Truncation of signal transducer and activator of transcription (STAT) 5 at the carboxy-terminal domain, either by genetic engineering or by proteolytic cleavage, results in generation of dominant-negative forms. A nuclear serine protease expressed in the myeloid precursor cells is known to mediate this cleavage, but other proteases responsible for this reaction were unknown. We found that calpain, a ubiquitously expressed cysteine protease, also trims STAT5 in vivo and in vitro, within the carboxy-terminal domain. Nuclear element is not necessary for calpain-mediated STAT5 cleavage, since this process occurs in platelets. We also found that STAT3 is a substrate for calpain in vivo and in vitro, indicating that calpain-mediated cleavage is a common feature of STAT3 and STAT5. Thus, our study reveals a novel pathway for posttranslational modification of STAT3 and STAT5.
Introduction
Many cytokines induce tyrosine phosphorylation and subsequent activation of signal transducers and activators of transcription (STATs) 5A and STAT5B, the products of 2 highly related STAT5 genes.1,2 Mice deficient in both genes were found to have defects in response to growth hormone and prolactin, and the functions of peripheral T lymphocytes were compromised.2Loss of STAT5 also led to severe anemia in fetal mice.3
Recently, much attention has been focused on the dominant-negative forms of STAT5A and STAT5B, which lack carboxy-terminal domains.4-9 A serine protease was reported to cleave both isoforms, and there is evidence for a role of the truncated STAT5s in myeloid differentiation.6-9 We previously found that STAT3 and STAT5 are present in platelets and that tyrosine phosphorylation is induced on platelet stimulation by thrombopoietin.10-12 In addition, by using platelets, we and others identified numerous substrates for calpain, including p60src, PTP1B, and focal adhesion kinase.13-17 Therefore, in this study, we used platelets to find a novel nuclear-free cleavage pathway of STATs. We found that both STAT5 and STAT3 were substrates for calpain in vivo and in vitro.
Study design
Calpeptin and μ-calpain were from Calbiochem18(San Diego, CA). An anti-pan STAT5 monoclonal antibody (MoAb) and an anti-STAT3 MoAb against the amino-terminal domain of STAT3 were from Transduction Laboratories (Lexington, KY). The anti-STAT5A and anti-STAT5B polyclonal antibodies against the carboxyl-terminal domains of each STAT were from R&D Systems (Minneapolis, MN). A polyclonal antibody against the carboxy-terminal domain of STAT5 that reacts with both isoforms and a polyclonal antibody against the carboxy-terminal domain of STAT3 were from Santa Cruz Biotechnology (Santa Cruz, CA). Dibucaine and human thrombin were from Sigma (St Louis, MO).
Platelet preparation
Platelet stimulation and protein analysis
Aliquots of platelets (0.5 mL; 3 × 108 cells/mL) were incubated in modified HEPES-Tyrode buffer supplemented with apyrase (0.6 U/mL) and Arg-Gly-Asp-Ser peptide (200 μg/mL).10,11 Preparation of platelet lysates and immunoblotting after sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) were done as described previously.10 11
In vitro cleavage of STATs by calpain
Results and discussion
On gradient SDS-PAGE gels, the STAT5 bands often resolved into 2 closely comigrating bands (Figure 1A, left panel). The upper band comigrated with a band recognized by anti-STAT5A (Figure 1A, right panel), whereas the lower band comigrated with the lower band, reactive with anti-STAT5B. These 2 bands likely corresponded to STAT5A and STAT5B. When platelets were treated with dibucaine, a recognized activator of calpain,13-16 we observed a significant loss of immunoreactive STAT5 and the generation of bands (Figure 1A, left panel, arrowheads) of lower molecular weights, reactive with anti-STAT5 (Figure 1A, middle lane). Inhibition of calpain activation by calpeptin resulted in inhibition of cleavage of STAT5 (Figure 1A, right panel). None of these STAT5-like molecules reacted with antibodies against the carboxy-terminal domains of STAT5A or STAT5B (Figure 1A, middle and right panels), thereby indicating that the bands were STAT5 molecules, which lack the carboxy-terminal domains. Thus, truncation of the carboxy-terminal domains of STAT5 can occur independently of nuclear elements; and calpain, a cysteine protease, is probably responsible for truncation of STAT5.
STAT5 is cleaved by calpain in vivo and in vitro.
(A) Dibucaine treatment of platelets leads to cleavage of STAT5. The platelets were incubated with either calpeptin (20 μM) or dimethyl sulfoxide (DMSO; vehicle of calpeptin; final, 0.1%) for 5 minutes. The platelets were then treated with dibucaine (1 mM) for 15 minutes. Whole-platelet lysates (1.5 × 107 cells/lane) were analyzed by 7.5% to 15% gradient SDS-PAGE. The separated proteins were electrophoretically transferred from the gel to nitrocellulose membranes. STAT5 was detected by immunoblotting with a pan anti-STAT5 MoAb (left panel), anti-STAT5A (middle panel), or anti-STAT5B (right panel). Arrows indicate the relative position of intact STAT5; arrowheads show the positions of cleaved products of STAT5. R indicates control resting platelets; D, dibucaine-treated cells after incubation in DMSO; D + C, dibucaine plus calpeptin; and IB, immunoblotting. (B) In vitro cleavage of STAT5. STAT5 was precipitated by either anti-STAT5A or anti-STAT5B. The immunoprecipitates were untreated or incubated with purified μ-calpain in the presence or absence of calcium ion. After denaturing in SDS, STAT5 was detected by immunoblotting with a pan anti-STAT5 MoAb. (C) Truncated STAT5 molecules cleaved in vitro were not recognized by the anticarboxy-terminal domain of STAT5. IP indicates immunoprecipitation. Methods were the same as those as described in the legend for Figure 1B except that STAT5 was recognized by an antibody against the carboxy-terminal domain of STAT5. (D) Cleavage of STAT5 during platelet aggregation. Platelets were stimulated with thrombin (1 U/mL) for 30 minutes with or without stirring. After addition of ethyleneglycotetraacetic acid (5 mM) and EDTA (5 mM), platelets were lysed by boiling in SDS sample buffer. STAT5 was detected by immunoblotting as described in the legend for Figure 1A by using the pan anti-STAT5 MoAb. Lane 1 shows resting platelets; lane 2, results after thrombin stimulation of platelets for 30 minutes, with stirring, in the absence of Arg-Gly-Asp-Ser; and lane 3, results after thrombin stimulation for 30 minutes without stirring. The arrow indicates full-length STAT5 and the arrowhead a cleaved form of STAT5.
STAT5 is cleaved by calpain in vivo and in vitro.
(A) Dibucaine treatment of platelets leads to cleavage of STAT5. The platelets were incubated with either calpeptin (20 μM) or dimethyl sulfoxide (DMSO; vehicle of calpeptin; final, 0.1%) for 5 minutes. The platelets were then treated with dibucaine (1 mM) for 15 minutes. Whole-platelet lysates (1.5 × 107 cells/lane) were analyzed by 7.5% to 15% gradient SDS-PAGE. The separated proteins were electrophoretically transferred from the gel to nitrocellulose membranes. STAT5 was detected by immunoblotting with a pan anti-STAT5 MoAb (left panel), anti-STAT5A (middle panel), or anti-STAT5B (right panel). Arrows indicate the relative position of intact STAT5; arrowheads show the positions of cleaved products of STAT5. R indicates control resting platelets; D, dibucaine-treated cells after incubation in DMSO; D + C, dibucaine plus calpeptin; and IB, immunoblotting. (B) In vitro cleavage of STAT5. STAT5 was precipitated by either anti-STAT5A or anti-STAT5B. The immunoprecipitates were untreated or incubated with purified μ-calpain in the presence or absence of calcium ion. After denaturing in SDS, STAT5 was detected by immunoblotting with a pan anti-STAT5 MoAb. (C) Truncated STAT5 molecules cleaved in vitro were not recognized by the anticarboxy-terminal domain of STAT5. IP indicates immunoprecipitation. Methods were the same as those as described in the legend for Figure 1B except that STAT5 was recognized by an antibody against the carboxy-terminal domain of STAT5. (D) Cleavage of STAT5 during platelet aggregation. Platelets were stimulated with thrombin (1 U/mL) for 30 minutes with or without stirring. After addition of ethyleneglycotetraacetic acid (5 mM) and EDTA (5 mM), platelets were lysed by boiling in SDS sample buffer. STAT5 was detected by immunoblotting as described in the legend for Figure 1A by using the pan anti-STAT5 MoAb. Lane 1 shows resting platelets; lane 2, results after thrombin stimulation of platelets for 30 minutes, with stirring, in the absence of Arg-Gly-Asp-Ser; and lane 3, results after thrombin stimulation for 30 minutes without stirring. The arrow indicates full-length STAT5 and the arrowhead a cleaved form of STAT5.
We next investigated whether calpain cleaves STAT5 in vitro. STAT5A and STAT5B were immunoprecipitated, and the precipitates were incubated with μ-calpain in the presence or absence of ionized calcium. The data from the blot analysis shown in Figure 1B indicate that, after incubation with calpain, the apparent molecular weights of both STAT5A and STAT5B decreased in a fashion dependent on ionized calcium, resulting in the generation of multiple bands reactive with an anti-STAT5 MoAb. None of these generated bands were recognized by a polyclonal antibody against the carboxy-terminal domain of STAT5, thereby indicating that all of the bands were truncated STAT5 molecules devoid of the carboxy-terminal domain (Figure 1C). When platelets were activated with thrombin and simultaneously stirred to induce platelet aggregation, which activates calpain,19 20 a significant loss of STAT5 immunoreactivity was observed (Figure 1D), resulting in appearance of a major cleaved band (as in Figure 1A). The band was not recognized by anti-STAT5 antibodies reactive with the carboxy-terminal domain of STAT5 (data not shown).
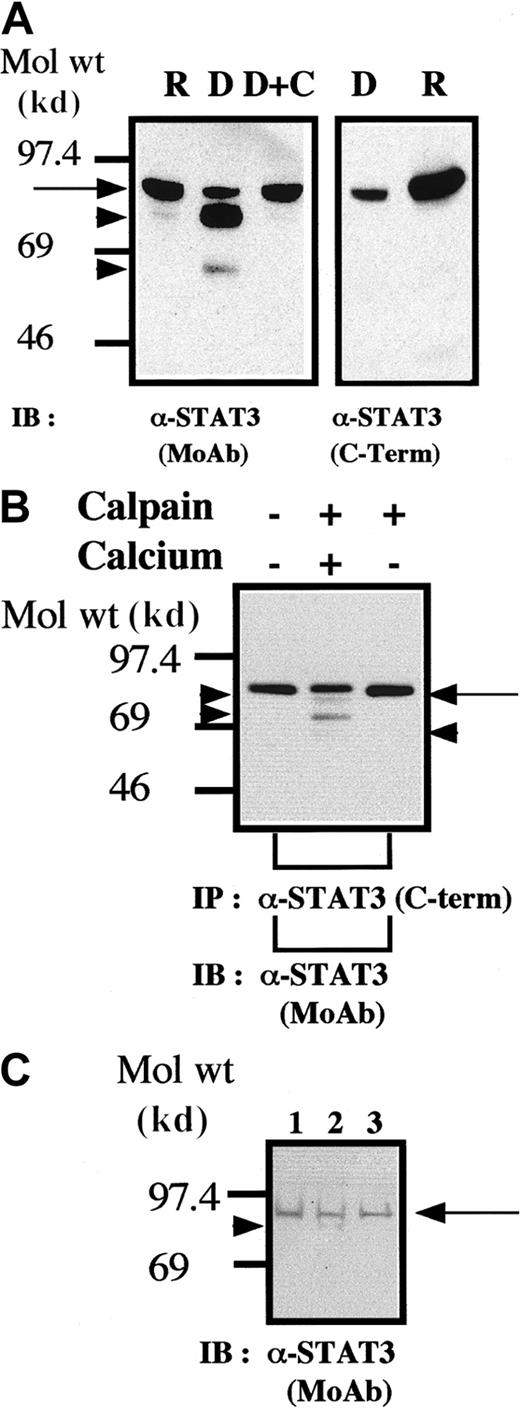
It is known that STAT3 variants (termed STAT3β) can be generated through alternative splicing leading to a loss or major defect in the carboxy-terminal domain of STAT3.20 We also conducted experiments to assess the possibility that STAT3 is a substrate for calpain in platelets. The results of the blot analysis shown in Figure2 clearly indicate that STAT3 is a substrate for calpain in vivo and in vitro. The anti-STAT3 MoAb used for the blotting analysis (Figure 2A, left panel, and Figure 2B) recognizes the amino-terminal domain of STAT3. Because the cleaved STAT3 molecules were not recognized by the anticarboxy-terminal domain of STAT3 (Figure 2A, right panel), these truncated STAT3 molecules must lack the carboxy-terminal domain while retaining a relatively intact amino-terminal domain. In addition, we found that cleavage of STAT3 also occurred during platelet aggregation (Figure 2C). Although these data do not directly indicate that calpain mediates cleavage of STATs on platelet aggregation, they are consistent with such a hypothesis.
STAT3 is cleaved by calpain in vivo and in vitro.
(A,B) Methods were the same as those described in the legends for Figures 1A and 1B, respectively, except that the blots were probed by using anti-STAT3 antibodies. (C) Cleavage of STAT3 during platelet aggregation. Methods were the same as those described in the legend for Figure 1C except that STAT3 was recognized by the anti-STAT3 MoAb as in Figure 2A.
STAT3 is cleaved by calpain in vivo and in vitro.
(A,B) Methods were the same as those described in the legends for Figures 1A and 1B, respectively, except that the blots were probed by using anti-STAT3 antibodies. (C) Cleavage of STAT3 during platelet aggregation. Methods were the same as those described in the legend for Figure 1C except that STAT3 was recognized by the anti-STAT3 MoAb as in Figure 2A.
To our knowledge, this is the first report of calpain-mediated cleavage of STAT3 and STAT5 in vivo and in vitro. Although we found that μ-calpain cleaves STATs, μ-calpain may also be involved in truncation of STATs in platelets, since A23187-induced or thrombin-induced cleavage of several substrates for calpain was observed in platelets from μ-calpain null mice.17
Because truncation of STATs occurred in platelets, the nuclear element is not necessary for calpain-mediated trimming of STATs. Although truncation of the carboxy-terminal domain of STAT5 by a serine protease resulted in generation of a dominant-negative form,6-9additional trimming of the carboxy-terminal domain of STAT5A due to alternative splicing has also been reported.21Overexpression of truncated STAT5A (devoid of transactivation and Src homology 2 domains) in interleukin 3 (IL-3)–dependent FDCP-1 cells increased DNA-binding activity of endogenous (wild-type) STAT5, enhanced proliferation, and prevented apoptosis after deprivation of IL-3.21 Our finding that cleavage of both STATs by calpain led to generation of multiple bands, some of which had molecular weights similar to those in earlier reports, indicates that the effects of truncation of STATs by calpain are complex.
Supported in part by grants-in-aid from the Ministry of Education, Science and Technology of Japan (HF), Human Frontier Science Program (AO), Kaiun Mishima Memorial Foundation (AO), Mochida Memorial Foundation (AO), Mitsui Insurance Welfare Foundation (AO), Daiwa Health Foundation (AO), Ichiro Kanehara Foundation (AO), and Welfide Medicinal Research Foundation (AO).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Atsushi Oda, Laboratory of Environmental Biology, Department of Preventive Medicine, Hokkaido University School of Medicine, N15W7, Kita-ku, Sapporo, 060-8638, Japan.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal