Human neutrophils have an important role in host defense against microbial infection. At different stages of an infectious process, neutrophils progressively up-regulate receptors and release various effector molecules. These are stored in several distinct types of granules with varying propensity to be secreted. Heparin-binding protein (HBP), also known as CAP37 or azurocidin, is a multifunctional, inactive serine-protease homologue. The present work shows that HBP is released from neutrophils on stimulation with secretagogues that do not trigger the secretion of azurophilic granule content. Therefore, the subcellular localization of HBP was investigated in more detail. Immunofluorescence microscopy revealed that HBP was localized close to the plasma membrane. Further analysis by fractionation of postnuclear supernatants from cavitated neutrophils showed that HBP is stored in azurophilic granules and secretory vesicles but that it is also detected to a minor extent in the plasma membrane. These findings were confirmed by immunoelectron microscopy showing that HBP colocalized with marker proteins of azurophilic granules and secretory vesicles. The presence of HBP in secretory vesicles possibly depends on the stage of cell differentiation, since the promyelocytic cell line HL-60 contains less HBP than mature neutrophils, stored exclusively in the less easily mobilized azurophilic granules. Our findings suggest that HBP can be synthesized or targeted to easily mobilized compartments at a late stage of neutrophil maturation. The ability of neutrophils to secrete HBP from secretory vesicles may be important for proinflammatory functions of this protein, such as the alteration of vascular permeability.
Introduction
Polymorphonuclear leukocytes (PMNs) have an important role in early host defense against invading microorganisms (for reviews, see references 1 and 2). Recruitment of these cells from the bloodstream to a site of infection involves their recognition of inflammatory mediators, their binding to adhesion molecules of the vascular endothelium, and their migration across the endothelial barrier.3 How efficiently neutrophils perform these tasks depends on a sophisticated mobilization mechanism that triggers the release of granule contents and the concomitant up-regulation of various receptors to the plasma membrane.4 Secretory processes are also important for the extravascular migration of neutrophils through tissues. Once the cells have reached the focus of infection, they are fully activated and are able to fight the infection by secreting reactive oxygen intermediates, antimicrobial peptides, and degradative enzymes.2 These substances can be preferentially targeted to phagosome compartments to achieve efficient killing and degradation of internalized microorganisms.
Lately, much interest has been focused on the various granule types of neutrophils and their sequential mobilization during the inflammatory process (for review, see reference 5). Analysis of these granules by electron microscopy and subcellular fractionation has demonstrated that neutrophils have at least 4 different granule or vesicle types.6-9 These are the primary or azurophilic granules that contain myeloperoxidase (MPO), bactericidal proteins, and proteinases; the secondary or specific granules that store lactoferrin and enzymes such as collagenase and gelatinase; the tertiary or gelatinase granules that, like specific granules, contain tissue-degrading enzymes; and the secretory vesicles, an easily mobilizable compartment, that contain alkaline phosphatase and plasma proteins such as human serum albumin. The 4 granule types are mobilized at different stages of the inflammatory process; secretory vesicles are more readily secreted than the other granule types. Currently, it is believed that secretory vesicles release their content when neutrophils establish the primary rolling contact with the endothelium. Because the membrane of secretory vesicles is enriched with proteins such as Mac-1, complement receptor 1 (CD35), and urokinase-type plasminogen activator receptor, the fusion of this compartment with the plasma membrane leads to an up-regulation of important receptors and adhesion factors to the neutrophil surface.5 Tertiary and secondary granules contain tissue-degrading enzymes and are less easily mobilized than secretory vesicles. Possibly, these compartments are involved in the regulation of the tissue remodeling processes that occur during the egress of neutrophils from the vasculature and into the tissue. Finally, azurophilic granules contain bactericidal proteins such as bactericidal permeability increasing protein, cathepsins, defensins, elastase, lysozyme, and proteinase 3. These proteins have important functions at the site of infection, where azurophilic granules fuse with phagosome compartments4 10 in some instances, these granules release their content extracellularly to achieve microbial killing.
Among the proteins stored in neutrophil granules, heparin-binding protein (HBP), also termed azurocidin or CAP37, has attracted much interest for its potential role in infectious diseases (for review, see reference 11). HBP belongs to a family of serine proteases with an overall structure homologous to the elastase fold.12 Other neutrophil proteins in this family are cathepsin G and proteinase 3. In contrast to these proteinases, HBP lacks enzymatic activity because of the exchange of 2 essential amino acids in the catalytic triad.13-15 Despite its lack of enzymatic activity, HBP is a multifunctional protein. Some of its functions, such as the antimicrobial activity of HBP, agree well with its proposed localization in the azurophilic granules.16 However, data suggest that HBP has important functions other than at the later stages of an inflammatory process. For example, HBP is able to recruit and activate monocytes,17,18 to mobilize T cells,19 and to induce detachment and homotypic aggregation of endothelial cells and fibroblasts.20Furthermore, endocytosis of HBP by monocytes enhances lipopolysaccharide-induced tumor necrosis factor–α production.21 Other studies have suggested that internalized HBP protects endothelial cells from apoptosis.22 More recently, HBP has been shown to have an exclusive role in mediating the alteration in vessel wall permeability evoked by chemoattractant-induced PMN activation.23 Some of these functions imply that HBP should be mobilized earlier than the content of azurophilic granules, perhaps before the egress of neutrophils from blood vessels. To elucidate how HBP is mobilized from neutrophils, we studied its secretion in response to different secretagogues. We also used subcellular fractionation, fluorescence microscopy, and electron microscopy techniques to demonstrate the subcellular localization of HBP in neutrophils.
Materials and methods
Materials
Neutrophil isolation medium was purchased from Cardinal Associates (Santa Fe, NM). RPMI 1640 medium with Glutamax I, minimum essential medium (MEM) with Earle salts and L-glutamine, fetal bovine serum, and penicillin (5000 U/mL)–streptomycin (5000 μg/mL) solution were purchased from Life Technologies (Täby, Sweden). Percoll was purchased from Pharmacia (Uppsala, Sweden). Cytochalasin B,P-nitrophenyl phosphate, nitro-blue tetrazolium, 5-bromo-4-chloro-3-indolylphosphate, phenylmethylsulfonyl fluoride (PMSF), and poly-L-lysine hydrobromide (average MWt, 99 500) were from Sigma Chemical (St Louis, MO). Ionomycin was obtained from Calbiochem (La Jolla, CA). ProLong Antifade Kit was from Molecular Probes (Eugene, OR). HEPES was from Merck (Whitehouse Station, NJ). Bovine serum albumin (BSA), aprotinin, leupeptin, and pepstatin A were from Boehringer Mannheim (Mannheim, Germany). Monoclonal antibodies against CD63, CD66b, and CD35 were purchased from Biodesign International (Kennebunk, ME), and monoclonal antibodies against myeloperoxidase and alkaline phosphatase were from BD PharMingen (San Diego, CA). Recombinant human HBP was produced using the baculovirus expression system in Sf9 insect cells (Invitrogen, Carlsbad, CA) and was purified as described.18 Human leukocyte elastase and human serum albumin (HSA) were from Sigma Chemical. Mouse monoclonal antibody 2F23C3 and rabbit antiserum (409A) to recombinant HBP were prepared and purified as described earlier.24 Sheep antibody against human elastase was from The Binding Site (Birmingham, England), and rabbit antibody against human cathepsin G was from Athens Research and Technology (Athens, GA). Monoclonal antibodies against human elastase and proteinase 3 were from Research Diagnostics (Flanders, NJ) and Wieslab AB (Lund, Sweden), respectively. Rabbit antibody against human gelatinase (MMP-9) was from Chemicon (Temecula, CA). Unconjugated and peroxidase-conjugated rabbit antibodies against HSA, rabbit antibody against human MPO, and AP-conjugated swine anti–rabbit immunoglobulin G (IgG) were from DAKO (Carpinteria, CA). Peroxidase-conjugated goat anti–rabbit IgG was from Bio-Rad Laboratories (Richmond, CA), and donkey anti–sheep IgG was from ICN Pharmaceuticals (Costa Mesa, CA). Donkey serum and the secondary antibodies used for immunofluorescence (fluorescein isothiocyanate [FITC]–conjugated donkey antirabbit and Cy3-conjugated donkey antimouse) were from Jackson ImmunoResearch (West Grove, PA). Aurion BSA-c (acetylated and partly linearized bovine serum albumin) and gold-labeled anti-mouse and anti-rabbit antibodies were from Aurion (Wageningen, The Netherlands).
Experimental media
Nominally bicarbonate-free solution MEM was supplemented with 13.8 mM NaCl, buffered with 10 mM HEPES, and its pH was adjusted to 7.3 with NaOH. Na+-based solution (Na-medium) was also buffered to pH 7.3 and contained 127 mM NaCl, 1.2 mM KH2PO4, 5.4 mM KCl, 0.8 mM MgSO4, 1.8 mM CaCl2, 5.6 mM glucose, and 10 mM HEPES. In calcium-free Na-medium, CaCl2 was replaced by 1 mM EGTA.
Coating of coverslips
Glass coverslips were washed with methanol and overlaid with 0.25 mL poly-L-lysine (0.2 mg/mL in water). After the added fluid was evaporated at 50°C to 65°C, the poly-L-lysine–coated coverslips were washed twice with distilled water.
Cell culture, neutrophil isolation, and protocol for stimulation of cells
Human promyelocytic HL-60 leukemia cells were the kind gift of Prof Inge Olsson (Haematology Research Laboratory, Lund University Hospital, Sweden). Cells were grown in suspension culture in RPMI 1640 Glutamax I medium, supplemented with 25 mM HEPES, 2% (vol/vol) penicillin-streptomycin, and 10% fetal bovine serum. Cell cultures were maintained at 37°C in a 5% CO2 humidified atmosphere. Exponentially growing cells were used for the experiments. Human neutrophils (more than 98% pure, as verified by fluorescence-activated cell sorter analysis) were isolated from fresh heparinized blood of healthy volunteers using neutrophil isolation medium, a single-step density gradient medium, according to the instructions supplied by the manufacturer. Neutrophils were counted with a hemocytometer, resuspended in MEM at 107 cells/mL, and maintained on rotation in this medium at room temperature until use. All experiments on isolated neutrophils were performed in Na-medium and were initiated within 1 hour of neutrophil isolation.
Enzyme-linked immunosorbent assay
Microtiter plates were coated by overnight incubation at 4°C with dilution series of experimental media containing HBP, elastase, cathepsin G, and proteinase 3. Starting dilutions were 50 μL media dissolved in 150 μL solution containing 15.9 mM Na2CO3 and 35 mM NaHCO3 (pH 9.6). Plates were washed 5 times with phosphate-buffered saline (PBS) (116.4 mM NaCl, 4.9 mM Na2HPO4, and 1.7 mM KH2PO4, pH 7.2) containing 0.05% (vol/vol) Tween 20 and were thereafter blocked with 200 μL/well washing buffer containing 2% (wt/vol) bovine serum albumin (incubation buffer) for 30 minutes at 37°C. This was followed by incubation with antibodies against HBP, elastase, cathepsin G, or proteinase 3 (200 μL/well, dilution 1:1000 in incubation buffer) for 1 hour at 37°C. Bound antibody was detected by a horseradish peroxidase–labeled secondary antibody against rabbit, goat, or mouse IgG (dilution 1:3000, 1 hour at 37°C in incubation buffer) and a chromogenic substrate solution (0.1% (wt/vol) diammonium-2,2'-azino-bis-(3-ethyl-2,3-dihydrobenzthiazoline)-6-sulfonate, 0.012% (vol/vol) H2O2 in 100 mM citric acid, 100 mM NaH2PO4, pH 4.5) for 30 minutes at 37°C. Each incubation step was followed by a washing step. To quantify HBP and elastase, plates were coated (200 μL/well) with 0.3 μg/mL antibody 2F23C3 or 0.25 μg/mL monoclonal antibody to elastase diluted in a solution containing 15.9 mM Na2CO3and 35 mM NaHCO3, pH 9.6, at 4°C. Plates were washed and blocked as described above, followed by incubation with 100 μL experimental media added to 100 μL incubation buffer for 1 hour at 37°C. In parallel, serial dilutions of purified protein (typical starting concentration, 100 ng/mL) were analyzed. After a washing step, microtiter plates were incubated with a polyclonal antibody against HBP (200 μL/well, 0.5 μg/mL), or elastase (200 μL/well, dilution 1:1000) for 1 hour at 37°C. Bound antibody was detected using a horseradish peroxidase–labeled secondary antibody against rabbit or goat IgG. After wash, the chromogenic substrate solution was applied as described above. For quantification of HSA, plates were coated with a polyclonal antibody to HSA (dilution 1:1000). Plates were incubated in washing buffer for 30 minutes at 37°C before incubation with 100 μL experimental media added to 100 μL washing buffer for 1 hour at 37°C. Bound HSA was detected using horseradish peroxidase–conjugated HSA (dilution 1:1000) diluted in washing buffer before detection as described above. In the figures, the releases of HBP, HSA, and elastase were corrected for the release observed in controls.
Immunofluorescence microscopy
After experimentation, cells were put on ice, washed twice in 1 mL cold PBS, and fixed with 0.5 mL 1% (vol/vol) paraformaldehyde solution (Becton Dickinson, Franklin Lakes, NJ) for 20 minutes on ice and for an additional 100 minutes at room temperature. After 2 washes in PBS, the cells were permeabilized in 0.5 mL cytoskeletal buffer containing 100 mM KOH, 2 mM MgCl2, 5 mM EGTA, 0.02% (vol/vol) Triton X-100, and 100 mM PIPES (pH 6.8) for 15 minutes on ice and were thereafter blocked for 30 minutes in PBS containing 5% (vol/vol) donkey serum (Sigma) at room temperature. After washing with PBS, cells were incubated at room temperature with the primary antibody (dilution 1:100-1:400) for 4 hours in PBS containing 1% (wt/vol) BSA. After washing, incubation with secondary antibody (dilution 1:800) was for 1 hour, also in PBS containing 1% (wt/vol) BSA. After washing, cells were adhered to poly-L-lysine–coated coverslips. Next, the samples were overlaid with ProLong Antifade reagent before mounting. Images were recorded on a Nikon Eclipse TE300 inverted fluorescence microscope equipped with a Hamamatsu C4742-95 cooled charge coupled device camera, using a Plan Apochromat 100× objective and a high NA oil condenser.
Subcellular fractionation
Subcellular fractionation of neutrophils was performed by density centrifugation on Percoll gradients, as described in the review by Kjeldsen et al.8 Neutrophils were suspended in disruption buffer (250 mM sucrose, 10 mM HEPES, 0.3 mM Na-EDTA) containing antiproteinases (1 μg/mL aprotinin, 0.5 μg/mL leupeptin, 1 μg/mL pepstatin A, 0.1 mg/mL PMSF) at 5 × 107cells/mL. Cells were disrupted by nitrogen cavitation (350 psi, 5 minutes), and the cavitate was collected by the drop into a solution of EGTA, pH 7.4, final concentration of 1.5 mM. Nuclei and unbroken cells were sedimented by centrifugation at 500g for 10 minutes at 4°C.
To isolate secretory vesicles from plasma membranes, a flotation gradient was used.25 The postnuclear supernatant (7 mL) was mixed with a heavy Percoll solution (7 mL, 1.12 g/mL). The mixture was layered under 14 mL light Percoll solution (1.04 g/mL). Five milliliters heavy Percoll solution (1.12 g/mL) was applied to the bottom of the tube. Relaxation buffer (5 mL) was applied on top of the gradient. The gradient was centrifuged at 37 000g for 35 minutes at 4°C using a fixed-angle Beckman JA-20 rotor. After centrifugation, 1-mL fractions were collected by aspiration from the bottom of the tube using a peristaltic pump. Localization of subcellular organelles in the gradients was determined by marker analysis of the fractions. Relative amounts of gelatinase (marker for the specific and gelatinase granules) and MPO (marker for the azurophil granules) in the fractions were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting using specific antibodies. Alkaline phosphatase (marker for secretory vesicles and plasma membranes) was measured by hydrolysis ofP-nitrophenyl phosphate (2 mg/mL) in the presence or absence of Triton X-100 (0.4%).26
To investigate the localization of HBP before and after cell stimulation, a 3-layer Percoll gradient was used. The postnuclear supernatant was applied on top of a 3 × 9 mL gradient (1.05/1.09/1.12 g/mL) and centrifuged at 37 000g for 30 minutes at 4°C in an SA-20 rotor (Beckman Instruments, Palo Alto, CA). Percoll densities were adjusted by mixing sucrose with precalculated amounts of Percoll and distilled water, according to instructions supplied by the manufacturer. After centrifugation, fractions (1 mL) were collected at 4°C by aspiration from the bottom of the tube, using a peristaltic pump and a fraction collector, and were analyzed by enzyme-linked immunosorbent assay (ELISA) for the presence of elastase, albumin, and HBP.
SDS-PAGE Western blotting, and immunoprinting
Proteins were separated by polyacrylamide gel electrophoresis in the presence of 1% (wt/vol) SDS.27 Molecular weight markers were from Sigma Chemical. Proteins were then transferred onto nitrocellulose or polyvinylidene difluoride membranes for 30 minutes at 100 mA.28 Membranes were blocked with PBS containing 5% (wt/vol) dry milk powder or 3% BSA and 0.05% (wt/vol) Tween 20, pH 7.4. Immunoprinting of the transferred proteins was performed according to Towbin et al.29 To stain for HBP, a polyclonal antibody, diluted 1:1000 in the blocking buffer, was used. Bound antibody was detected using a peroxidase-conjugated secondary antibody against rabbit IgG (dilution 1:3000) followed by a chemiluminescence detection method or the peroxidase substrate VIP (Vector Laboratories, Burlingame, CA). To stain for gelatinase and MPO, respectively, polyclonal rabbit antibodies (dilution 1:1000) and AP-conjugated secondary antibody were used. Densitometric analysis of the immunoblots was performed using a UMAX C12 scanner and the public domain NIH Image program (developed at the National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/).
Electron microscopy
Samples for electron microscopy were prepared by pelleting 5 × 106 cells at 4°C immediately after the addition of fixative (4% paraformaldehyde + 0.1% glutaraldehyde). After incubation at room temperature for 1 hour, the fixed pellets were subsequently dehydrated in ethanol and further processed for Lowicryl embedding.30 Sections were cut with a microtome and mounted on nickel grids. For immunostaining, the grids were floated on top of drops of immune reagents displayed on a sheet of parafilm. Free aldehyde groups were blocked with 50 mM glycine, and the grids were then incubated with 5% (vol/vol) donkey serum in incubation buffer (0.2% BSA-c in PBS, pH 7.6) for 15 minutes. This blocking procedure was followed by overnight incubation with primary antibodies (dilution 1:100) at 4°C. After washing the grids in a large volume (200 mL) of incubation buffer, floating on drops containing the gold conjugate reagents (diluted 1/20 in incubation buffer) was performed for 60 minutes at room temperature. After further washes in a large volume of incubation buffer, the sections were postfixed in 2% glutaraldehyde. Finally, sections were washed with distilled water and poststained with uranyl acetate and lead citrate and examined under the electron microscope.
Results
HBP is easily mobilized from neutrophils
To investigate neutrophil secretion of HBP, elastase, cathepsin G, and proteinase 3 after stimulation with strong secretagogues, cells were treated with formyl-methionyl-leucyl-phenylalanine (fMLP) and cytochalasin B for 30 minutes at 37°C. As a control, nonstimulated neutrophils were incubated under the same experimental conditions. Figure 1 shows that fMLP and cytochalasin B triggered the release of all 4 proteins into the culture medium, as measured semiquantitatively in an indirect ELISA. Unexpectedly, high amounts of HBP accumulated in the culture medium of nonstimulated neutrophils, whereas only smaller amounts of cathepsin G and almost no release of elastase and proteinase 3 were observed. Hence, we conclude that the release of HBP occurs more readily than the mobilization of cathepsin G, elastase, and proteinase 3.
Release of HBP, elastase, cathepsin G, and proteinase 3 from purified neutrophils.
Neutrophils were incubated in the absence (■) or presence of fMLP (100 nM) and cytochalasin B (10 μM) (⋄) for 30 minutes at 37°C. Cells were centrifuged, and 50 μL supernatant was applied in serial dilutions (2n) to microtiter plates. This was followed by detection with antibodies to HBP (A), elastase (B), cathepsin G (C), and proteinase 3 (D). Results are the mean ± SD of 3 independently performed experiments.
Release of HBP, elastase, cathepsin G, and proteinase 3 from purified neutrophils.
Neutrophils were incubated in the absence (■) or presence of fMLP (100 nM) and cytochalasin B (10 μM) (⋄) for 30 minutes at 37°C. Cells were centrifuged, and 50 μL supernatant was applied in serial dilutions (2n) to microtiter plates. This was followed by detection with antibodies to HBP (A), elastase (B), cathepsin G (C), and proteinase 3 (D). Results are the mean ± SD of 3 independently performed experiments.
Secretion patterns of HBP and elastase differ on stimulation with different agonists
Neutrophil granules are classified, among other criteria, on the basis of their propensity to undergo exocytosis and their protein content.5 For example, the release of proteins such as MPO and elastase from azurophilic granules is not as readily triggered as the exocytosis of the content of secretory vesicles, in which plasma proteins, including HSA, are stored.31 To compare the secretion of HBP with the secretion of marker proteins for azurophilic granules and secretory vesicles, sandwich ELISAs for HBP, elastase, and HSA were established. In a series of experiments, neutrophils were treated at 37°C with fMLP, fMLP and cytochalasin B, phorbol 12-myristate 13-acetate (PMA), or ionomycin for 30 minutes, or they were incubated for 10, 20, or 30 minutes without stimulation. Quantification of the amounts of HBP, HSA, and elastase in the culture medium revealed that HBP showed a similar exocytosis pattern to that of HSA (Figure 2A-B). Furthermore, the pattern of elastase secretion in response to the tested secretagogues (Figure 2C) was distinct from those of HBP and HSA, and elastase was not released from nonstimulated neutrophils. These data indicate that the secretion mechanisms of elastase differ from those that regulate the release of HBP.
Release of HBP, HSA, and elastase from purified neutrophils.
Neutrophils were stimulated with fMLP (100 nM), cytochalasin B (10 μM), PMA (100 nM), and ionomycin (0.5 μM) for 30 minutes at 37°C. Cells were incubated in the absence of a stimulus for 10, 20, and 30 minutes at 37°C. Cells were pelleted, and the concentrations of HBP (A), HSA (B), and elastase (C) in the culture medium were determined by ELISA. Results are the mean ± SD of 3 independently performed experiments, each performed in duplicate.
Release of HBP, HSA, and elastase from purified neutrophils.
Neutrophils were stimulated with fMLP (100 nM), cytochalasin B (10 μM), PMA (100 nM), and ionomycin (0.5 μM) for 30 minutes at 37°C. Cells were incubated in the absence of a stimulus for 10, 20, and 30 minutes at 37°C. Cells were pelleted, and the concentrations of HBP (A), HSA (B), and elastase (C) in the culture medium were determined by ELISA. Results are the mean ± SD of 3 independently performed experiments, each performed in duplicate.
HBP is stored in organelles close to the plasma membrane
Next, we localized HBP by immunofluorescence microscopy and compared its staining pattern with that of markers for azurophilic granules (CD63) and secretory vesicles (CD35). As shown in Figure3, only partial colocalization of these markers with HBP was observed. Especially CD63 differed in its staining pattern from HBP in that the punctate staining of CD63 was not as peripherally located in the cell as that of HBP (Figure 3A-B). In addition, the staining pattern of CD35 did not completely colocalize with that of HBP (Figure 3D-E). Resolution of the wide-field fluorescence microscope did not allow a discrimination between HBP localized in vesicular compartments close to the plasma membrane and staining caused by the possible binding of released HBP to the surfaces of the cells. We could not resolve this issue using confocal microscopy either (results not shown). However, because the secretion of HBP can be modulated by signal transduction inhibitors such as genistein and wortmannin (data not shown), vesicular localization of HBP seems more likely.
Subcellular localization of HBP by immunofluorescence microscopy.
Indirect immunolocalization of HBP and markers for azurophilic granules and secretory vesicles. Neutrophils were fixed, permeabilized, and stained, as described in “Materials and methods,” before attachment to poly-L-lysine–coated coverslips and recording of images. (A-C) Cells were incubated with a rabbit anti-HBP antibody and a mouse monoclonal antibody against the azurophilic granule marker, CD63. (D-F) Cells were incubated with a rabbit anti-HBP antibody and a mouse monoclonal antibody against the secretory vesicle marker, CD35. Thereafter, the cells were stained with FITC-labeled anti-rabbit and Cy3-labeled anti-mouse secondary antibodies. Staining of HBP (A, D), CD63 (B), and CD35 (E). Corresponding Nomarski images (C, F). Results are representative of more than 5 separate experiments. (Bar = 10 μM). (inset) Specificity of the rabbit anti-HBP antibody was verified by Western blot analysis of neutrophil lysates (inset, B). As a control, purified recombinant HBP was used in inset A.
Subcellular localization of HBP by immunofluorescence microscopy.
Indirect immunolocalization of HBP and markers for azurophilic granules and secretory vesicles. Neutrophils were fixed, permeabilized, and stained, as described in “Materials and methods,” before attachment to poly-L-lysine–coated coverslips and recording of images. (A-C) Cells were incubated with a rabbit anti-HBP antibody and a mouse monoclonal antibody against the azurophilic granule marker, CD63. (D-F) Cells were incubated with a rabbit anti-HBP antibody and a mouse monoclonal antibody against the secretory vesicle marker, CD35. Thereafter, the cells were stained with FITC-labeled anti-rabbit and Cy3-labeled anti-mouse secondary antibodies. Staining of HBP (A, D), CD63 (B), and CD35 (E). Corresponding Nomarski images (C, F). Results are representative of more than 5 separate experiments. (Bar = 10 μM). (inset) Specificity of the rabbit anti-HBP antibody was verified by Western blot analysis of neutrophil lysates (inset, B). As a control, purified recombinant HBP was used in inset A.
HBP is stored in neutrophil azurophil granules and secretory vesicles
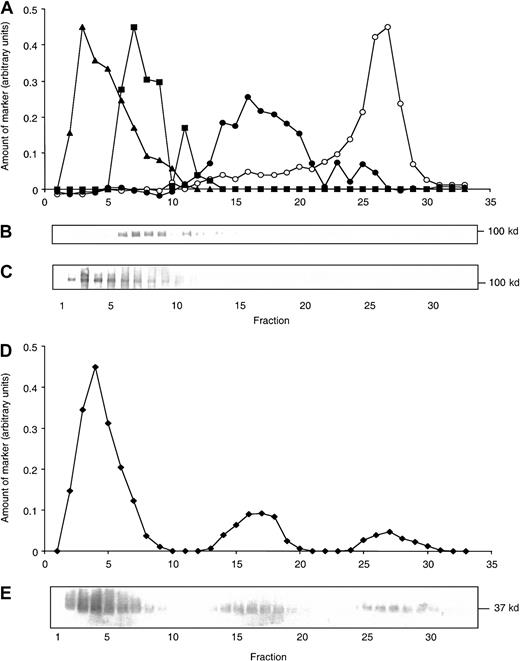
The fact that HBP was easily secreted on the stimulation of neutrophils and showed partial colocalization with CD35, a marker for the secretory vesicles, suggested that HBP may be localized in these organelles. To investigate the subcellular localization of HBP by biochemical means, we fractionated neutrophils on a Percoll gradient. Because secretory vesicles are similar in density to plasma membrane vesicles, a gradient in which these light membranes or vesicles flotate was used, allowing a clear separation of the 2 compartments. In Figure4, the marker profile of a flotation gradient is shown. The plasma membrane is localized in the upper part of the gradient (fractions 25-28), shown by the presence of nonlatent (measured in the absence of detergent) alkaline phosphatase (ALP). Secretory vesicles are denser and are localized in the middle fractions,14-20 shown by the peak of ALP seen only in the presence of detergent (latent ALP).32 Neutrophil gelatinase is present in specific and gelatinase granules and mark the presence of these organelles in the lower part of the gradient (fractions 6-11), whereas the azurophil granules are shown by the presence of MPO in fractions 2 to 4. Staining each fraction with antibodies to HBP showed a single band at 37 kd localized by a major part in the azurophil granules. However, HBP was also localized in the secretory vesicles, and a minor part was found in the fractions holding the plasma membrane. By densitometric measurements, the relative amounts of HBP in the fractions were estimated (Figure 4). Calculations based on these measurements showed that approximately 74% of the HBP are stored in the azurophil granules, whereas the remaining part is divided between the secretory vesicles (18%) and the plasma membrane (8%).
Localization of HBP in neutrophil subcellular fractions.
Human neutrophils were fractionated in a flotation gradient as described in “Materials and methods.” Localization of neutrophil organelles in the gradient is shown by marker analysis of the fractions. (A) Nonlatent alkaline phosphatase (activity measured in the absence of detergent; marker for the plasma membrane; open circles), latent alkaline phosphatase (difference between activity measured in the presence and absence of detergent; marker for the secretory vesicles; closed circles), gelatinase (densitometric curve from panel B; squares), and MPO (densitometric curve from panel C; triangles). (B, C) Western blots of the fractions probed with antibodies to gelatinase and MPO, respectively. (D) Presence of HBP in the gradient is shown as a densitometric curve measured from the immunoblot (E) probed with anti-HBP antibodies.
Localization of HBP in neutrophil subcellular fractions.
Human neutrophils were fractionated in a flotation gradient as described in “Materials and methods.” Localization of neutrophil organelles in the gradient is shown by marker analysis of the fractions. (A) Nonlatent alkaline phosphatase (activity measured in the absence of detergent; marker for the plasma membrane; open circles), latent alkaline phosphatase (difference between activity measured in the presence and absence of detergent; marker for the secretory vesicles; closed circles), gelatinase (densitometric curve from panel B; squares), and MPO (densitometric curve from panel C; triangles). (B, C) Western blots of the fractions probed with antibodies to gelatinase and MPO, respectively. (D) Presence of HBP in the gradient is shown as a densitometric curve measured from the immunoblot (E) probed with anti-HBP antibodies.
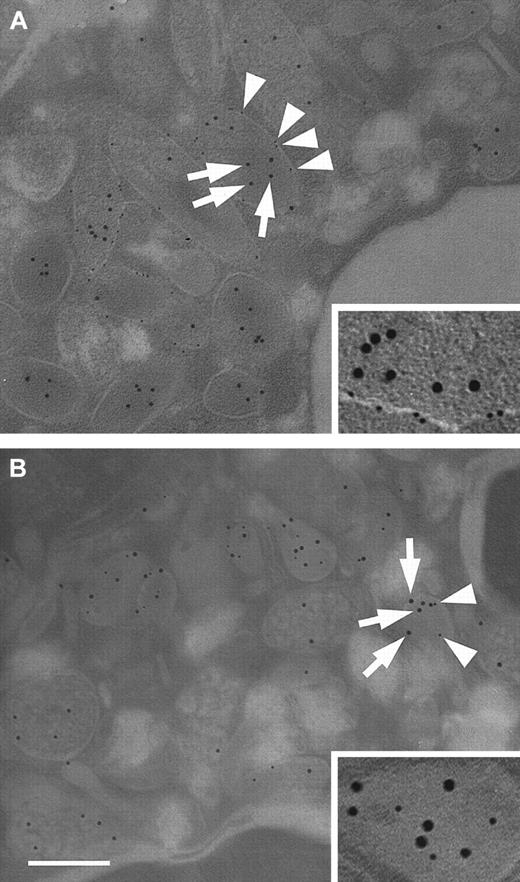
Immunoelectron microscopy was used to confirm the results of the subcellular fractionation experiments. To this end, antibodies against ALP and MPO were used to identify secretory vesicles and azurophil granules, respectively. Figure 5A shows a representative double immunostaining of a thinly sectioned neutrophil using antibodies against HBP and ALP. The micrographs reveal that HBP is stored in organelles that show ALP membrane staining. In addition, staining in ALP-negative granules was observed. When neutrophils were double-stained with antibodies against HBP and MPO, colocalization of HBP and MPO was found in organelles with a different morphology than those that were ALP positive (Figure 5B). Significantly, some HBP-positive organelles did not stain for MPO, and their morphology resembled ALP-positive organelles. Taken together, subcellular fractionation and immunoelectron microscopy studies demonstrated that HBP is stored in 2 different compartments, namely azurophil granules and secretory vesicles.
Subcellular localization of HBP by electron microscopy.
Neutrophils were fixed and prepared for transmission electron microscopy as described in “Materials and methods.” (A) Neutrophils were immunostained with a monoclonal antibody against ALP and a rabbit polyclonal antibody against HBP. Bound antibody was detected with gold-labeled secondary antibodies against mouse IgG (6-nm gold particles) and rabbit IgG (10-nm gold particles), respectively. Arrows indicate HBP, arrowheads indicate ALP. (inset) Secretory vesicle containing HBP at a higher magnification. (B) Neutrophils were immunostained with a monoclonal antibody against MPO and a polyclonal antibody against HBP, as described above. Arrows indicate HBP, arrowheads indicate MPO. (inset) Azurophil granule containing HBP at a higher magnification. Images are representative of 4 separate experiments. Bar = 200 nm.
Subcellular localization of HBP by electron microscopy.
Neutrophils were fixed and prepared for transmission electron microscopy as described in “Materials and methods.” (A) Neutrophils were immunostained with a monoclonal antibody against ALP and a rabbit polyclonal antibody against HBP. Bound antibody was detected with gold-labeled secondary antibodies against mouse IgG (6-nm gold particles) and rabbit IgG (10-nm gold particles), respectively. Arrows indicate HBP, arrowheads indicate ALP. (inset) Secretory vesicle containing HBP at a higher magnification. (B) Neutrophils were immunostained with a monoclonal antibody against MPO and a polyclonal antibody against HBP, as described above. Arrows indicate HBP, arrowheads indicate MPO. (inset) Azurophil granule containing HBP at a higher magnification. Images are representative of 4 separate experiments. Bar = 200 nm.
HBP is secreted from secretory vesicles on weak stimulation
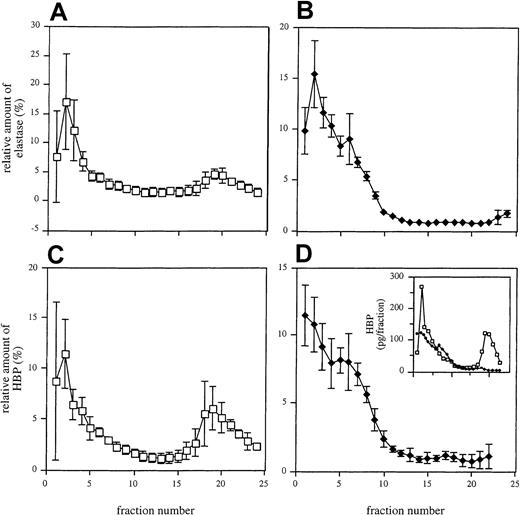
In another set of experiments, the distinct exocytosis patterns of HBP and elastase was investigated. To address this issue, nonstimulated neutrophils and neutrophils treated with a weak secretagogue (100 nM fMLP) were disrupted by nitrogen cavitation and were subjected to subcellular fractionation on 3-layer Percoll gradients. When analyzing elastase in the eluted fractions from nonstimulated neutrophils, a major peak was found in the first fractions, previously shown to contain azurophilic granule constituents.8 31 Additionally, a smaller peak was detected in the upper fractions holding the light membranes (secretory vesicles and plasma membranes) (Figure6A). After stimulation of the cells with fMLP, the elution profile of elastase changed in that the first peak became slightly broader and the second peak disappeared (Figure 6B). Analysis of the subcellular distribution of HBP revealed that this protein was also found in 2 peaks in fractions from nonstimulated neutrophils (Figure 6C). However, the second peak was larger than the corresponding elastase peak, suggesting that a substantial fraction of the HBP is stored in compartments lacking high amounts of elastase. After treatment with fMLP, the HBP profile was similar to that of elastase (Figure 6D). These data show that the secretable fraction of the protein is derived from the secretory vesicles.
Subcellular localization of elastase and HBP in neutrophils.
Unstimulated (A, C) and fMLP (100 nM)-stimulated (B, D) neutrophils were fractionated as described in “Materials and methods.” Each fraction was analyzed for elastase (A, B) and HBP (C, D) by ELISA. Mean ± SD of 3 independent experiments is shown. (inset) Absolute amounts of HBP in one representative experiment with (♦) and without (■) stimulation of cells.
Subcellular localization of elastase and HBP in neutrophils.
Unstimulated (A, C) and fMLP (100 nM)-stimulated (B, D) neutrophils were fractionated as described in “Materials and methods.” Each fraction was analyzed for elastase (A, B) and HBP (C, D) by ELISA. Mean ± SD of 3 independent experiments is shown. (inset) Absolute amounts of HBP in one representative experiment with (♦) and without (■) stimulation of cells.
In HL-60 cells, HBP is exclusively stored in azurophilic granules
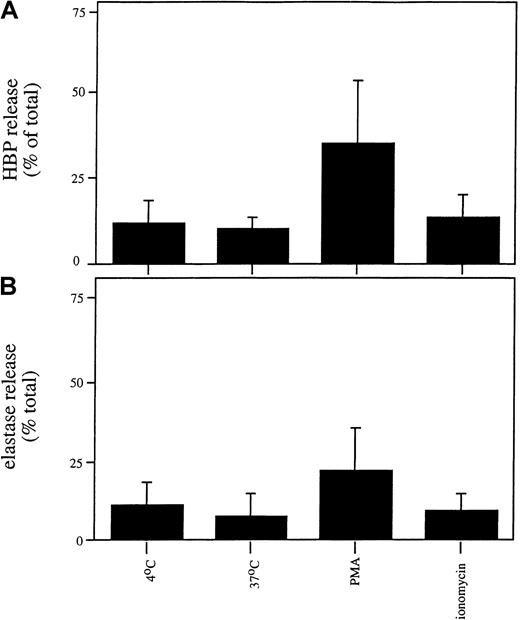
We next investigated whether the release or localization of HBP in the promyelocytic cell line HL-60 differed from that observed in mature neutrophils because the HL-60 cell line is arrested in maturation at a stage after the formation of azurophilic granules and before the formation of other granule types. We observed that HL-60 cells contained less HBP than mature neutrophils (0.51 ± 0.14 ng HBP/106 HL-60 cells compared with 162 ± 69 ng/106 mature neutrophils). As shown in Figure7, stimulation of HL-60 cells with PMA triggered the release of HBP and elastase, whereas treatment with ionomycin or temperature elevation did not cause the release of HBP or elastase. This result contrasts with what we observed in mature neutrophils (Figure 2), suggesting that HL-60 cells store HBP in the same compartment as elastase and that there is no easily secretable pool of HBP in these cells. In accordance with this hypothesis, similar staining patterns of HBP and a marker for azurophilic granules were observed in HL-60 cells in indirect immunofluorescence microscopy (Figure8A-B). As expected, no staining was observed using the secretory vesicle marker, CD35 (Figure 8E). Thus, the low amount of HBP in HL-60 cells appears to be exclusively stored in azurophil granules, in marked contrast to the localization of HBP in the mature human neutrophil.
Release of HBP and elastase from HL60 cells.
HL60 cells were incubated in the absence of a stimulus or in the presence of PMA (100 nM) or ionomycin (0.5 μM) for 30 minutes at 37°C. Cells were spun down, and the concentrations of HBP (A) and elastase (B) in the supernatants were determined by ELISA. Data represent the relative release of HBP and elastase (compared with the total amount of these proteins in lysates) and are the mean ± SD of 4 experiments, each performed in duplicate.
Release of HBP and elastase from HL60 cells.
HL60 cells were incubated in the absence of a stimulus or in the presence of PMA (100 nM) or ionomycin (0.5 μM) for 30 minutes at 37°C. Cells were spun down, and the concentrations of HBP (A) and elastase (B) in the supernatants were determined by ELISA. Data represent the relative release of HBP and elastase (compared with the total amount of these proteins in lysates) and are the mean ± SD of 4 experiments, each performed in duplicate.
Localization of HBP by immunofluorescence microscopy in HL60 cells.
Indirect immunolocalization of HBP and markers for azurophilic granules and secretory vesicles. HL60 cells were fixed, permeabilized, and stained, as described in “Materials and methods,” before attachment to poly-L-lysine–coated coverslips and recording of images. (A-C) Cells were incubated with a rabbit anti-HBP antibody and a mouse monoclonal antibody against the azurophilic granule marker, CD63. (D-F) Cells were incubated with a rabbit anti-HBP antibody and a mouse monoclonal antibody against the secretory vesicle marker, CD35. Thereafter, the cells were stained with FITC-labeled anti-rabbit and Cy3-labeled anti-mouse secondary antibodies. Staining of HBP (A, D), CD63 (B), and CD35 (E). Corresponding Nomarski images (C, F). Results are representative of 3 separate experiments. Bar = 10 μM.
Localization of HBP by immunofluorescence microscopy in HL60 cells.
Indirect immunolocalization of HBP and markers for azurophilic granules and secretory vesicles. HL60 cells were fixed, permeabilized, and stained, as described in “Materials and methods,” before attachment to poly-L-lysine–coated coverslips and recording of images. (A-C) Cells were incubated with a rabbit anti-HBP antibody and a mouse monoclonal antibody against the azurophilic granule marker, CD63. (D-F) Cells were incubated with a rabbit anti-HBP antibody and a mouse monoclonal antibody against the secretory vesicle marker, CD35. Thereafter, the cells were stained with FITC-labeled anti-rabbit and Cy3-labeled anti-mouse secondary antibodies. Staining of HBP (A, D), CD63 (B), and CD35 (E). Corresponding Nomarski images (C, F). Results are representative of 3 separate experiments. Bar = 10 μM.
Discussion
The current work was performed to explore the mechanisms that lead to the secretion of HBP from neutrophils and to identify the compartments, in which the protein is stored. Our investigations demonstrated that HBP is more readily mobilized than elastase, a protein closely related to HBP and known to be a component of azurophilic granules.5 This finding is in line with reports from Pereira et al,11,17 who found a massive release of HBP from neutrophils phagocytosing Staphylococcus aureus, whereas only minor amounts of CAP57 (cationic antimicrobial protein of molecular mass 57 kd also known as BPI (bactericidal permeability increasing protein) was secreted, which is stored in azurophilic granules. In Pereira et al's17study, however, no characterization of the compartments that contain HBP was performed.
In the current work, we show by fractionation of postnuclear supernatants from cavitated neutrophils that HBP is found in 2 distinct intracellular compartments. As expected, the densest fractions containing the azurophilic granules showed the presence of HBP. However, HBP was also detected in fractions containing the secretory vesicles (18%), and it colocalized to a minor extent with the plasma membranes (8%). The presence of HBP in the plasma membrane was verified also by fluorescence-activated cell sorter analysis using nonstimulated intact cells, and additional experiments showed that the HBP in the plasma membrane was not mobilized or released on stimulation with fMLP (not shown). The finding that only a small amount of HBP bound to the membrane was confirmed by immunoelectron microscopy, demonstrating that HBP is almost exclusively stored in intracellular organelles. The HBP anchored to the plasma membrane was not further investigated because the current study focused on the mechanism of release of HBP and its intracellular localization.
Identification by electron microscopy of the compartments that contain HBP is difficult because of the morphologic heterogeneity of neutrophil granules. Recent investigations on the ultrastructural localization of MPO in azurophilic granules using immuno-cryoultramicrotomy have shown that MPO-containing granules can be divided into 5 groups with different morphologic characteristics.33 In addition, the morphology of secretory vesicles can vary, depending on the method used to prepare the cells for electron microscopy. Moreover, these compartments can change to tubular structures after stimulation, probably because of the fusion of individual granules.7Hence, for electron microscopy identification of HBP-containing compartments in neutrophils, we used double-immunostaining with antibodies against ALP and MPO, proteins exclusively stored in secretory vesicles and azurophil granules, respectively. Our data clearly show that HBP is found in secretory vesicles and in azurophil granules.
Proteinase 3 has also been localized in compartments other than azurophilic granules, namely specific granules and secretory vesicles.34 In contrast to HBP, which is released into the culture medium on stimulation, proteinase 3 becomes attached to the plasma membrane. Witko-Sarsat et al speculate that membrane-bound proteinase 3 plays an important role in inflammatory processes, especially in antineutrophil cytoplasmic antibody-associated vasculitis.34 Like proteinase 3, the serine proteinases elastase and cathepsin G can bind to the plasma membrane of stimulated neutrophils.35 36 However, our studies indicate that after stimulation of neutrophils, the secreted HBP is released extracellularly rather than bound to the plasma membrane.
The targeting of neutrophil proteins to granules has been studied extensively in recent years.24,37-40 It is thought that a granule contains proteins synthesized when a compartment is formed.5,37,39 According to this concept, azurophilic granules are already present in myeloblasts and promyelocytes. Specific and gelatinase granules are generated in myelocytes, metamyelocytes, and band cells, whereas secretory vesicles appear at the end of neutrophil maturation.5 Northern blot analysis of myeloid cells at different stages of maturation demonstrate that the mRNA profiles of proteins stored in different compartments are in agreement with this hypothesis.40 Unfortunately, HBP was not included in this study. However, the finding by Lindmark et al24 that HBP is not expressed at high levels in the promyelocytic HL-60 and NB4 cells might indicate that the protein is also expressed at a later stage of neutrophil development. This observation is in line with our results showing that HL-60 cells contain lower amounts of HBP than mature neutrophils. Our experiments also show that HBP colocalizes with a marker of azurophilic granules in HL-60 cells, but only partial colocalization is observed in mature neutrophils. This implies that HBP produced during the promyelocytic stage of neutrophil development is targeted to the azurophilic granules. Furthermore, the protein may also be expressed at a later stage of neutrophil maturation and, consequently, may be targeted to other compartments, such as the plasma membrane. This is a plausible explanation for the localization of HBP to the secretory vesicles. It has been suggested that these organelles are formed by endocytosis during the final stages of neutrophil maturation, and they contain many plasma membrane components and plasma proteins.41Additional studies are needed to address the biosynthesis, processing, and sorting mechanisms that target HBP to the secretory vesicles.
In conclusion, the dual localization of HBP in azurophil granules and secretory vesicles indicates that the protein has several functions in the neutrophil. The azurophil-granule–localized HBP probably comes in close contact with internalized bacteria after fusion of the azurophil granules with the phagosome, possibly resulting in important antibacterial effects. On the other hand, the release of easily mobilized HBP from secretory vesicles could have important functions during early events in inflammatory processes. Of note, in a recent study, Gautam et al23 demonstrate that HBP is the only constituent in neutrophils capable of provoking increased permeability in endothelial cell monolayers in vitro and in vascular endothelium in vivo. Olofsson et al22 show that endothelial cells rapidly internalize HBP and locate it to the mitochondrial compartment, thereby protecting the endothelial cells from apoptosis. HBP also has proinflammatory chemotactic effects toward monocytes and T cells.20,21 42 Given that the major fraction of HBP in neutrophils is easily mobilized, our findings indicate that these processes or other functions of HBP are important in early inflammation, possibly already before or during the egress of neutrophils from the blood.
We thank Monica Heidenholm, Gunnel Karlsson, and Marie Samuelsson for excellent technical assistance.
Supported in part by the Swedish Medical Research Council (grants 12182, 12613, and 13413), the Magnus Bergvall Foundation, the Crafoord Foundation, the Lars Hiertas Minne Foundation, the Greta and Johan Kock Foundation, the Kungliga Fysiografiska Sällskapet, the Tore Nilson Foundation, the Åke Wiberg Foundation, and the AlfredÖsterlund Foundation.
One of the authors, H.F., is employed by Leukotech A/S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Heiko Herwald, Dept of Cell and Molecular Biology, Section for Molecular Pathogenesis, BMC, B14, Lund University, Tornavägen 10, SE-221 84 Lund, Sweden; e-mail:heiko.herwald@medkem.lu.se.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal