Abstract
The impact of elevated vascular endothelial growth factor (VEGF) expression on the course of chronic myeloid leukemia (CML) is unknown. By radioimmunoassay, we measured pretreatment cellular VEGF protein in bone marrow samples from 184 (148 chronic and 36 accelerated/blastic phases) CML patients and found the levels to be 1.6-fold higher than in 31 normal control bone marrow samples (P = .000 01). No significant differences were found in VEGF levels by different phases of CML (P = .1). VEGF levels correlated with older age (P = .01) and higher platelet count (P = .0003), but also with smaller spleen size (P = .004), lower white blood cell count (P = .0006), and lower percentage of peripheral blasts (P = .04). With the use of Cox proportional hazard model and VEGF levels as a continuous variable, high VEGF levels correlated with shorter survival of patients in chronic CML (P = .008). Multivariate analysis showed that VEGF was not independent of the synthesis stage (P = .09). These data suggest that VEGF plays a role in the biology of CML and that VEGF inhibitors should be investigated in CML.
Introduction
Increased microvessel density has been documented in the bone marrow of patients with several hematologic cancers, including chronic myeloid leukemia (CML). Vascular endothelial growth factor (VEGF) plasma levels have also been shown to be significantly higher in CML patients compared with normal controls.1Lundberg et al2 reported the number of VEGF+bone marrow cells to be significantly higher in samples from CML patients than in normal controls and to correlate with bone marrow vascularity. Ratajczak et al3 reported that VEGF was produced by granulocyte-macrophage colony-stimulating factor/interleukin 3–supported cell colonies derived from CML patients and that VEGF costimulated colony formation of cells obtained from approximately 15% of CML patients studied. Furthermore, VEGF receptor–1 messenger RNA was detected in all 15 chronic phase CML samples examined.3 The clinical relevance of VEGF expression on CML outcome is unknown. Several therapeutic approaches targeting the VEGF/VEGF-receptor (VEGF-R) system have recently been developed.4 Thus, it is important to understand the significance of VEGF in CML to better define the potential role of these approaches in CML therapy. In this study, we examined cellular VEGF protein expression in the bone marrow of patients in different phases of CML and assessed its prognostic significance.
Study design
Patients and controls
Pretreatment cellular VEGF protein concentrations were measured in bone marrow samples collected from 184 patients with CML at presentation to the University of Texas M D Anderson Cancer Center, and in 31 “normal” control bone marrow samples obtained as a part of staging from patients with suspected lymphoma but without evidence of lymphoma in the bone marrow. Although the control samples are actually not normal, they show cell populations similar to those seen in normal bone marrows and are the closest to normal available to us. All samples were obtained under protocols approved by the Internal Review Board and with the patient's written informed consent. The characteristics of patients are shown in Table 1.
Study patients
| Disease phase . | No. . |
|---|---|
| Chronic (early/late) | 148 (118/30) |
| Accelerated | 25 |
| Blastic | 11 |
| Characteristics of patients with chronic phase CML, % | |
| Age at least 60 years | 17 |
| Spleen at least 10 cm below costal margin | 10 |
| White blood cell count at least 30 × 109/L | 57 |
| Platelet count at least 700 × 109/L | 17 |
| Peripheral blasts at least 3% | 11 |
| Marrow blasts at least 5% | 5 |
| Peripheral basophils at least 7% | 17 |
| Marrow basophils at least 3% | 25 |
| Predictors of shorter survival of patients with chronic phase CML (univariate analysis), P | |
| VEGF level | .008 |
| Risk-group assignment | .01 |
| Age | .1 |
| Spleen size | .2 |
| White blood cell count | .9 |
| Platelet count | .002 |
| Hemoglobin level | .9 |
| Percentage of peripheral basophils | .1 |
| Disease phase . | No. . |
|---|---|
| Chronic (early/late) | 148 (118/30) |
| Accelerated | 25 |
| Blastic | 11 |
| Characteristics of patients with chronic phase CML, % | |
| Age at least 60 years | 17 |
| Spleen at least 10 cm below costal margin | 10 |
| White blood cell count at least 30 × 109/L | 57 |
| Platelet count at least 700 × 109/L | 17 |
| Peripheral blasts at least 3% | 11 |
| Marrow blasts at least 5% | 5 |
| Peripheral basophils at least 7% | 17 |
| Marrow basophils at least 3% | 25 |
| Predictors of shorter survival of patients with chronic phase CML (univariate analysis), P | |
| VEGF level | .008 |
| Risk-group assignment | .01 |
| Age | .1 |
| Spleen size | .2 |
| White blood cell count | .9 |
| Platelet count | .002 |
| Hemoglobin level | .9 |
| Percentage of peripheral basophils | .1 |
CML indicates chronic myeloid leukemia; VEGF, vascular endothelial growth factor.
Protein extraction and Western blot analysis
Solid-phase radioimmunoassay
Solid-phase radioimmunoassay (RIA) was used to measure VEGF protein, as described previously.5
Statistical considerations
Associations among variables were assessed by means of the Spearman rank correlation analysis. The Kruskal-Wallis test was used to compare various groups of data. The log-rank test was used to study correlation with patient survival when a cutoff point was used for a given variable. The Cox proportional hazard model was used to study correlation with patient survival when a variable was used as a continuum. The sample size of the chronic phase patient group was chosen on the basis of our goal of analyzing survival. The chronic phase group had 68 events, adequate for fitting a multiple regression model with the 6 covariates (based on the role of approximately 10 events per covariate to protect against overfitting of the data). This covers most of the known important covariates in CML. The blast-crisis and accelerated phase patients were simply patients in these stages that were seen during the same period as chronic phase patients.
Results and discussion
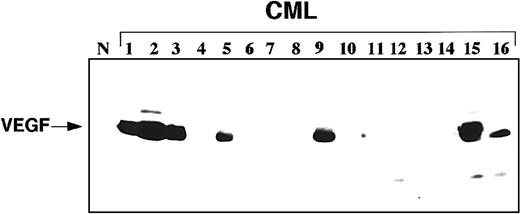
Western blot analysis showed detectable VEGF protein in samples with significantly increased VEGF protein (Figure1). RIA, a more sensitive assay, showed VEGF protein in all bone marrow samples. VEGF levels in 184 CML samples were normalized to the median VEGF level in 31 normal control bone marrow samples, which was assigned a value of 1. The median VEGF value in CML samples was 1.6-fold (range, 0.8–11.3-fold) higher than in normal control samples (P = .000 01). The normal control group was comparable to the CML group in age (P = .1). There were no significant differences of VEGF levels among 118 patients in early chronic, 30 patients in late chronic, 25 patients in accelerated, and 11 patients in blastic phase (P = .1). With the use of Cox proportional hazard model and VEGF levels as a continuous variable, increasing VEGF protein levels correlated with poorer survival of patients in chronic phase (P = .008), but not in accelerated/blastic phases (P = .5). Since the number of patients in accelerated/blastic phase group is small, no definitive conclusions should be made about this group (rather, our results should be verified in a larger group of accelerated/blastic phase patients). The survival was measured from the date the sample was obtained.
Representative Western blot showing VEGF protein expression in samples from patients with CML.
Western blot includes 1 sample from a normal control (N) donor and 16 CML patient samples.
Representative Western blot showing VEGF protein expression in samples from patients with CML.
Western blot includes 1 sample from a normal control (N) donor and 16 CML patient samples.
Correlation analyses of VEGF levels and characteristics of patients with chronic phase CML showed that high VEGF levels had a direct correlation with older age (P = .01) and higher platelet count (P = .0003) and an inverse correlation with spleen size (P = .004), white blood cell count (P = .0006), and percentage of peripheral blasts (P = .04). There was no correlation between VEGF level and hemoglobin (P = .5), percentage of marrow blasts (P = .4), and percentage of peripheral or marrow basophils (P = .3 and P = .7, respectively). Univariate analysis of characteristics associated with survival in chronic phase CML (Table 1) showed, in addition to VEGF levels, a significantly worse prognosis for patients with elevated platelet counts (P = .002) and higher risk-group assignment by the synthesis staging system (P = .01). There was no effect of age (P = .1), spleen size (P = .2), hemoglobin level (P = .9), white blood cell count (P = .9), or percentage of peripheral basophils (P = .1). The synthesis staging system accounts for the following variables: age, spleen size, percentage of blasts in blood or bone marrow, percentage of basophils in blood or marrow, cytogenetic clonal evolution, and platelet count.6 The VEGF effect on survival was reanalyzed in multivariate analysis after synthesis-stage stratification. Multivariate analysis showed that VEGF was not independent of the synthesis stage (P = .09).
A number of studies have confirmed the presence of high VEGF levels in samples from patients with leukemias.1,2,7-9 The prognostic significance of VEGF expression has been analyzed in chronic lymphocytic leukemia and in acute myeloid leukemia (AML).5,7,10 In AML, high levels of cellular VEGF were independent predictors of shorter survival and lower complete remission rates.5 In this study, we showed cellular VEGF protein levels to be highly expressed in all phases of CML and to have significant influence on survival in patients with chronic phase CML. The mechanism behind this observation is not clear. Previous studies have suggested the presence of both autocrine and paracrine VEGF/VEGF-R systems in leukemia.3,8,11 The CML progenitor cell has been recently suggested to arise from a hemangioblastic progenitor cell, the progeny of which are malignant blood cells and genotypically clonal endothelial cells.12 If this is correct, then malignant endothelial cells might play a role in the increased marrow vascularity in CML, and VEGF might be pathophysiologically linked to CML development. Thus, the examination of endothelial cell mitogens as well as surface markers in the bone marrow of CML patients may contribute to our understanding of its pathophysiology and offer targets for new type of treatments. Results of our previous work in AML5 and current findings in CML suggest that targeting VEGF might be a potential therapeutic strategy in myeloid leukemias.
Supported by grant T32-CA09666 from the National Institutes of Health (S.V.); J.C. is Leukemia and Lymphoma Society Scholar in Clinical Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maher Albitar, Department of Hematopathology, The University of Texas M D Anderson Cancer Center, 1515 Holcombe Blvd, Box 72, Houston, TX 77030; e-mail: malbitar@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal