requires host antigens to be presented to donor T cells by antigen-presenting cells (APCs). Recent evidence has suggested that only host APCs can interact with donor T cells in the induction of GVHD. Because CD52 has been reported to be expressed on DCs, we reasoned that pretransplant Campath-1G might have a direct effect on circulating DCs in addition to any effects on donor T cells. Using direct immunostaining, we demonstrated expression of CD52 on DCs and that Campath-1G killed purified DCs in vitro. In vivo Campath also depleted DCs. Twenty-four hours after the first dose of Campath-1G, circulating DCs were reduced by a mean of 79% (range, 44%-96%). By day 0 after 5 doses of Campath-1G and chemoradiotherapy conditioning, DCs became undetectable in 7 of 9 cases, whereas in 6 of 7 patients receiving conditioning therapy without Campath-1G, host DCs were still detectable. The reconstitution of circulating DCs after transplantation was not affected by Campath-1G and in both groups DC1 (CD11c+) recovered more rapidly than DC2 (CD11c−). Analysis of chimerism confirmed that the DCs recovering after transplantation in patients receiving Campath-1G were of donor origin. We conclude that in vivo Campath-1G causes a rapid depletion of host circulating DCs and that this may, in part, explain the low incidence of acute GVHD. The reconstitution of donor DCs was not delayed, which may be important in preserving immune reconstitution.
Introduction
High-dose chemoradiotherapy followed by the allogeneic transplantation of hematopoietic stem cells from either bone marrow (BM) or peripheral blood stem cells (PBSCs) is widely used in the treatment of malignant and nonmalignant hematologic diseases. However, allogeneic transplantation is frequently associated with the development of acute graft-versus-host disease (GVHD), which is thought to occur as a result of donor T-cell activation damaging host tissues particularly affecting the liver, skin, and gastrointestinal tract.1 2
Current therapeutic approaches to the prevention of acute GVHD after transplantation include immunosuppression with agents such as cyclosporin and methotrexate. However, despite these measures GVHD still remains a significant cause of morbidity and mortality.3-5 Alternative strategies for the prevention of acute GVHD have focused on the depletion of donor T cells from the graft before infusion into the host.6-8 Although it has been recognized for many years that T-cell depletion decreases the risk of GVHD development,6,9 it also increases the risk of leukemia relapse in chronic myeloid leukemia10 and graft rejection.11 A number of different approaches to T-cell depletion have been used including CD34+ cell selection12,13 and ex vivo treatment of the graft with monoclonal antibodies. For this purpose, Campath-1M, which recognizes the CD52 antigen, has been widely used.9,14,15 CD52 is highly expressed on human lymphocytes and monocytes and is a good target for cell lysis by antibody with human complement.16,17 Although ex vivo treatment with Campath-1M is effective in preventing acute and chronic GVHD, the clinical benefit in reduction in GVHD is offset by an increased risk of graft rejection by residual host T cells and by an increased risk of leukemic relapse in CML due to the loss of a graft-versus-leukemia effect.10 Graft rejection in the setting of T-cell–depleted grafts can be reduced by increasing the intensity of pretransplant immunosuppression by using the rat IgG2b antibody Campath-1G to deplete residual host T cells.15 In vivo treatment with Campath-1G has also been used to prevent acute GVHD, particularly following unrelated donor transplantation.15,18-20 Originally, the Campath-1G was given both before and after transplantation with the intention that the pretransplant antibody would prevent rejection and the posttransplant antibody would suppress GVHD by the elimination of donor T cells via antibody-dependent cell cytotoxicity (ADCC).20 More recently, pretransplant treatment alone has been reported to be highly effective in the prevention of acute GVHD following either unrelated transplantation21 or transplantation from HLA-matched siblings.22 In fact, we found that pretransplant treatment with Campath-1G from day −5 to −1 was as effective in preventing acute GVHD following unrelated donor bone marrow transplantation (BMT) as combined pretransplant and posttransplant antibody therapy.21 The efficacy of in vivo pretransplant Campath-1H (the humanized equivalent of Campath-1G) in preventing acute GVHD has also been established in the setting of nonmyeloablative transplants using fludarabine and melphalan conditioning.18
Induction of acute GVHD requires host antigens to be presented to donor T cells by antigen-presenting cells (APCs) in the context of the major histocompatibility complex molecules. Recent evidence in a murine allogeneic BMT model showed that only host CD11c+APCs can present antigen to donor CD8+ T cells and that these cells are critical in the induction of GVHD despite the presence of numerous donor APCs,23 suggesting that donor DCs cannot efficiently present host antigens necessary for the development of acute GVHD. These results also suggested that the depletion of host APCs before allogeneic transplantation might abrogate acute GVHD without requiring prolonged posttransplant immunosuppression.23 DCs are potent APCs that play an important role in initiating the immune response, as a consequence of their potent ability to induce T-cell activation and proliferation.24 Because the CD52 antigen has been reported to be expressed on DCs,25 we reasoned that the administration of in vivo Campath to the recipient prior to allogeneic transplantation might result in the depletion of host DCs prior to the infusion of donor cells and that this could contribute to the reduction of acute GVHD.21 22
We have therefore assessed the effect of in vivo pretransplant Campath-1G on circulating DCs before and after the completion of conditioning chemoradiotherapy. We have also analyzed the effect of pretransplant Campath-1G on the origin and kinetics of reconstitution of DCs after transplantation.
Material and methods
Samples
Peripheral blood samples were taken into EDTA from patients undergoing allogeneic hemopoietic stem cell transplantation (HSCT) for DC enumeration and the DC separation prior to DNA preparation. All samples were processed within 6 hours. Samples were taken from 2 groups of patients, those receiving pretransplant Campath 1-G and those not so treated.
For patients receiving pretransplant Campath-1G, Campath-1G was given at a dose of 10 mg daily from day −5 to −1 inclusive. The first dose of Campath was given 24 hours before starting conditioning chemoradiotherapy. Chemoradiotherapy was given during days −4 to −1. For conditioning, patients received either cyclophosphamide 60 mg/kg for 2 doses plus fractionated total body irradiation (TBI; 14.4 Gy) for unrelated donor transplantation (n = 6) or BCNU, etoposide, cytosine arabinoside, melphalan (BEAM)/Campath conditioning for patients undergoing reduced-intensity sibling donor transplantation for lymphoproliferative disorders (n = 4), as previously described.22
Patients who did not receive Campath-1G received either a matched sibling allogeneic transplant with cyclophosphamide 60 mg/kg for 2 doses and fractionated TBI (12 Gy; n = 3) or an autologous transplant with BEAM conditioning (n = 4).
In both groups peripheral blood DCs were enumerated before starting Campath/conditioning, and on day 0 following completion of conditioning (with or without Campath therapy). In the Campath group peripheral blood DCs were also measured on day −4, that is, 24 hours after the first dose of Campath-1G but before starting conditioning therapy. DCs were also enumerated in the peripheral blood at various time points in the posttransplant period and in the peripheral blood of a group of healthy volunteers (n = 19).
Monoclonal antibodies
Allophycocyanin-conjugated anti-CD11c and mouse IgG2b isotype controls were purchased from Becton Dickinson (San Jose, CA). Fluorescein isothiocyanate (FITC)–conjugated anti-CD52 and rat IgG2b isotype controls were purchased from Serotec (Oxford, United Kingdom). Campath-1G was provided by Professor G. Hale, (University of Oxford, United Kingdom). All other monoclonal antibodies were purchased from Pharmingen (San Diego, CA).
DC enumeration in peripheral blood
Peripheral blood was stained without further separation to minimize selective loss. Erythrocytes were lysed after staining using Ortho-mune lysing reagent (Ortho Diagnostic Systems, Raritan, NJ) according to the manufacturer's instruction. DCs were identified as negative for the cocktail antibodies of phycoerythrin (PE) conjugated specific for lineage markers on T cells (CD3), B cells (CD19, CD20), monocytes (CD14), neutrophils (CD16), hematopoietic stem cells (CD34), and natural killer (NK) cells (CD16, CD56) and positive for FITC-conjugated anti–HLA-DR. DC1 and DC2 were identified using allophycocyanin-conjugated anti-CD11c. Three- color analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson). The number of total white blood cells (WBCs) in the samples was determined using an automated WBC counter. The absolute number of total DCs and their subsets was calculated from the WBC count multiplied by the proportion of each subset among the WBC, as determined by flow cytometric analysis.
CD52 expression by DCs
To identify the expression of the CD52 antigen by DCs, a peripheral blood sample from a healthy volunteer donor was used. FITC-conjugated Campath-1G was used to stain the DC population together with PE-conjugated cocktail antibodies (anti-CD3, CD14, CD16, CD19, CD20, CD34, and CD56), allophycocyanin-conjugated anti-CD11c, and cychrome (PEcy5)–conjugated anti-HLA-DR. Four-color analysis was performed using a FACSCalibur flow cytometer. The expression of CD52 on T cells was also determined using Campath-1G as a positive control. A FITC-conjugated rat IgG2b was also used as an isotype negative control.
The effect of Campath-1G on DCs in vitro
Mononuclear cells were separated from normal peripheral blood by density gradient centrifugation technique using Histopaque (Sigma, St Louis, MO). Cells were stained with PE-conjugated anti-CD3, CD14, CD16, CD19, CD20, CD34, and CD56 and FITC-conjugated anti–HLA-DR. The HLA-DR+, lineage-minus (HLA-DR+/Lin−) cells were sorted using EpicALTRA High-Performance Cell Sorting System (Beckman Coulter, Fullerton, CA). Purified DCs were incubated for 20 minutes with 75 μg/mL Campath-1G in complete RPMI-HEPES supplemented with 10% heat-inactivated fetal calf serum (First Link, Wolverhampton, United Kingdom). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 ng/mL; (Pharmingen) and 50 ng/mL recombinant human interleukin (IL)-3 (R & D Systems, Abingdon, United Kingdom) were added to the culture medium because GM-CSF and IL-3 are essential for the survival of DCs.26 Rat IgG2b (75 μg/mL; Serotec) was also used as a control antibody in culturing purified DCs without Campath-1G. Twenty percent of autologous serum was added and cells were cultured for 24 hours. DCs were also cultured with Campath-1G without autologous serum to determine whether complement is needed for the induction of cell death. Cells were then washed twice with phosphate-buffered saline (PBS) and resuspended with PBS. Cell suspension was incubated with 20 μg/mL (final concentration) of 7-amino-actinomycin D (7-AAD, Sigma) at 4°C for 20 minutes in the dark and analyzed for the numbers of live cells using FACSCalibur flow cytometer according to the previous studies by Philpott et al27
DC separation for microsatellite polymerase chain reaction
Peripheral blood mononuclear cells (PBMCs) were separated from EDTA blood by a density gradient centrifugation technique. PBMCs were stained with PE-conjugated anti-CD3, CD14, CD16, CD19, CD20, CD34, and CD56. Cells were then labeled with anti-PE magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Labeled cells were depleted on a high-gradient magnetic separation column (MiniMACS, Miltenyi Biotec). The negative fraction was then stained with anti–HLA-DR magnetic microbeads and positively selected in the same way. The immunomagnetic separation was performed according to the manufacturer's instructions. The entire procedure lasted 4 to 6 hours, during which cells were kept at 4°C. DCs were resuspended in 200 μL PBS. DNA was extracted using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). DNA samples were kept at −20 °C until microsatellite polymerase chain reaction (PCR) was performed.
Microsatellite PCR
Sequential samples were assessed for donor chimerism status using fluorescent microsatellite PCR for Rb1 or D6S264. One or both of these markers had previously been shown to be informative by analyzing DNA from donor-recipient pairs before transplantation. Forward (F) and reverse (R) primers specific for Rb128 and D6S26429 were Rb1(F) 5′CTCCTCCCTACTTACTTGT, Rb1(R) 5′AATTAACAAGGTGTGGTGGTACACG, D6S264(F) 5′AGCTGACTTTATGCTGTTCCT, D6S264(R) 5′TTTTCCATGCCCTTCTATCA with the forward primer being 5′end-labeled with FAM. Typically, 100 ng DC DNA was amplified in a 25-μL reaction volume using 1 U Amplitaq Gold (Applied Biosystems, Foster City, CA), one times PCR buffer II, and 0.125 mM dNTPs (Amersham Life Science, Cleveland, OH). Rb1 PCR had 35 cycles with annealing at 55°C and 2.0 mM MgCl2. D6S264 amplifications were conducted in 1.5 mM MgCl2 for 30 cycles and annealing at 55°C.
Analysis of PCR products
Fluorescent PCR products were diluted in molecular grade water (1/5 or 1/10) and electrophoresed through 4.2% sequencing gels (Thistle Scientific, Coatbridge, United Kingdom) on an ABI Prism 377 DNA sequencer (Applied Biosystems). Electrophoresis was for 5 hours at 40 W and 48°C with a GS-500 marker (Applied Biosystems) in each lane. Data were analyzed using Genescan Analysis software and quantitation of percentage of donor chimerism was as described by Miflin et al.30
Statistics
Statistical analysis (unpaired t test) was performed using the statistics package (GraphPad Prism software, West Wycombe, United Kingdom).
Results
DC enumeration in peripheral blood of healthy volunteers
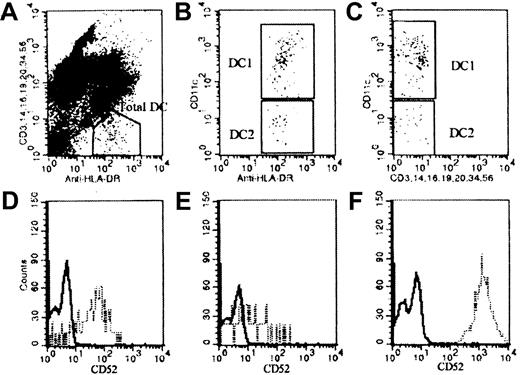
To establish the reference range of DCs in peripheral blood, peripheral blood samples from 10 healthy men and 9 healthy women, aged 23 to 48, were stained. DCs were identified as HLA-DR+ and HLA-DR− for lineage markers of T cells, monocytes, NK cells, granulocytes, B cells, and CD34+ progenitor cells (Figure 1A). The 2 subsets of DCs were analyzed using antibody against the adhesion molecule CD11c. DC1 were identified as CD11c+ cells, whereas DC2 were negative for CD11c (Figure 1B,C). The mean absolute number of DCs in normal peripheral blood using this method was 27.7 ± 13.3 × 106/L (mean ± 1 SD) with DC1 of 15.4 ± 7.5 × 106/L and DC2 of 12.3 ± 7.5 × 106/L.
DC enumeration and CD52 staining on DCs and T cells.
Peripheral blood sample from a healthy donor was stained with anti–HLA-DR and the mixture of antibodies specific for lineage markers CD3, CD14, CD16, CD19, CD20, CD34, and CD56. Anti-CD11c was also used to separate DCs into 2 subsets. (A) Total DC enumeration. (B,C) DC1 (CD11c+) and DC2 (CD11c−) staining. Peripheral blood DCs were also stained for the expression of CD52. (D) D52 expression on DC1. (E) CD52 expression on DC2. (F) CD52 expression on T cells where the closed line represents isotype control staining and broken line represents CD52 staining.
DC enumeration and CD52 staining on DCs and T cells.
Peripheral blood sample from a healthy donor was stained with anti–HLA-DR and the mixture of antibodies specific for lineage markers CD3, CD14, CD16, CD19, CD20, CD34, and CD56. Anti-CD11c was also used to separate DCs into 2 subsets. (A) Total DC enumeration. (B,C) DC1 (CD11c+) and DC2 (CD11c−) staining. Peripheral blood DCs were also stained for the expression of CD52. (D) D52 expression on DC1. (E) CD52 expression on DC2. (F) CD52 expression on T cells where the closed line represents isotype control staining and broken line represents CD52 staining.
CD52 expression on DCs
To determine whether peripheral blood DCs express CD52 antigen, normal peripheral blood was stained as described. The CD52 antigen was found to be expressed on both DC1 and DC2 (Figure 1D,E). Studies of 5 healthy controls showed that a median of 81% of DC1 expressed the CD52 antigen (range, 59%-97%), whereas a median of 64% of DC2 expressed CD52 (range, 46%-91%). In this experiment T cells were also stained for CD52 expression as the positive control for this antibody (Figure 1F).
Campath-1G causes DC death in vitro
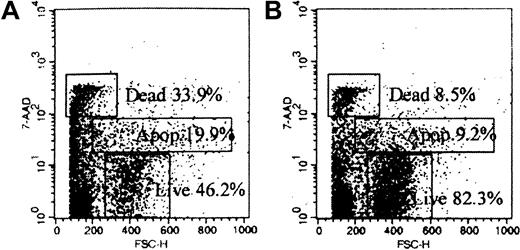
The effects of Campath-1G on DCs in vitro were studied by culturing purified DCs with Campath-1G. Staining with 7-AAD was used to analyze the numbers of dead, apoptotic, and live cells as shown in Figure 2. The proportion of dead, apoptotic, and live cells was compared in the cells cultured with Campath-1G and the control cells cultured with rat IgG2b. The results showed that after incubating purified with Campath-1G for 24 hours in the presence of autologous complement, the proportion of surviving cells cultured with Campath-1G (median = 36.5%) was significantly lower than the proportion of live cells after culturing with control antibody (median = 76.2%; n = 4, P = .002). Cells were also cultured with Campath-1G without autologous serum and we found that there was no difference in the proportion of surviving cells between cells cultured with control antibody and cells cultured with Campath-1G alone (median = 71.6%). This suggested Campath-1G could induce complement-dependent cytotoxicity in DCs.
Scattergrams of cells stained with 7-AAD show specific cell death induced by Campath-1G.
(A) Purified DCs cultured with Campath-1G. (B) Purified DC culture with control antibody Campath-1G.
Scattergrams of cells stained with 7-AAD show specific cell death induced by Campath-1G.
(A) Purified DCs cultured with Campath-1G. (B) Purified DC culture with control antibody Campath-1G.
Pretransplant Campath causes rapid depletion of circulating DCs
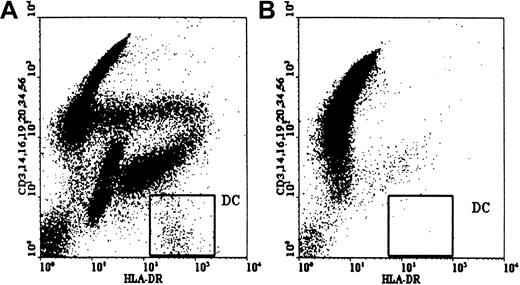
The effects of pretransplant Campath-1G on circulating DCs were analyzed using flow cytometry for DC enumeration at different time points before transplant as described. In the Campath-treated group, pretransplant Campath-1G (10 mg) depleted circulating DCs by a mean of 79% (range, 44%-96%; n = 9) 24 hours after the first dose of antibody and before starting any chemoradiotherapy conditioning (Figure3 and Table1). At this time point the total number of circulating DCs had fallen from 12.6 ± 7.3 × 106/L to 2.4 ± 2.3 × 106/L. At day 0, following the completion of Campath-1G and the conditioning therapy, circulating DCs in the Campath group were undetectable in 7 of 9 cases (Table 1). In contrast, on day 0 peripheral blood DCs in the non-Campath group were depleted but could still be detected in 6 of 7 cases (P = .029).
Depletion of DCs by in vivo Campath.
Circulating host DCs were depleted 24 hours after the first dose of Campath antibody. In this case the number of DCs fell from 19.19 × 106/L (A) to 0.80 × 106/mL (B).
Depletion of DCs by in vivo Campath.
Circulating host DCs were depleted 24 hours after the first dose of Campath antibody. In this case the number of DCs fell from 19.19 × 106/L (A) to 0.80 × 106/mL (B).
The effect of in vivo Campath and conditioning therapy on circulating DC numbers before transplant
| Patient age/sex . | Diagnosis . | Conditioning . | Number of DCs (× 106/L) . | % Depletion after 1st dose of Campath . | ||
|---|---|---|---|---|---|---|
| Pretransplant . | 24 h after 1st dose of Campath . | Day 0 . | ||||
| 51/M | NHL | BEAM/Campath | 5.56 | 0.48 | 0 | 91 |
| 20/F | AML | TBI/Cy/Campath | 10.38 | 3.97 | 0 | 61 |
| 37/M | MM | TBI/Cy/Campath | 10.89 | 1.25 | 0 | 88 |
| 20/M | ALL | TBI/Cy/Campath | 5.48 | 1.09 | 0.20 | 80 |
| 63/F | MM | BEAM/Campath | 4.61 | 0.64 | 0 | 80 |
| 47/F | AML | TBI/Cy/Campath | 18.48 | 2.08 | 0 | 88 |
| 58/F | AML | TBI/Cy/Campath | 13.22 | 7.34 | 0 | 44 |
| 43/F | NHL | BEAM/Campath | 5.34 | — | 0.09 | — |
| 20/M | ALL | TBI/Cy/Campath | 25.87 | 3.90 | 0 | 85 |
| 55/M | NHL | BEAM/Campath | 19.19 | 0.80 | — | 96 |
| 53/M | NHL | BEAM | 6.92 | — | 1.42 | — |
| 29/M | NHL | TBI/Cy | 23.62 | — | 3.00 | — |
| 49/M | NHL | BEAM | 32.93 | — | 0 | — |
| 49/F | NHL | BEAM | 8.30 | — | 0.26 | — |
| 26/F | NHL | BEAM | 21.22 | — | 0.23 | — |
| 51/F | NHL | BEAM | 11.45 | — | 0.15 | — |
| 32/M | NHL | TBI/Cy | 0.54 | — | 0.35 | — |
| Patient age/sex . | Diagnosis . | Conditioning . | Number of DCs (× 106/L) . | % Depletion after 1st dose of Campath . | ||
|---|---|---|---|---|---|---|
| Pretransplant . | 24 h after 1st dose of Campath . | Day 0 . | ||||
| 51/M | NHL | BEAM/Campath | 5.56 | 0.48 | 0 | 91 |
| 20/F | AML | TBI/Cy/Campath | 10.38 | 3.97 | 0 | 61 |
| 37/M | MM | TBI/Cy/Campath | 10.89 | 1.25 | 0 | 88 |
| 20/M | ALL | TBI/Cy/Campath | 5.48 | 1.09 | 0.20 | 80 |
| 63/F | MM | BEAM/Campath | 4.61 | 0.64 | 0 | 80 |
| 47/F | AML | TBI/Cy/Campath | 18.48 | 2.08 | 0 | 88 |
| 58/F | AML | TBI/Cy/Campath | 13.22 | 7.34 | 0 | 44 |
| 43/F | NHL | BEAM/Campath | 5.34 | — | 0.09 | — |
| 20/M | ALL | TBI/Cy/Campath | 25.87 | 3.90 | 0 | 85 |
| 55/M | NHL | BEAM/Campath | 19.19 | 0.80 | — | 96 |
| 53/M | NHL | BEAM | 6.92 | — | 1.42 | — |
| 29/M | NHL | TBI/Cy | 23.62 | — | 3.00 | — |
| 49/M | NHL | BEAM | 32.93 | — | 0 | — |
| 49/F | NHL | BEAM | 8.30 | — | 0.26 | — |
| 26/F | NHL | BEAM | 21.22 | — | 0.23 | — |
| 51/F | NHL | BEAM | 11.45 | — | 0.15 | — |
| 32/M | NHL | TBI/Cy | 0.54 | — | 0.35 | — |
NHL indicates non-Hodgkin lymphoma; Cy, cyclophosphamide; AML, acute myeloid leukemia; MM, multiple myeloma.
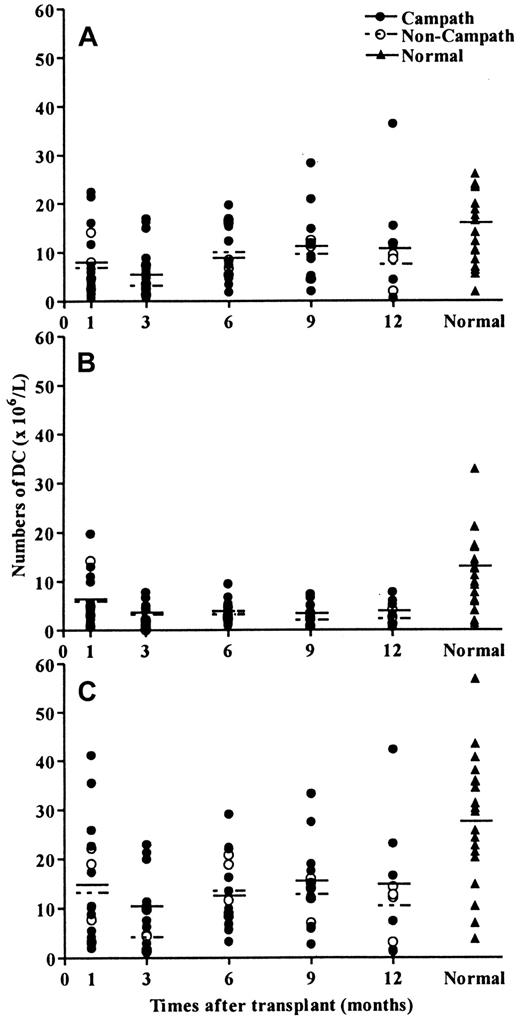
DC reconstitution in peripheral blood is not delayed by pretransplant Campath
Because Campath-1G administered before transplantation has been shown to circulate at the time of marrow infusion,22 it was possible that the regeneration of donor DCs would be delayed. We therefore examined the kinetics of circulating DC reconstitution at various time points up to 1 year after transplantation. There was a slow recovery of both DC1 and DC2 subsets in both patient groups and the levels of both total DC numbers and DC subsets remained depressed up to 12 months after transplantation (Figure4). However, comparison between the 2 groups of patients suggested that the posttransplant recovery of both DC1 and DC2 was not affected by pretransplant Campath (Figure4).
DC reconstitution after allogeneic transplantation.
Regeneration of peripheral blood DCs after transplantation was not affected by pretransplant Campath. (A) DC1 regeneration. (B) C2 regeneration. (C) Total DC regeneration.
DC reconstitution after allogeneic transplantation.
Regeneration of peripheral blood DCs after transplantation was not affected by pretransplant Campath. (A) DC1 regeneration. (B) C2 regeneration. (C) Total DC regeneration.
Peripheral blood DCs regenerating after transplant are of donor origin
Peripheral blood DCs were isolated after transplant and analyzed for chimerism. At 8 weeks after transplant, 4 of 4 patients who had received pretransplant Campath had 100% donor DCs by variable number of tandem repeats (VNTR) analysis. Two of 2 patients with no pretransplant Campath treatment also had 100% donor DCs. However, at 12 weeks after transplant, one patient in the non-Campath group had mixed chimerism with 90% of DCs being of donor origin. This patient later had a relapse at 5 months, whereas at this time point all patients in the Campath group had DCs of donor origin.
Discussion
The induction of acute GVHD requires host antigen to be presented in the context of HLA to donor T cells by APCs. Recent reports have suggested that host APCs may be critical to donor CD8+T-cell activation and the induction of acute GVHD,23suggesting that depletion of host APCs before allogeneic transplantation might abrogate acute GVHD. Because pretransplant serotherapy with Campath-1G has been highly effective in the prevention of both acute GVHD and graft rejection following either unrelated donor or sibling donor transplantation,21,22 we reasoned that this effect might be mediated by a direct effect of Campath on host APCs. In this study, we focused on the effect of Campath-1G on DCs, which are one of the most potent APCs. The CD52 antigen has been reported to be expressed on CMRF-56+ peripheral blood DCs25 and we initially studied the expression of CD52 on circulating DCs. These studies confirmed that the majority of peripheral blood DCs, enumerated as HLA-DR+/Lin−, also express the CD52 antigen, although the level of expression was less than seen on T cells. By using anti-CD11c as a marker to separate these DC into 2 subsets, DC1 (CD11c+) and DC2 (CD11c−), we also found that the CD52 antigen was expressed on both subsets of DCs. Our method found a higher number of DCs in healthy donors compared to the enumeration of DCs using the antibody against CMRF44, an activation antigen expressed on DCs, as described by Fearnley et al.31 This may relate to the direct staining of unmanipulated whole blood in our method, whereas the use of the CMRF44 antibody required the culture of mononuclear cell population to induce the CMRF44 expression on DCs.31 The DC population enumerated using our method comprised 2 distinct subsets of DCs, DC1, shown to express myeloid antigen, CD11c, and the lymphoid DCs (DC2), which have been recently described in human peripheral blood and lymphoid tissues as HLA-DR+/lin−/CD11c− plasmacytoid cells.32-34
Studies of the effect of Campath-1G therapy on peripheral blood DCs demonstrated that pretransplant Campath reduced the number of circulating DCs by a mean of 79% (range, 44%-96%) after one dose. At day 0, following the completion of 5 doses of Campath-1G and the conditioning therapy, peripheral blood DCs were undetectable in 7 of 9 patients. In contrast, peripheral blood DCs in 6 of 7 patients receiving conditioning therapy for BMT without Campath could still be detected on day 0. The data demonstrate that in vivo Campath-1G causes rapid depletion of peripheral blood DCs and in conjunction with high-dose chemotherapy or chemoradiotherapy totally cleared DCs from the peripheral blood in the majority of patients. Furthermore, we also showed a direct in vitro effect of Campath-1G in killing purified DCs by complement-dependent cytotoxicity suggesting that the in vivo effect of Campath-1G was a direct effect and not merely due to pooling of DCs into the extravascular space. These effects of Campath on DCs may in part explain the lack of severe acute GVHD observed using pretransplant Campath.18,19,21 However, our studies have been confined to the effects on circulating DCs and we have not studied whether CD52 is expressed on tissue DCs or whether in vivo Campath-1G depletes these cells as efficiently as peripheral blood DCs. Furthermore, other mechanisms are probably involved in the suppression of acute GVHD by pretransplant Campath-1G because we have previously demonstrated that the antibody has a half-life of approximately 13 hours and we found that at the time of marrow infusion plasma levels of Campath were sufficient to deplete donor T cells by ADCC in the majority of cases.22 Thus we cannot be certain whether depletion of host DCs contributes to the prevention of acute GVHD by pretransplant Campath antibodies because it is impossible to separate this effect from T-cell depletion of the graft by residual Campath-1G. As well as effects on host DCs we considered whether Campath antibodies could delay donor DC reconstitution after transplant. Our results show that there is a slow recovery of both DC1 and DC2 following allogeneic transplantation. However, there were no significant differences between the Campath and non-Campath groups (Figure 4), which suggested that the regeneration of DCs after transplant was not adversely affected by pretransplant Campath-1G.
Because host DCs persisting after transplant might induce acute GVHD, we considered it important to determine the origin of DCs regenerating after transplantation. In all patients who received pretransplant Campath-1G, peripheral blood DCs were totally of donor origin. None of these patients had evidence of GVHD at the time of analysis. We have not specifically studied DC chimerism in patients undergoing transplantation with established acute GVHD, but early reports from others suggest that this is associated with a pattern of mixed chimerism,35 which is in keeping with the role of residual host DCs in mediating GVHD.
In summary, we found that peripheral blood DCs express the CD52 antigen and that Campath-1G induces DC death on in vitro culture. In vivo Campath when used in combination with conditioning therapy totally depletes circulating host DCs, which contrasts with conditioning regimens not including Campath where the majority of patients still had circulating host DCs at the time of transplantation. These observations may therefore in part explain the low incidence of acute GVHD in patients receiving pretransplant Campath-1G. Furthermore, these results support the concept that antibodies that specifically target host DCs may be of value in the prevention of acute GVHD without the prolonged immunosuppression caused by T-cell depletion.
The authors would like to thank Dr Adrian Robins and Dr Heather Judge from the Division of Molecular and Clinical Immunology, School of Clinical Laboratory Sciences, University of Nottingham, Nottingham, United Kingdom, for their kind help in purifying dendritic cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nigel H. Russell, Haematology Department, Nottingham City Hospital, Hucknall Rd, Nottingham NG5 1PB, United Kingdom; e-mail: nigel.russell@nottingham.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal