In a recent issue of Blood, Greco et al1reported on the expression of a novel structurally altered P2X1 receptor in platelets and in megakaryocytic cell lines. This P2X1 variant lacks 17 amino acids in its extracellular domain due to a deletion within exon 6 of theP2X1 gene (GenBank accession no. 17481172). The authors showed that, after heterologous expression in the 1321N1 astrocytoma cell line, P2X1del subunits constitute a channel preferentially activated by adenosine diphosphate (ADP). In reverse transcriptase–polymerase chain reaction (RT-PCR) analyses, they described this variant as the major P2X1mRNA of platelets, thus claiming that P2X1del may play an important role as an ADP-activated ion channel in these cells. These conclusions are in contradiction with other studies2,3 that show that the functional platelet P2X1 receptor is an adenosine triphosphate (ATP)–gated ion channel that is unresponsive to high-performance liquid chromatography–purified ADP. Indeed, the activation of the P2X1 receptor by ATP or by its stable analogs, α,β-methylene ATP and L-β,γ–methylene ATP, produces a rapid, quickly desensitized Ca2+ influx4 that is responsible for reversible platelet shape change,3,5and that also plays a pivotal role during platelet aggregation induced by collagen.3 These platelet responses to ATP were found to be antagonized by ADP similarly to the inward current produced by ATP in Xenopus oocytes expressing wild-type P2X1 receptors (P2X1wt).3In addition, platelet receptors for ADP have been well characterized and are identified as 2 P2Y receptors: P2Y1 and P2Y12 (reviewed in Gachet6). Both receptors are required for normal platelet responses to ADP, a conclusion recently corroborated in P2Y1−/− and P2Y12−/− knock-out mice.7,8 Thus, the existence in platelets of an ADP-activated variant of the P2X1 ion channel as the major platelet P2X representative, as hypothesized by Greco et al,1 can be questioned.
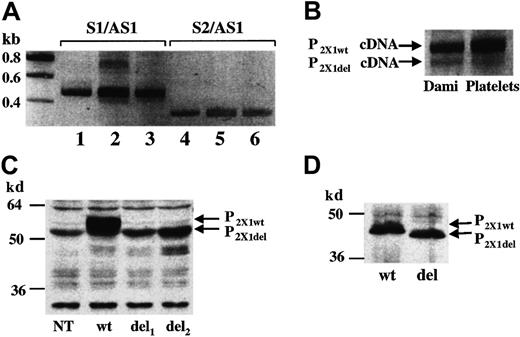
In this letter, we present data that argue against the possibility for a role of P2X1del in platelet function. First, RT-PCR analyses of independent platelet RNA samples followed by sequencing of the PCR products showed major abundance of the P2X1wt mRNA (Figure 1A, lane 2; Figure 1B), whereas the platelet P2X1del mRNA appeared as a minor product. In contrast to the findings of Greco et al,1 we found comparable relative amounts of the P2X1del mRNA in platelets and in the Dami megakaryocytic cell line (Figure 1A, lane 3; Figure 1B). Second, in order to further demonstrate the presence of P2X1del transcripts in platelets the authors designed 2 different sets of primers, S1/AS1 to amplify both P2X1wt and P2X1del cDNAs and S2/AS1 to amplify only P2X1del cDNAs. However, this strategy does not enable a quantitative assessment of P2X1del levels. Indeed, when the pcDNA3-P2X1wtplasmid was used as a PCR template, we showed that the primer set S2/AS1 annealed to the P2X1wt cDNA leading to artificial amplification of the 51 base pair deleted cDNA-encoding P2X1del (Figure 1A, lane 4, as confirmed by sequencing). This phenomenon evidently also can occur during RT-PCR analyses of platelet and Dami cell RNA samples containing both P2X1wtand P2X1del mRNA (Figure 1A, lane 5 and 6, respectively). Third, Western blotting experiments performed after transient transfection of 1321N1 cells with a pcDNA3 vector containing either the P2X1del cDNA (del) or the P2X1wt cDNA (wt) revealed only low amounts of P2X1del proteins at the expected size in comparison to the P2X1wt protein levels (Figure 1C). To ensure that the antibody used in this detection would recognize the 2 proteins with equal sensitivity the P2X1del(del) and P2X1wt (wt) proteins were synthesized in an in vitro T7-coupled transcription/translation rabbit reticulocyte system. Western blotting analyses revealed identical amounts of the 2 in vitro–translated (nonglycosylated) proteins (Figure 1D). These data thus suggest that the P2X1del protein is not properly produced or is mainly unstable in the transfected 1321N1 cells.
Analyses of P2X1del mRNA and protein.
(A) RT-PCR of platelet (lanes 2 and 5) and Dami cell (lanes 3 and 6) RNA; the primer sets S1/AS1 and S2/AS1 are described by Greco et al.1 In lanes 1 and 4, the pcDNA3-P2X1wtplasmid was used as a PCR template. (B) Enlarged view of lanes 2 and 3. For these experiments, platelets were isolated from freshly drawn blood of at least 10 unrelated healthy volunteers. (C) Western blots of P2X1del and P2X1wt in transfected 1321N1 total cell extracts. The pcDNA3-P2X1del vector was transfected in 2 independent experiments (del1 and del2) in parallel with the pcDNA3-P2X1wt vector (wt). A nontransfected cell extract is also shown (NT). (D) Western blots of P2X1del (del) and P2X1wt (wt) proteins synthesized in a in vitro T7-coupled transcription/translation rabbit reticulocyte system. The rabbit polyclonal anti–human P2X1antibody used in these experiments was previously described.9 Bands corresponding to P2X1del and P2X1wt PCR products and proteins are indicated. Molecular weight ladder is shown on the left.
Analyses of P2X1del mRNA and protein.
(A) RT-PCR of platelet (lanes 2 and 5) and Dami cell (lanes 3 and 6) RNA; the primer sets S1/AS1 and S2/AS1 are described by Greco et al.1 In lanes 1 and 4, the pcDNA3-P2X1wtplasmid was used as a PCR template. (B) Enlarged view of lanes 2 and 3. For these experiments, platelets were isolated from freshly drawn blood of at least 10 unrelated healthy volunteers. (C) Western blots of P2X1del and P2X1wt in transfected 1321N1 total cell extracts. The pcDNA3-P2X1del vector was transfected in 2 independent experiments (del1 and del2) in parallel with the pcDNA3-P2X1wt vector (wt). A nontransfected cell extract is also shown (NT). (D) Western blots of P2X1del (del) and P2X1wt (wt) proteins synthesized in a in vitro T7-coupled transcription/translation rabbit reticulocyte system. The rabbit polyclonal anti–human P2X1antibody used in these experiments was previously described.9 Bands corresponding to P2X1del and P2X1wt PCR products and proteins are indicated. Molecular weight ladder is shown on the left.
Taken together, our data indicate that the P2X1del variant is unlikely to be a major protein in platelets. Moreover, the fact that Greco et al present this variant as a potential ADP-activated channel is not consistent with all the previous molecular and functional studies of platelet P2 receptors.6The quantitative and functional relevance of the platelet P2X1del variant should therefore be reconsidered.3 4
Functional adenosine diphosphate–activated P2X1delreceptor
Oury et al have confirmed our recent identification of a P2X1del variant of the P2X1wt receptor RNA in platelets and megakaryocytic DAMI cells.1-1 The complex array of nucleotide receptors they describe in different cell types suggests that questions of identity and function may not be fully resolved. What we have done, no more and no less, is to show that transfection of nonresponsive 1321 cells with the P2X1del-variant cDNA results in the expression in these cells of a selective homomeric receptor sensitive to adenosine diphosphate (ADP).1-1
Unfortunately, Oury et al have not provided adequate information for an evaluation of their polymerase chain reaction (PCR) results. We have previously shown that stringent annealing temperatures (60°C) are needed to minimize nonspecific binding due to the high degree of homology of the S2 and AS1 PCR primers.1(Fig1 legend)There are similar difficulties in evaluating their protein blots because their antibody appears to recognize multiple proteins in nontransfected cells.1(Fig 1C, lane 1, “NT”)In addition, Oury et al do not take into account the cross-reactivity of available antibodies for the P2X1wt and P2X1del receptors. We do not feel that their conclusion that “the P2X1del protein is not properly produced or is mainly unstable in the transfected 1321N1 cells” can be deduced from these results.
Furthermore, contrary to the comments of Oury et al we have made no claims that “the P2X1del variant is a major protein in platelets.” In fact, data from the literature1-2 would suggest that the P2X receptor would be of low abundance in platelets. However, our Ca2+ influx studies clearly show that the P2X1del receptor is an ADP-activated channel, not a potential ADP-activated channel. Consideration of these problems, taken together with the extensive data reported in our original paper,1-1 shows that the question posed by Oury et al as the title of their communication must be answered in the affirmative.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal