We analyzed factors having an impact on response to treatment and survival in 78 consecutive patients with chronic myeloid leukemia (CML) in blastic transformation (BT) referred to the Hammersmith Hospital from January 1995 to December 2000. BT was defined as the presence of at least 30% blasts in blood or marrow or extramedullary blastic deposits. Immunophenotyping of blasts showed 57 myeloid, 19 lymphoid, and 2 biphenotypic. The median age of the patients was 39.1 years (range, 11.3-73.4 years), with 55 males and 23 females. The median survival for all patients after onset of BT was 8.2 months (95% CI, 6.4-10). Patients in lymphoid BT survived longer than those in myeloid BT (median, 11.2 months versus 6.9 months, P = .052). Initial treatment varied; 41 patients received cytotoxic drugs, 8 underwent allogeneic or autologous transplantation procedures, 21 received STI571 (imatinib mesylate, Gleevec), 1 received radiotherapy, and 7 received no therapy. Of the 25 (32%) patients who achieved a “second chronic phase” with first therapy, 6 of 21 (29%) were treated with STI571 and 19 of 50 (38%) were treated with chemotherapy, transplantation, or radiotherapy. Patients who achieved a second chronic phase survived longer than those who did not (median time from onset of BT 12.0 months versus 6.3 months, P = .0004). In multivariate analysis the finding of more than 50% blast cells in the blood and the presence of cytogenetic progression were independent adverse prognostic variables for survival. We conclude that survival after onset of BT has improved in recent years but is still unsatisfactory. We speculate that the combined use of STI571 with cytotoxic drugs may offer additional benefit.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder, characterized by the constitutively activated BCR-ABL tyrosine kinase. The course of CML is generally triphasic; the disease is usually diagnosed in chronic phase, changes spontaneously after a variable interval to an accelerated phase, and then proceeds to a phase of blastic transformation (BT) or blast crisis. The BT resembles superficially an acute leukemia arising de novo, but is in fact a distinct entity characterized by marked refractoriness to treatment and dismal outcome with a median survival of only 3 to 6 months from the onset.1
There is no standard therapy for patients in BT. The use of chemotherapy regimens of variable intensities offer short-term benefit at best,2-8 and allogeneic stem cell transplantations are usually, though not invariably, unsuccessful.9 10 The preliminary experience with the Abl kinase inhibitor STI571 (imatinib mesylate, Gleevec) has renewed enthusiasm to define the best treatment options for BT of CML.
We describe here our experience with the treatment of patients with CML in BT over recent years. The patients have been treated variously, with traditional chemotherapy regimens or STI571 or stem cell transplantation (SCT). We also describe their clinical and cytogenetic features and the prognostic factors for response and survival.
Patients and methods
Patients
The records of patients with CML with onset of BT between January 1995 and December 2000 were reviewed. BT was defined as the presence of at least 30% blasts in peripheral blood (PB) or bone marrow (BM) or evidence of extramedullary disease.11Clinical and laboratory features at the time of diagnosis of CML and subsequently at the time of BT were recorded. Cytogenetic data were reviewed at diagnosis and at the time of BT. The blast cell phenotype was confirmed by immunophenotyping.
Before treatment, informed consent was provided by all patients in accordance with the Declaration of Helsinki. Patients who received STI571 were treated in accordance with studies approved by the Local Research Ethics Committee. Response to treatment was defined by restoration of hematologic features of chronic phase disease, which we have designated “second” chronic phase (with < 5% blasts in PB and BM). Inability to achieve a “second” chronic phase was considered as failure of therapy. After the initial treatment the details of subsequent treatment designed to induce or consolidate chronic phase, for example, high-dose chemotherapy with autologous or allogeneic stem cell rescue, were recorded and analyzed.
Chemotherapy
Forty-one patients received one or other form of chemotherapy starting as soon as possible after the diagnosis of BT was confirmed. Details of this chemotherapy are summarized in Table1.
Chemotherapy regimens used to treat BT
| Regimen . | No. of patients . |
|---|---|
| AML-like | 7 |
| Daunorubicin, cytarabine, 6-thioguanine (DAT); mitoxantrone, cytarabine, etoposide (MAE); cytarabine, daunorubicin, etoposide (ADE) | |
| ALL-like | 16 |
| Vincristine, daunorubicin, prednisolone l-asparaginase, Intrathecal MTX under UKALL or MRC protocol | |
| FLAG-IDA | 4 |
| Fludarabine, high-dose cytarabine, G-CSF, idarubucin | |
| Other combinations | 9 |
| Idarubicin, cytarabine, etoposide (ICE), carboplatin, mitoxantrone, cytarabine; mitoxantrone, cytarabine; cytarabine, interferon; 6-thioguanine, hydroxyurea, interferon; mitoxantrone, hydroxyurea | |
| Single agents | 5 |
| Mitoxantrone, 6-mercaptopurine, etoposide |
| Regimen . | No. of patients . |
|---|---|
| AML-like | 7 |
| Daunorubicin, cytarabine, 6-thioguanine (DAT); mitoxantrone, cytarabine, etoposide (MAE); cytarabine, daunorubicin, etoposide (ADE) | |
| ALL-like | 16 |
| Vincristine, daunorubicin, prednisolone l-asparaginase, Intrathecal MTX under UKALL or MRC protocol | |
| FLAG-IDA | 4 |
| Fludarabine, high-dose cytarabine, G-CSF, idarubucin | |
| Other combinations | 9 |
| Idarubicin, cytarabine, etoposide (ICE), carboplatin, mitoxantrone, cytarabine; mitoxantrone, cytarabine; cytarabine, interferon; 6-thioguanine, hydroxyurea, interferon; mitoxantrone, hydroxyurea | |
| Single agents | 5 |
| Mitoxantrone, 6-mercaptopurine, etoposide |
UKALL indicates United Kingdom Acute Lymphoid Leukaemia; MRC, Medical Research Council.
STI571
Twenty-one patients received STI571 as the first treatment for myeloid BT at a dose of 400 mg daily, modified subsequently based on the response and toxicity.
Allografts
Twenty-three patients received an allograft after the onset of blast crisis (2 as front-line therapy). The conditioning regimen for the majority of patients consisted of cyclophosphamide-total body irradiation (Cy-TBI; n = 19). In other patients it was either busulfan-cyclophosphamide (Bu-Cy; n = 1), Bu-Cy-TBI (n = 1), FLAG-IDA (n = 1), or etoposide-TBI (n = 1). Prophylaxis for graft-versus-host disease (GVHD) consisted of cyclosporin A and methotrexate (MTX) for HLA-identical sibling donors, and additional Campath-1G or Campath-1H for “matched” unrelated donor transplants.12 Patients received a median total nucleated cells (TNC) of 3.22 × 108/kg.
Autografts
Six patients received autografts as front-line treatment and a further 8 patients received autografts only after other therapy for blast crisis. The conditioning regimens used were highly variable: 16 mg busulphan in 3, 8 mg busulphan in 5, FLAG-IDA in 5, and FLA-Bu in 1 patient. Patients received a median TNC of 5.0 × 108/kg.
Statistical methods
Response rates with different first-line treatments were compared using the χ2 test. Survival curves were plotted using the Kaplan-Meier method, and compared using the log-rank test. Cox regression analysis was performed to try and identify prognostic factors for response and survival. P ≤ .05 was considered significant.
Results
Seventy-eight patients fulfilled the criteria for a diagnosis of BT with men outnumbering women at a ratio of 55:23. These patients had been treated from original diagnosis by hydroxyurea, busulfan, interferon, cytarabine, SCT or other (eg, 6-thioguanine, splenectomy, etc), alone or in various combinations. Clinical and laboratory features at the diagnosis of BT are summarized in Table2. Immunophenotyping showed that 57 (73%) patients had myeloid blasts, 19 (24%) had lymphoid blasts, and 2 had biphenotypic (mixed myeloid and lymphoid) blast cells. Of the 21 patients treated with STI571 (16 men, 5 women), the median age was 51.3 years (range, 20.5-78.5 years) and the median duration of disease at onset of BT was 40.5 months (range, 3-131 months). Only 2 patients had evidence of cytogenetic evolution at BT.
Patient and disease characteristics at diagnosis of BT
| Variable . | Median (range) or No. (%) . |
|---|---|
| Patient, age, y | 39.1 (11.3-73.4) |
| Duration of disease, mo | 24 (0-253) |
| Hemoglobin level, g/dL | 10.3 (5.3-15.2) |
| WBC count, × 109/L | 33.7 (1.5-800) |
| Platelet count, × 109/L | 118 (7-1623) |
| PB blasts % | 38 (2-100) |
| PB basophils % | 1 (0-34) |
| BM blasts % | 47 (3-100) |
| Splenomegaly, % | |
| No | 21 (42) |
| Yes | 29 (58) |
| BM fibrosis grade, % | |
| 0 | 4 (10) |
| 1 | 5 (12) |
| 2 | 6 (15) |
| 3 | 10 (24) |
| 4 | 16 (39) |
| No. of symptoms,* % | |
| 0 | 8 (16) |
| 1 | 11 (22) |
| 2 | 11 (22) |
| More than 2 | 21 (41) |
| Variable . | Median (range) or No. (%) . |
|---|---|
| Patient, age, y | 39.1 (11.3-73.4) |
| Duration of disease, mo | 24 (0-253) |
| Hemoglobin level, g/dL | 10.3 (5.3-15.2) |
| WBC count, × 109/L | 33.7 (1.5-800) |
| Platelet count, × 109/L | 118 (7-1623) |
| PB blasts % | 38 (2-100) |
| PB basophils % | 1 (0-34) |
| BM blasts % | 47 (3-100) |
| Splenomegaly, % | |
| No | 21 (42) |
| Yes | 29 (58) |
| BM fibrosis grade, % | |
| 0 | 4 (10) |
| 1 | 5 (12) |
| 2 | 6 (15) |
| 3 | 10 (24) |
| 4 | 16 (39) |
| No. of symptoms,* % | |
| 0 | 8 (16) |
| 1 | 11 (22) |
| 2 | 11 (22) |
| More than 2 | 21 (41) |
Fever, night sweats, weight loss, malaise, bleeding, bone pain, abdominal discomfort, focal swellings.
At diagnosis of BT the various treatments used included chemotherapy, STI571, or autologous or allogeneic SCT. The chemotherapy regimens are presented in Table 1. Single-agent therapies were given with palliative intent.
Cytogenetic data were available for all the patients at diagnosis; 70 patients had a classical t(9;22)(q34;q11) and 8 patients had a variant translocation. Fifty-one of 78 patients (65%) had evidence of cytogenetic evolution at BT. The major additional changes (n = 28) consisted of a second Philadelphia (Ph) chromosome (n = 5), trisomy 8 (n = 7), isochromosome 17q (n = 7), either alone or in various combinations (n = 9). All other so-called minor additional changes were present in the remaining patients either alone or in addition to major changes. All the chromosomes were involved in the process of cytogenetic evolution to varying degrees.
When patients were classified by blast cell phenotype, it emerged that those with lymphoid blasts were significantly younger and had an earlier onset of BT than those with myeloid blasts. However, when the 3 patients with diagnosis of BT at the start of their disease were excluded, the duration of disease before BT no longer differed in the 2 groups (Table 3). There was no difference in the interferon treatment received in chronic phase for these 2 groups (10 of 19 versus 35 of 57, P = .50).
Patient and disease features by type of BT
| Feature . | Myeloid BT (n = 57) (median, range) . | Lymphoid BT (n = 19) (median, range) . |
|---|---|---|
| Patient, age, y3-150 | 44 (18-79) | 36 (14-54) |
| Sex, male/female | 37/16 | 13/6 |
| Disease duration, mo3-150 | 30 (2-253) | 10 (0-109) |
| Hemoglobin, g/dL | 10.6 (5-15) | 10.5 (8-15) |
| WBC, × 109/L | 41 (2-800) | 31 (1.5-254) |
| Platelet, × 109/L | 118 (7-1623) | 103 (13-1170) |
| PB blast, % | 35 (2-92) | 38 (3-100) |
| BM blast, % | 40 (3-90) | 90 (32-100) |
| Basophil, % | 1 (0-34) | 0 (0-3) |
| Spleen size, cm | 2 (0-24) | 4 (0-15) |
| Feature . | Myeloid BT (n = 57) (median, range) . | Lymphoid BT (n = 19) (median, range) . |
|---|---|---|
| Patient, age, y3-150 | 44 (18-79) | 36 (14-54) |
| Sex, male/female | 37/16 | 13/6 |
| Disease duration, mo3-150 | 30 (2-253) | 10 (0-109) |
| Hemoglobin, g/dL | 10.6 (5-15) | 10.5 (8-15) |
| WBC, × 109/L | 41 (2-800) | 31 (1.5-254) |
| Platelet, × 109/L | 118 (7-1623) | 103 (13-1170) |
| PB blast, % | 35 (2-92) | 38 (3-100) |
| BM blast, % | 40 (3-90) | 90 (32-100) |
| Basophil, % | 1 (0-34) | 0 (0-3) |
| Spleen size, cm | 2 (0-24) | 4 (0-15) |
P < .05 (Mann-Whitney test).
Predictors of onset of BT
The median time to onset of BT was only 24 months (range, 0-253 months). The various features at diagnosis of CML that were analyzed for their impact on timing of BT included age, sex, number of initial symptoms, disease status at diagnosis, spleen size, hemoglobin, white blood cell (WBC) count, platelet count, and the initial treatment received during chronic or accelerated phase. From univariate analyses 3 factors predicted an earlier onset of blastic transformation: (1) at least 2 symptoms at disease diagnosis (P = .039), (2) any degree of splenomegaly (P = .06), and (3) treatment other than SCT or interferon (P = .02).
Response to therapy
Different types of first therapy were used at the time of BT in an attempt to induce a second chronic phase (Table4). Chemotherapy was used in 41 patients with a response in 13 (32%). Twenty-one patients with myeloid BT received STI571 resulting in a chronic phase of variable duration in 6 (29%). Eight patients received high-dose or low-intensity conditioning regimen first followed by either autologous (n = 6) or allogeneic (n = 2) stem cell rescue. Five of them (3 and 2, respectively, in the 2 groups) entered a second chronic phase (62%). One patient with extramedullary disease received external beam radiotherapy and had a complete response. Thus first-line therapy resulted in a second chronic phase in 35% (25 of 71) of patients. The response rate was 31% for myeloid and 42% for the lymphoid phenotype (P = .87). One of the 2 patients with biphenotypic BT responded. Seven patients were not evaluable, either due to early death or because they made a single visit to our department.
Response to first treatment for BT
| Treatment . | No. . | Response (%) . |
|---|---|---|
| Chemotherapy | 41 | 13 (32) |
| AML-like | 7 | 3 |
| ALL-like | 16 | 6 |
| FLAG-IDA | 4 | 1 |
| Other combinations | 9 | 3 |
| Single agents | 5 | 0 |
| STI571 | 21 | 6 (29) |
| Allo-SCT | 2 | 2 (100) |
| Auto-SCT | 6 | 3 (50) |
| Radiotherapy | 1 | 1 |
| Overall | 71 | 25 (35) |
| Treatment . | No. . | Response (%) . |
|---|---|---|
| Chemotherapy | 41 | 13 (32) |
| AML-like | 7 | 3 |
| ALL-like | 16 | 6 |
| FLAG-IDA | 4 | 1 |
| Other combinations | 9 | 3 |
| Single agents | 5 | 0 |
| STI571 | 21 | 6 (29) |
| Allo-SCT | 2 | 2 (100) |
| Auto-SCT | 6 | 3 (50) |
| Radiotherapy | 1 | 1 |
| Overall | 71 | 25 (35) |
Thirty-one patients received a second cycle of chemotherapy, to either induce a second chronic phase or for consolidation of chronic phase. Seven patients achieved a second chronic phase, and thus overall 34 patients achieved a second chronic phase (25 with first therapy, 7 with second course of chemotherapy, 2 with second-line SCT). None of the nonresponders to first and second therapy responded to a third attempt.
The median duration of remission of chronic phase in patients with myeloid BT who received chemotherapy as the first therapy with or without a subsequent SCT was 198 days (range, 61-546 days). In the STI571 group, it was 205 days (range, 29-288 days).
Factors predicting achievement of second chronic phase
Univariate analyses to identify prognostic factors for response to first therapy identified only one factor as significant—patients with less than 50% blasts cells in their marrow at time of BT were more likely to enter into second chronic phase with first therapy.
SCT for BT
After initial therapy, 37 patients underwent a SCT (Table5); 23 patients underwent an allogeneic SCT. One patient suffered a primary graft failure, 6 had moderate to severe acute GVHD, 7 had clinical features to suggest veno-occlusive disease (VOD) of the liver, and 21 had clinical or laboratory evidence of infection. Other posttransplantation complications included hemorrhagic cystitis (n = 1) and cardiac problems (n = 4). Sixteen patients achieved molecular remission or chronic phase. The median survival after the onset of BT was 11.6 months (range, 10.7-12.5 months). Median duration of survival after undergoing SCT was 6.2 months (range, 2.9-9.6 months). At the time of analysis, 16 of these 23 patients had died.
SCT for BT
| Factor . | SCT (n = 37) . | Allo-SCT (n = 23) . | Auto-SCT (n = 14) . |
|---|---|---|---|
| Median age, y (range) | 36.3 (17.1-57.2) | 31.3 (17.1-50.5) | 45.8 (31-57.2) |
| Source of stem cells | |||
| BM | 20 | 19 | 1 |
| PBSC | 17 | 4 | 13 |
| Disease status at time of SCT | |||
| 2nd CP | 7 | 6 | 1 |
| ≥ 3rd CP | 6 | 5 | 1 |
| Refractory | 13 | 7 | 6 |
| Untreated | 7 | 2 | 5 |
| Relapsed | 4 | 3 | 1 |
| Median TNC, × 108/kg (range) | 3.8 (0.3-11) | 3.2 (0.3-11) | 5.0 (1.5-11) |
| Response to SCT (%) | |||
| Chronic phase | 20 (54) | 16 (69) | 4 (28) |
| Refractory | 12 (32) | 4 (17) | 8 (57) |
| Not evaluable | 5 (13) | 3 (13) | 2 (14) |
| Median survival after BT, mo (95% CI) | 11.0 (7.7-14) | 11.6 (10.7-12.5) | 5.8 (3.5-8.0) |
| Factor . | SCT (n = 37) . | Allo-SCT (n = 23) . | Auto-SCT (n = 14) . |
|---|---|---|---|
| Median age, y (range) | 36.3 (17.1-57.2) | 31.3 (17.1-50.5) | 45.8 (31-57.2) |
| Source of stem cells | |||
| BM | 20 | 19 | 1 |
| PBSC | 17 | 4 | 13 |
| Disease status at time of SCT | |||
| 2nd CP | 7 | 6 | 1 |
| ≥ 3rd CP | 6 | 5 | 1 |
| Refractory | 13 | 7 | 6 |
| Untreated | 7 | 2 | 5 |
| Relapsed | 4 | 3 | 1 |
| Median TNC, × 108/kg (range) | 3.8 (0.3-11) | 3.2 (0.3-11) | 5.0 (1.5-11) |
| Response to SCT (%) | |||
| Chronic phase | 20 (54) | 16 (69) | 4 (28) |
| Refractory | 12 (32) | 4 (17) | 8 (57) |
| Not evaluable | 5 (13) | 3 (13) | 2 (14) |
| Median survival after BT, mo (95% CI) | 11.0 (7.7-14) | 11.6 (10.7-12.5) | 5.8 (3.5-8.0) |
PBSC indicates peripheral blood stem cell; CP, chronic phase.
Of the 14 patients who received an autologous SCT, 1 patient suffered grade 1 autologous acute GVHD involving the skin, 1 had clinical features to suggest VOD, and 12 patients had clinical or laboratory evidence of infection. Two patients suffered from hemorrhagic cystitis. Four patients achieved chronic phase. The median survival after the onset of BT was 5.8 months (range, 13.5-8.0 months). Median duration of survival after undergoing SCT was 4 months (range, 1.2-6.8 months). At the time of analysis, 10 of these 14 patients are dead.
Survival
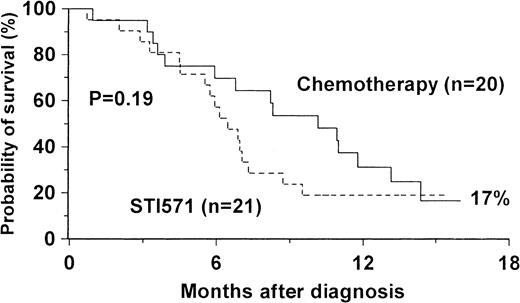
The median survival of patients after BT was 8.2 months (95% CI, 6.4-10). The probability of survival at 24 months was 15% (Figure1). The features associated with improved survival are shown in Table 6. The adverse prognostic factors for survival were patient age over 50 years, WBC count more than 60 × 109/L at BT, PB blasts more than 50%, evidence of cytogenetic evolution at BT, failure to achieve a second chronic phase with first- or second-line therapy, not undergoing high-dose chemotherapy followed by SCT, and heavy treatment before onset of BT in chronic or accelerated phase of CML. On comparing survival in the groups of patients receiving first therapy for myeloid BT with chemotherapy (n = 20) or STI571 (n = 21), we found that the median survival durations were 10.1 months (95% CI, 6.3-14) and 6.5 months (95% CI, 5-8), respectively (P = .19, Figure2). Of no prognostic value for survival in univariate analyses were patient sex, duration of disease before BT, symptoms at BT, spleen size, hemoglobin, platelet count, marrow blasts or fibrosis, type of additional cytogenetic abnormality (double Ph, trisomy 8, or isochromosome 17), and the type of first treatment (including chemotherapy or STI571). Multivariate analysis revealed that 2 factors were independent predictors of mortality. The presence of cytogenetic evolution was associated with an increased risk when compared with patients with no evolution (RR = 2.6; 95% CI, 1.4-4.8;P = .003), whereas a PB blast count at diagnosis of BT of more than 50% had a relative risk of 2.7 (95% CI, 1.4-5.5,P = .004).
Univariate analysis of prognostic factors for survival after BT
| Factor . | No. . | Median survival (95% CI) . | P . |
|---|---|---|---|
| Patient age, y | |||
| 50 or younger | 49 | 8.8 (7-10) | .04 |
| Older than 50 | 25 | 5.5 (4-7) | |
| WBC count at BT, × 109/L | |||
| No higher than 60 | 43 | 8.7 (5-12) | .04 |
| Higher than 60 | 19 | 5.6 (3-8) | |
| PB blasts at BT, % | |||
| No more than 50 | 35 | 8.2 (6-10) | .025 |
| More than 50 | 15 | 3.6 (0-7) | |
| Cytogenetic evolution | |||
| No | 24 | 11.8 (9-15) | .012 |
| Yes | 50 | 6.3 (5-7) | |
| Response to first/second therapy | |||
| CP | 34 | 13 (10-17) | .00001 |
| No CP | 40 | 5.7 (5-7) | |
| Pre-BT treatment | |||
| No more than 2 agents | 37 | 8.7 (6-11) | .05 |
| More than 2 agents | 35 | 6.8 (5-8) | |
| SCT | |||
| Yes | 37 | 11 (8-14) | .039 |
| No | 37 | 6.8 (5-8) | |
| Type of SCT | |||
| Allo | 23 | 11.6 (10.7-12.5) | .024 |
| Auto | 14 | 5.8 (3.5-8) |
| Factor . | No. . | Median survival (95% CI) . | P . |
|---|---|---|---|
| Patient age, y | |||
| 50 or younger | 49 | 8.8 (7-10) | .04 |
| Older than 50 | 25 | 5.5 (4-7) | |
| WBC count at BT, × 109/L | |||
| No higher than 60 | 43 | 8.7 (5-12) | .04 |
| Higher than 60 | 19 | 5.6 (3-8) | |
| PB blasts at BT, % | |||
| No more than 50 | 35 | 8.2 (6-10) | .025 |
| More than 50 | 15 | 3.6 (0-7) | |
| Cytogenetic evolution | |||
| No | 24 | 11.8 (9-15) | .012 |
| Yes | 50 | 6.3 (5-7) | |
| Response to first/second therapy | |||
| CP | 34 | 13 (10-17) | .00001 |
| No CP | 40 | 5.7 (5-7) | |
| Pre-BT treatment | |||
| No more than 2 agents | 37 | 8.7 (6-11) | .05 |
| More than 2 agents | 35 | 6.8 (5-8) | |
| SCT | |||
| Yes | 37 | 11 (8-14) | .039 |
| No | 37 | 6.8 (5-8) | |
| Type of SCT | |||
| Allo | 23 | 11.6 (10.7-12.5) | .024 |
| Auto | 14 | 5.8 (3.5-8) |
Survival after diagnosis of myeloid BT CML by initial treatment with chemotherapy or STI571.
Survival after diagnosis of myeloid BT CML by initial treatment with chemotherapy or STI571.
The median survival of patients after the onset of myeloid BT was 6.9 months (95% CI, 5.8-7.9) and those with lymphoid BT 11.2 months (95% CI, 6.4-16). The 2 patients with biphenotypic BT died at 8.4 months and 17.2 months after BT. In univariate analysis lower WBC (≤ 60 × 109/L, lower BM blast percentage (≤ 50%), and achievement of chronic phase with first therapy were associated with improved survival in patients with myeloid BT. No significant prognostic factors were identified with lymphoid BT.
The median duration of survival after SCT was 5.4 months (95% CI, 3.3-7.6). Survival after SCT was significantly better for those in CP at the time of transplantation (8.2 months in chronic phase versus 2.6 months for refractory/relapsed patients, P = .015) and for patients who received chemotherapy or initial transplantation as first treatment for BT compared to STI571 (4 months versus 8 months versus 2.6 months, respectively, P = .026).
At the time of analysis (July 2001), 55 of the 78 patients had died. Disease progression was the most common cause of death (54 of 78, 69%). Thirteen patients survived beyond 12 months (range, 13.2-33.9 months), but no outstanding prognostic features were identified in this group.
Discussion
Our results confirm the overall poor prognosis for patients with BT of CML. The definition of BT is somewhat arbitrary11and the definition of response that we have used, namely, restoration of a hematologic picture resembling chronic phase, is clearly specious because small numbers of cells able to regenerate blastic phase disease must persist in almost every case. Moreover, the patient population we have studied is clearly selected in that they were younger than a typical CML patient population in the Western world, they had shorter durations of chronic phase disease than an average population, and they were treated more intensively than might generally have been expected. These points notwithstanding, their median survival (8.2 months) did not differ appreciably from the survival of patients reported previously by other workers (Table 7). We have, however, confirmed that patients whose blast cells had a lymphoid phenotype13 14 have a better overall prognosis than patients with myeloid phenotypes. Patients who achieved second chronic phase survived longer than those who did not. We also showed that relatively high percentages of blasts in the peripheral blood and the finding of evidence of cytogenetic progression were adverse prognostic factors for survival.
Previous major publications of CML in BT
| Author . | No. . | Overall response, % . | Median survival, mo . | Prognostic factors . |
|---|---|---|---|---|
| Coleman et al, 19808 | 202 | 34 | 3 | Response to therapy |
| Kantarjian et al, 198726 | 242 | 36 | 4.5 | Hemoglobin, platelets, BM blasts, type of BT, clonal evolution |
| Anger et al, 19886 | 69 | 40 | 2.5 | Clonal evolution, type of BT, BM blasts + promyelocytes, response to therapy |
| Derderian et al, 199314 | 296 | 20 | 3 | Type of BT |
| (LBC-68) | (49) | (9) | ||
| Griesshammer et al, 199629 | 90 | 29 | 2.8 | Response to therapy, presence of trisomy 8 |
| Sacchi et al, 19991 | 162 | 22 | 5.5 | Decitabine therapy particularly in older patients |
| Present study | 78 | 46 | 8.2 | Cytogenetic evolution, PB blasts |
| Author . | No. . | Overall response, % . | Median survival, mo . | Prognostic factors . |
|---|---|---|---|---|
| Coleman et al, 19808 | 202 | 34 | 3 | Response to therapy |
| Kantarjian et al, 198726 | 242 | 36 | 4.5 | Hemoglobin, platelets, BM blasts, type of BT, clonal evolution |
| Anger et al, 19886 | 69 | 40 | 2.5 | Clonal evolution, type of BT, BM blasts + promyelocytes, response to therapy |
| Derderian et al, 199314 | 296 | 20 | 3 | Type of BT |
| (LBC-68) | (49) | (9) | ||
| Griesshammer et al, 199629 | 90 | 29 | 2.8 | Response to therapy, presence of trisomy 8 |
| Sacchi et al, 19991 | 162 | 22 | 5.5 | Decitabine therapy particularly in older patients |
| Present study | 78 | 46 | 8.2 | Cytogenetic evolution, PB blasts |
LBC indicates lymphoid blastic crisis.
In this retrospective analysis, the first-line treatment that patients received was highly variable and included cytotoxic drug combinations appropriate to acute myeloid leukemia/acute lymphoid leukemia (AML/ALL), autografting with various regimens, and allografting. We were unable to demonstrate any unequivocal benefit for any of the approaches we used. This variability of treatment approaches as applied in previous reports of the management of CML in BT presumably reflects the fact that no particular approach (before the advent of STI571) seemed to offer any particular therapeutic advantage.
Autologous SCTs have been used to support hematopoiesis during high-dose therapy for CML BT.15,16 In 1996, we reported the use of a reduced intensity regimen using busulfan alone for autografts in patients in BT, which resulted in restoration of chronic phase in 5 of 9 patients, with relatively little toxicity.17 This regimen has subsequently been used to treat some patients with BT as initial therapy and in some to consolidate the second chronic phase, with acceptable toxicity and comparable results to other chemotherapy regimens. The approach should probably be studied further. Allogeneic SCT induces durable remission in less than 10% of patients.18,19 However, better results are achieved when SCT is done after achieving chronic phase rather than in blastic phase itself.9 20 Our study also supports this notion.
The new ABL kinase inhibitor STI571 has proved extremely valuable in the management of patients with CML in chronic phase21 and is effective also for treating patients with CML in advanced phases.22 In a recently reported phase I study of patients with myeloid BT, the overall response rate to STI571 was 55%; there was a reduction of BM blasts to less than 5% in 21% of the patients. We obtained comparable results with STI571 as first-line therapy for BT. We compared our results using STI571 as first-line treatment with our results using “conventional” chemotherapy, though there were differences in the 2 patients cohorts; the STI571-treated patients were older than the conventionally treated patients, they had a lower incidence of additional cytogenetic changes, and their duration of follow-up was relatively short. These differences notwithstanding, there was no appreciable difference in survival between patients treated with STI571 and those undergoing chemotherapy.
Chromosomal changes in addition to the Ph chromosome have been reported in more than 70% of patients with BT.23 Most authors have shown that the finding of additional changes is associated with reduced survival, but this is not universally agreed.24-29 In this study approximately 65% of patients had additional cytogenetic changes and evidence of cytogenetic evolution was an independent adverse prognostic factor for survival. Its presence, however, had no impact on response to first therapy.
In summary, the management of patients with CML in BT remains highly unsatisfactory. Although the “up-front” use of STI571 as monotherapy is valuable in the short-term, it appears not to have made a fundamental difference to the longer term clinical outcome. One may speculate how the clinical results of using this exciting new agent for blastic phase CML could be improved. First, it could be used as initial agent for a finite period of time, after which the patient might be eligible for an elective allograft or autograft procedure. Second, in vitro data with CML BT cell lines suggest that combination of STI571 with other drugs (eg, cytarabine, etoposide) displays synergistic activity,30 so its clinical use as initial therapy in combination with these or other cytotoxic drugs including decitabine, troxacitabine, or differentiating agents such as arsenic trioxide, all-trans retinoic acid, or homoharringtonine could prove highly effective.
We thank the many hematologists who referred patients included in this study. We thank also the junior medical staff and nursing staff who played a major role in the care of these patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John M. Goldman, Department of Haematology, Imperial College at Hammersmith Campus, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: jgoldman@ic.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal