Based on previous reports for impaired hematopoiesis in rheumatoid arhrtitis (RA), and in view of the current interest in exploring the role of autologous stem cell transplantation (ASCT) as an alternative treatment in patients with resistant disease, we have evaluated bone marrow (BM) progenitor cell reserve and function and stromal cell function in 26 patients with active RA. BM progenitor cells were assessed using flow cytometry and clonogenic assays in short-term and long-term BM cultures (LTBMCs). BM stroma function was assessed by evaluating the capacity of preformed irradiated LTBMC stromal layers to support the growth of normal CD34+ cells. We found that RA patients exhibited low number and increased apoptosis of CD34+ cells, defective clonogenic potential of BM mononuclear and purified CD34+ cells, and low progenitor cell recovery in LTBMCs, compared with healthy controls (n = 37). Patient LTBMC stromal layers failed to support normal hematopoiesis and produced abnormally high amounts of tumor necrosis factor alpha (TNFα). TNFα levels in LTBMC supernatants inversely correlated with the proportion of CD34+ cells and the number of colony-forming cells, and positively with the percentage of apoptotic CD34+ cells. Significant restoration of the disturbed hematopoiesis was obtained following anti-TNFα treatment in 12 patients studied. We concluded that BM progenitor cell reserve and function and BM stromal cell function are defective in RA probably due, at least in part, to a TNFα-mediated effect. The role of these abnormalities on stem cell harvesting and engraftment in RA patients undergoing ASCT remains to be clarified.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized mainly by chronic destructive polyarthritis. Although the main target tissue of the disease is the synovium, evidence has been accumulated that bone marrow (BM) may be also actively involved in the pathophysiology of RA by providing immune and inflammatory cells in the affected synovial membranes.1-3It has been suggested that during the inflammatory stages of the disease, BM progenitor cells or even BM-derived immature mesenchymal cells enter the joint microenvironment through mechanisms similar to those of mature inflammatory cells, and gradually replace normal synovial cells.4,5 Moreover, on the basis of findings from experimental models, it has been proposed that RA might even begin within the BM and might be regarded as a BM disorder.6 7However, this hypothesis has not been adequately addressed in humans.
It is now well established that lymphocytes, macrophages, and fibroblastlike cells found in rheumatoid synovium originate from or have their counterpart in the BM. These cells are capable of producing a variety of cytokines that may act not only as mediators of inflammation but also as regulators of hematopoiesis. It is then possible that BM, even if not primarily affected, may display impaired function in RA as a consequence of abnormal interactions between immune and hematopoietic cells or as a result of an aberrant, local or systemic, cytokine production potentially affecting the survival, proliferation, and optimal growth of hematopoietic cells. Indeed, previous studies have shown an altered phenotype and abnormal growth activity of BM myeloid progenitor cells in RA, at least at sites adjacent to the affected joints.2,8-10 It has also been reported that elevated levels of locally produced proinflammatory cytokines, such as tumor necrosis factor alpha (TNFα), may modulate the frequency of erythroid progenitor cells in the BM and may contribute to the pathogenesis of anemia frequently seen in patients with RA.11 The observation that BM stromal cell lines established from RA patients displayed abnormal support of B-cell growth, is additional evidence for a possible defect of BM microenvironment in RA.12
However, stem cells per se, in terms of their number and functional characteristics, and BM microenvironment, in terms of its capacity to support hematopoietic progenitor cell growth, have not been extensively studied in RA. In view of the current interest in exploring the use of high-dose immunosuppression followed by autologous hematopoietic stem cell transplantation (ASCT) in patients with severe, resistant autoimmune diseases including RA,13-16 it is important to know whether BM stem cell compartment and/or BM microenvironment are already affected by the inflammatory process in these patients. The aim of the present study was to evaluate hematopoietic progenitor cell reserve and function and BM stromal cell hematopoiesis supporting capacity in patients with severe, active RA.
Patients, materials, and methods
Patients
We studied 26 white patients with RA, 18 females and 8 males, aged 28 to 71 years (median, 53.5 years). All patients satisfied the 1987 revised diagnostic criteria of the American College of Rheumatology for RA17 and had evidence of active disease according to established criteria.18 Health-related quality of life and functional status of the patients were determined using the Health Assessment Questionnaire (HAQ) index.19Patient characteristics are summarized in Table1. At the time of study, 6 patients were on treatment only with nonsteroidal anti-inflammatory drugs (NSAIDs) and had never been previously treated with corticosteroids or disease-modifying antirheumatic drugs (DMARDs), 4 patients were on a low-dose oral corticosteroid regimen receiving 5 mg to 10 mg prednisolone daily, 18 were taking methotrexate at a dose ranging from 15 mg to 20 mg weekly, and 14 were receiving other DMARDs including 400 mg hydroxychloroquine daily and/or 2000 mg sulfasalazine-A daily, or 150 mg cyclosporine-A daily. Patients had discontinued any medication for at least 24 hours prior to BM aspiration. Of the patients, 12 were also studied 6 to 8 months after initiation of treatment with cA2 (Remicade, Infliximab; Schering-Plough, Kenilworth, NJ), a human/murine chimeric monoclonal antibody (Mab) of IgG1κ isotype, with specificity for recombinant and natural human TNFα.20 Patients were receiving the antibody in a 2-hour intravenous infusion at a dose of 3 mg/kg on weeks 0, 2, and 6, and then every 2 months, without discontinuing previous medication. As controls, 37 hematologically healthy subjects undergoing surgery for lumbar fixation or healthy volunteers, age- and sex-matched with the patients, were studied. Institutional ethics committee approval was granted prior to the study. Informed consent was provided according to the Declaration of Helsinki.
Clinical and laboratory data of the patients studied
| Patient . | Age/Sex . | Duration (mo) . | Arthritis . | HAQ score . | CRP (mg/dL) . | ESR (mm/h) . | RF (mg/dL) . | Peripheral blood counts† . | Bone marrow† . | Medication‡ . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hgb (g/dL) . | WBC . | Neutro . | Lympho . | Mono . | Plt . | NSAID . | MTX . | PDN . | Other (DMARDs) . | |||||||||
| No. tender/swollen joints . | (×109/L) . | Cellularity . | ||||||||||||||||
| 1 | 71/F | 180 | 56/12 | ND | 8.6 | 61 | 166 | 11.1 | 9.7 | 7.1 | 1.8 | 0.6 | 252 | Normal | No | Yes | 5 mg/d | None |
| 2 | 37/M | 5 | 40/22 | ND | 4.6 | 50 | 153 | 14.2 | 8.4 | 5.7 | 1.8 | 0.8 | 255 | Normal | Yes | No | No | None |
| 3* | 35/M | 12 | 22/10 | ND | 0.6 | 2 | 55 | 15.0 | 6.4 | 3.4 | 2.2 | 0.5 | 244 | Increased | No | 20 mg/w | No | HQ |
| 4 | 53/F | 96 | 26/20 | ND | 0.7 | 42 | 293 | 12.2 | 5.7 | 3.6 | 1.6 | 0.4 | 210 | Normal | No | No | No | HQ |
| 5* | 55/F | 24 | 55/37 | 0.600 | 0.9 | 20 | 155 | 12.0 | 6.9 | 6.2 | 0.5 | 0.1 | 239 | Increased | No | 20 mg/w | 10 mg/d | No |
| 6* | 55/M | 276 | 27/20 | 1.750 | 0.6 | 42 | 137 | 12.4 | 8.6 | 6.1 | 1.7 | 0.6 | 383 | Increased | No | 15 mg/w | No | CSA |
| 7* | 58/F | 60 | 58/50 | 1.125 | 0.6 | 58 | 51 | 14.1 | 9.3 | 4.2 | 4.1 | 0.7 | 458 | Normal | No | 15 mg/w | No | SSZ |
| 8* | 30/F | 10 | 39/39 | 0.375 | 0.1 | 45 | 20 | 12.6 | 7.6 | 5.5 | 1.4 | 0.4 | 400 | Normal | No | 20 mg/w | No | SSZ, HQ |
| 9 | 67/F | 96 | 49/47 | 1.500 | 0.8 | 41 | 41 | 11.4 | 8.4 | 5.0 | 2.5 | 0.7 | 383 | Decreased | No | 20 mg/w | No | SSZ |
| 10* | 55/F | 48 | 57/54 | 1.250 | 1.2 | 17 | 37 | 14.2 | 6.6 | 4.6 | 1.3 | 0.5 | 108 | Increased | No | 15 mg/w | No | SSZ |
| 11 | 44/F | 240 | 53/46 | 0.500 | 1.3 | 45 | 43 | 13.0 | 6.8 | 3.8 | 2.4 | 0.4 | 256 | Normal | No | 15 mg/w | No | SSZ, HQ |
| 12 | 49/F | 120 | 58/55 | 1.250 | 0.4 | 73 | 49 | 10.4 | 4.8 | 2.1 | 2.0 | 0.5 | 371 | Normal | No | 20 mg/w | No | SSZ |
| 13 | 60/M | 96 | 8/8 | ND | 0.4 | 24 | 51 | 13.3 | 8.0 | 5.8 | 1.5 | 0.6 | 363 | Normal | No | 15 mg/w | No | No |
| 14 | 31/M | 120 | 32/31 | 1.250 | 0.7 | 43 | 50 | 10.1 | 7.2 | 2.6 | 1.5 | 1.9 | 153 | Decreased | No | 15 mg/w | No | CSA |
| 15* | 54/M | 24 | 43/31 | 0.375 | 0.2 | 67 | 21 | 9.9 | 7.4 | 4.7 | 1.7 | 0.8 | 430 | Increased | No | 15 mg/w | No | SSZ |
| 16* | 54/F | 96 | 55/38 | 1.250 | 0.8 | 55 | 31 | 11.3 | 4.6 | 3.1 | 1.1 | 0.3 | 320 | Decreased | No | 15 mg/w | No | SSZ, HQ |
| 17 | 49/F | 12 | 62/21 | 0.250 | 6.3 | 62 | 292 | 11.3 | 8.6 | 5.9 | 2.0 | 0.3 | 292 | Normal | Yes | No | No | None |
| 18* | 62/F | 22 | 40/22 | 0.750 | 0.8 | 69 | 111 | 12.5 | 7.8 | 5.0 | 2.2 | 0.5 | 293 | Normal | No | 15 mg/w | No | SSZ, HQ |
| 19* | 33/F | 84 | 36/26 | 0.250 | 0.8 | 58 | 230 | 11.8 | 9.8 | 7.1 | 1.7 | 0.9 | 221 | Increased | No | 15 mg/w | No | None |
| 20* | 68/F | 72 | 22/18 | 1.375 | 1.2 | 23 | 51 | 12.5 | 6.8 | 3.5 | 2.4 | 0.7 | 253 | Decreased | No | 15 mg/w | 5 mg/d | SSZ, HQ |
| 21 | 71/F | 120 | 21/19 | ND | 0.3 | 21 | 68 | 13.7 | 5.2 | 3.5 | 1.2 | 0.3 | 229 | Decreased | No | 15 mg/w | No | None |
| 22 | 49/M | 6 | 40/12 | 0.250 | 1.1 | 128 | 786 | 15.0 | 6.3 | 3.5 | 2.4 | 0.4 | 185 | Normal | Yes | No | No | None |
| 23 | 47/F | 6 | 35/31 | 0.250 | 1.6 | 33 | 25 | 11.3 | 3.0 | 1.5 | 1.3 | 0.2 | 186 | Normal | Yes | No | No | None |
| 24 | 28/F | 12 | 33/20 | 0.375 | 1.2 | 85 | 110 | 12.3 | 5.0 | 2.8 | 1.6 | 0.6 | 220 | Normal | Yes | No | No | None |
| 25 | 35/M | 10 | 22/12 | 0.375 | 0.8 | 43 | 137 | 13.0 | 6.8 | 3.1 | 2.4 | 0.8 | 250 | Normal | Yes | No | No | None |
| 26* | 65/F | 240 | 50/45 | 1.375 | 0.9 | 85 | 155 | 11.2 | 5.7 | 3.6 | 1.6 | 0.5 | 125 | Decreased | No | 15 mg/w | 5 mg/d | None |
| Patient . | Age/Sex . | Duration (mo) . | Arthritis . | HAQ score . | CRP (mg/dL) . | ESR (mm/h) . | RF (mg/dL) . | Peripheral blood counts† . | Bone marrow† . | Medication‡ . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hgb (g/dL) . | WBC . | Neutro . | Lympho . | Mono . | Plt . | NSAID . | MTX . | PDN . | Other (DMARDs) . | |||||||||
| No. tender/swollen joints . | (×109/L) . | Cellularity . | ||||||||||||||||
| 1 | 71/F | 180 | 56/12 | ND | 8.6 | 61 | 166 | 11.1 | 9.7 | 7.1 | 1.8 | 0.6 | 252 | Normal | No | Yes | 5 mg/d | None |
| 2 | 37/M | 5 | 40/22 | ND | 4.6 | 50 | 153 | 14.2 | 8.4 | 5.7 | 1.8 | 0.8 | 255 | Normal | Yes | No | No | None |
| 3* | 35/M | 12 | 22/10 | ND | 0.6 | 2 | 55 | 15.0 | 6.4 | 3.4 | 2.2 | 0.5 | 244 | Increased | No | 20 mg/w | No | HQ |
| 4 | 53/F | 96 | 26/20 | ND | 0.7 | 42 | 293 | 12.2 | 5.7 | 3.6 | 1.6 | 0.4 | 210 | Normal | No | No | No | HQ |
| 5* | 55/F | 24 | 55/37 | 0.600 | 0.9 | 20 | 155 | 12.0 | 6.9 | 6.2 | 0.5 | 0.1 | 239 | Increased | No | 20 mg/w | 10 mg/d | No |
| 6* | 55/M | 276 | 27/20 | 1.750 | 0.6 | 42 | 137 | 12.4 | 8.6 | 6.1 | 1.7 | 0.6 | 383 | Increased | No | 15 mg/w | No | CSA |
| 7* | 58/F | 60 | 58/50 | 1.125 | 0.6 | 58 | 51 | 14.1 | 9.3 | 4.2 | 4.1 | 0.7 | 458 | Normal | No | 15 mg/w | No | SSZ |
| 8* | 30/F | 10 | 39/39 | 0.375 | 0.1 | 45 | 20 | 12.6 | 7.6 | 5.5 | 1.4 | 0.4 | 400 | Normal | No | 20 mg/w | No | SSZ, HQ |
| 9 | 67/F | 96 | 49/47 | 1.500 | 0.8 | 41 | 41 | 11.4 | 8.4 | 5.0 | 2.5 | 0.7 | 383 | Decreased | No | 20 mg/w | No | SSZ |
| 10* | 55/F | 48 | 57/54 | 1.250 | 1.2 | 17 | 37 | 14.2 | 6.6 | 4.6 | 1.3 | 0.5 | 108 | Increased | No | 15 mg/w | No | SSZ |
| 11 | 44/F | 240 | 53/46 | 0.500 | 1.3 | 45 | 43 | 13.0 | 6.8 | 3.8 | 2.4 | 0.4 | 256 | Normal | No | 15 mg/w | No | SSZ, HQ |
| 12 | 49/F | 120 | 58/55 | 1.250 | 0.4 | 73 | 49 | 10.4 | 4.8 | 2.1 | 2.0 | 0.5 | 371 | Normal | No | 20 mg/w | No | SSZ |
| 13 | 60/M | 96 | 8/8 | ND | 0.4 | 24 | 51 | 13.3 | 8.0 | 5.8 | 1.5 | 0.6 | 363 | Normal | No | 15 mg/w | No | No |
| 14 | 31/M | 120 | 32/31 | 1.250 | 0.7 | 43 | 50 | 10.1 | 7.2 | 2.6 | 1.5 | 1.9 | 153 | Decreased | No | 15 mg/w | No | CSA |
| 15* | 54/M | 24 | 43/31 | 0.375 | 0.2 | 67 | 21 | 9.9 | 7.4 | 4.7 | 1.7 | 0.8 | 430 | Increased | No | 15 mg/w | No | SSZ |
| 16* | 54/F | 96 | 55/38 | 1.250 | 0.8 | 55 | 31 | 11.3 | 4.6 | 3.1 | 1.1 | 0.3 | 320 | Decreased | No | 15 mg/w | No | SSZ, HQ |
| 17 | 49/F | 12 | 62/21 | 0.250 | 6.3 | 62 | 292 | 11.3 | 8.6 | 5.9 | 2.0 | 0.3 | 292 | Normal | Yes | No | No | None |
| 18* | 62/F | 22 | 40/22 | 0.750 | 0.8 | 69 | 111 | 12.5 | 7.8 | 5.0 | 2.2 | 0.5 | 293 | Normal | No | 15 mg/w | No | SSZ, HQ |
| 19* | 33/F | 84 | 36/26 | 0.250 | 0.8 | 58 | 230 | 11.8 | 9.8 | 7.1 | 1.7 | 0.9 | 221 | Increased | No | 15 mg/w | No | None |
| 20* | 68/F | 72 | 22/18 | 1.375 | 1.2 | 23 | 51 | 12.5 | 6.8 | 3.5 | 2.4 | 0.7 | 253 | Decreased | No | 15 mg/w | 5 mg/d | SSZ, HQ |
| 21 | 71/F | 120 | 21/19 | ND | 0.3 | 21 | 68 | 13.7 | 5.2 | 3.5 | 1.2 | 0.3 | 229 | Decreased | No | 15 mg/w | No | None |
| 22 | 49/M | 6 | 40/12 | 0.250 | 1.1 | 128 | 786 | 15.0 | 6.3 | 3.5 | 2.4 | 0.4 | 185 | Normal | Yes | No | No | None |
| 23 | 47/F | 6 | 35/31 | 0.250 | 1.6 | 33 | 25 | 11.3 | 3.0 | 1.5 | 1.3 | 0.2 | 186 | Normal | Yes | No | No | None |
| 24 | 28/F | 12 | 33/20 | 0.375 | 1.2 | 85 | 110 | 12.3 | 5.0 | 2.8 | 1.6 | 0.6 | 220 | Normal | Yes | No | No | None |
| 25 | 35/M | 10 | 22/12 | 0.375 | 0.8 | 43 | 137 | 13.0 | 6.8 | 3.1 | 2.4 | 0.8 | 250 | Normal | Yes | No | No | None |
| 26* | 65/F | 240 | 50/45 | 1.375 | 0.9 | 85 | 155 | 11.2 | 5.7 | 3.6 | 1.6 | 0.5 | 125 | Decreased | No | 15 mg/w | 5 mg/d | None |
HAQ indicates Health Assessment Questionnaire; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RF, rheumatoid factor; Hgb, hemoglobin; WBC, white blood cells; Neutro, neutrophils; Lympho, lymphocytes; Plt, platelets; Mono, monocytes; ND, not done; NSAID, nonsteroidal anti-inflammatory drugs; MTX, methotrexate; PDN, prednizone; DMARDs, disease-modifying antirheumatic drugs; HQ, hydroxychloroquine (400 mg/d); SSZ, sulfasalazine-A (2 g/d); CSA, cyclosporin-A (150 mg/d).
These patients were also studied 6 to 8 months after initiation of anti-TNFα treatment.
At the time of study.
During the last 6 months.
BM samples
BM cells obtained from posterior iliac crest aspirates from patients and healthy controls, were immediately diluted 1:1 in Iscoves modified Dulbecco medium (IMDM; Gibco BRL Life Technologies, Paisley, Scotland), supplemented with 100 IU/mL penicilline-streptomycin (PS; GibcoBRL Life Technologies) and 10 IU/mL preservative-free heparin (Sigma, St Louis, MO). Diluted BM samples were centrifuged on Lymphoprep (Nycomed Pharma AS, Oslo, Norway) (density 1.077 g/cm3) at 400g for 30 minutes at room temperature to obtain the bone marrow mononuclear cells (BMMCs). Cell number and viability were assessed after staining with trypan blue.
Immunophenotyping and 7-amino-actinomycin D staining
An indirect immunofluorescence technique was used to quantitate BM CD34+ cells. In brief, 1 × 106 BMMCs were stained with phycoerythrin (PE)–conjugated mouse antihuman CD34 Mab (QBEND-10; Immunotech, Marseille, France) and fluorescein isothiocyanate (FITC)–conjugated mouse antihuman CD38 Mab (T16; Immunotech) for 30 minutes on ice. PE- and FITC-conjugated mouse IgG isotype-matched controls were used as negative controls. Cells were washed twice in phosphate buffered saline (PBS) containing 1% fetal calf serum (FCS; Gibco BRL, Life Technologies) and 0.05% azide, and fixed in 500 μL 2% paraformaldeyde solution (Sigma).
Aliquots of 1 × 106 BMMCs stained with PE-conjugated anti-CD34 Mab and FITC-conjugated mouse antihuman Fas (CD95) Mab (LOB 3/17; Serotec, United Kingdom) as above, were further stained prior to fixation with 7-amino-actinomycin D (7AAD; Calbiochem-Novabiochem, La Jolla, CA) as previously described.21 In brief, 100 μL 7AAD solution (200 μg/mL) was added to the cells, suspended in 1 mL PBS, and incubated for 20 minutes on ice protected from the light. Following centrifugation, the supernatant was removed and cells were fixed as above. Unstained fixed cells were used as negative controls.
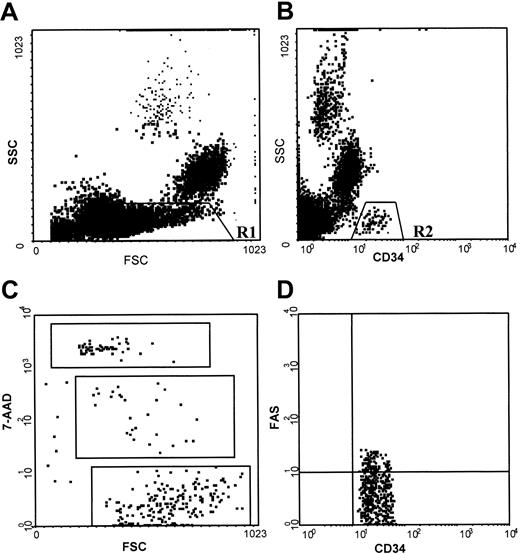
Cell samples were analyzed on an Epics Elite model flow cytometer (Coulter, Miami, FL) within 30 minutes of fixation. Data were acquired and processed on 500 000 events. After creating a scattergram combining forward and right-angle light scatter for the whole population, a region was drawn around BMMCs (low forward and low right scatter properties) for CD34+ and CD38+ cell estimation. When cells were stained for CD34 antigen, Fas antigen, and 7AAD, a scattergram was created by combining right-angle light scatter with CD34 fluorescence in the gate of BMMCs and a second scattergram was generated by combining CD34 and Fas fluorescence in the gate of CD34+ cells. Finally, a scattergram was generated by combining forward light scatter with 7AAD fluorescence to quantitate 7AAD-negative (live), -dim (apoptotic), and -bright (dead) cells in the gate of CD34+, CD34+/Fas+, and CD34+/Fas− cell populations. Regions were drawn around clear-cut populations, and the proportion of cells within each region was calculated excluding cell debris (Figure 1).
Flow cytometric analysis of normal BMMCs stained with anti-CD34 antibody, anti-Fas (CD95) antibody, and 7AAD.
(A) Scattergram of forward light scatter (FSC) versus right-angle light scatter (SSC), to allow gating on the BMMCs (low FSC and low SSC properties) (R1). (B) Scattergram of anti-CD34 fluorescence versus SSC gated on R1, to allow gating on CD34+ cells (R2). (C) Scattergram of FSC versus 7AAD fluorescence gated on R2, showing 7AADbright (dead), 7AADdim (apoptotic), and 7AAD− (live) cells. (D) Scattergram of anti-CD34 versus anti-Fas fluorescence gated on R2, showing Fas+ and Fas− CD34+ cells. Similar staining patterns were obtained for RA BM samples.
Flow cytometric analysis of normal BMMCs stained with anti-CD34 antibody, anti-Fas (CD95) antibody, and 7AAD.
(A) Scattergram of forward light scatter (FSC) versus right-angle light scatter (SSC), to allow gating on the BMMCs (low FSC and low SSC properties) (R1). (B) Scattergram of anti-CD34 fluorescence versus SSC gated on R1, to allow gating on CD34+ cells (R2). (C) Scattergram of FSC versus 7AAD fluorescence gated on R2, showing 7AADbright (dead), 7AADdim (apoptotic), and 7AAD− (live) cells. (D) Scattergram of anti-CD34 versus anti-Fas fluorescence gated on R2, showing Fas+ and Fas− CD34+ cells. Similar staining patterns were obtained for RA BM samples.
Purification of CD34+ cells
CD34+ cells were isolated from BMMCs by indirect magnetic labeling (magnetic activated cell sorting; MACS isolation kit, Mitenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the manufacturer's protocol. In each experiment, purity of CD34+ cells was more than 96% as estimated by flow cytometry.
Clonogenic progenitor cell assays
BMMCs or CD34+ BM cells were cultured in 1 mL IMDM supplemented with 30% FCS, 1% bovine serum albumin (BSA; Gibco BRL Life Technologies), 10−4 M mercaptoethanol (Sigma), 0.075% sodium bicarbonate (Gibco BRL Life Technologies), 2 mMl-glutamine (Sigma), 0.9% methylcellulose (StemCell Technologies, Vancouver, BC, Canada), in the presence of 5 ng granulocyte macrophage–colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN), 50 ng interleukin-3 (IL-3; R&D Systems), and 2 IU erythropoietin (EPO; Janssen-Ciliag, Bucks, United Kingdom), at a concentration of 105 BMMCs or 3 × 103CD34+ cells/mL of culture medium. Cultures were set up in duplicate in 35-mm petri dishes and incubated at a 37°C, 5% CO2, fully humidified atmosphere. On day 14, colonies were scored and classified as granulocyte colony-forming units (CFU-G), macrophage colony-forming units (CFU-M), granulocyte-macrophage colony-forming units (CFU-GM), erythroid burst-forming units (BFU-E) and colonies containing both granulocyte-macrophage and erythroid elements (CFU-GEM) according to established criteria.22Results were expressed as total numbers of CFU-GM (CFU-G plus CFU-M plus CFU-GM) and total numbers of BFU-E (BFU-E plus CFU-GEM).
Long-term bone marrow cultures
Long-term bone marrow cultures (LTBMCs) from 107BMMCs were grown according to the standard technique23 24in 10 mL IMDM supplemented with 10% FCS, 10% horse serum (Gibco BRL Life Technologies), 100 IU/mL PS, 2 mmol l-glutamine and 10−6 mol hydrocortisone sodium succinate (Sigma), and incubated at a 33°C, 5% CO2, fully humidified atmosphere. At weekly intervals, cultures were examined for stromal layer formation using an inverted microscope and fed by removing half of the medium and replacing it with equal volume of fresh IMDM supplemented as above. Nonadherent cells were counted and assayed for CFU-GM and BFU-E committed progenitor cells as described above. Colony results were expressed as total numbers of colony-forming cells (CFCs) (CFU-GM plus BFU-E). When mentioned, a mouse antihuman TNFα monoclonal neutralizing antibody (R&D Systems) was added weekly to the cultures at a concentration of 1.8 μg/mL. According to the manufacturer, the neutralization dose50 for this antibody is 0.02 μg/mL to 0.04 μg/mL in the presence of 0.25 ng/mL TNFα. On confluence (week 3-4), cell-free supernatants were harvested and stored at −70°C for TNFα quantification by means of a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Biosource International, Camarillo, CA). According to the manufacturer, the sensitivity of this assay is less than 0.09 pg/mL.
Assessment of BM stromal cell function
A 2-stage culture procedure was used to test the capacity of BM stromal layers to support normal hematopoiesis. Confluent stromal layers from patients and healthy controls, grown in standard LTBMCs, were irradiated (10 Gy) and recharged with 5 × 104normal allogeneic CD34+ BM cells as previously described.25 In each experiment, flasks were recharged in triplicate and the CD34+ cells from the same healthy control were used to test patient and healthy control cultures. Cultures were fed weekly by demidepopulation and supernatants were monitored by determining the total number of nonadherent cells and CFC frequency.
Statistical analysis
Data were analyzed in the GraphPad Prism statistical PC program (GraphPad Software, San Diego, CA) by means of the nonparametric Mann-Whitney test, the Student t test for paired samples, and the Pearson coefficient of correlation test. Standard 2-way analysis of variance test was used to define differences in the number of nonadherent cells and the number of CFCs generated in LTBMCs in the groups tested. The F value represents the ratio of variance between the groups and the total variance of values in the study. Statistically significant differences for a certain F value are given in specific tables on the basis of the respective degrees of freedom.26
Results
Flow-cytometric data
The percentage of BM CD34+ cells and their subpopulations in patients and healthy controls are presented in Table2. Patients with RA displayed significantly lower proportions of CD34+ cells within the BMMCs, compared with the controls (P = .0016). This decrease probably reflected the reduction of the committed CD34+/CD38+ cells (P = .0017), because no statistically significant difference was found between patients and healthy controls in the number of the more primitive CD34+/CD38− cells (P = .1890).
Flow-cytometric analysis of bone marrow mononuclear cells
| . | Untreated RA patients* . | Treated RA patients† . | Total RA patients* . | Healthy controls . |
|---|---|---|---|---|
| BMMC fraction | ||||
| % CD34+cells | 1.18 ± 0.44‡ | 1.49 ± 1.06 | 1.42 ± 0.94 | 2.13 ± 1.02 |
| Median (range) | 1.30 (0.55-1.80) | 1.20 (0.40-4.10) | 1.20 (0.40-4.10) | 1.90 (0.70-4.80) |
| n | 6 | 20 | 26 | 37 |
| P2-153 | .0142 | .0091 | .0016 | |
| P2-155 | .9519 | |||
| % CD34+/CD38+ cells | 0.92 ± 0.32 | 1.04 ± 0.76 | 1.03 ± 0.69 | 1.69 ± 0.89 |
| Median (range) | 1.00 (0.46-1.30) | 1.84 (0.20-3.00) | 0.92 (0.20-3.00) | 1.60 (0.30-4.50) |
| n | 6 | 20 | 26 | 37 |
| P2-153 | .0439 | .0038 | .0017 | |
| P2-155 | .8550 | |||
| % CD34+/CD38− cells | 0.26 ± 0.14 | 0.41 ± 0.34 | 0.36 ± 0.31 | 0.44 ± 0.28 |
| Median (range) | 0.25 (0.09-0.50) | 0.30 (0.10-1.40) | 0.25 (0.09-1.40) | 0.38 (0.10-1.18) |
| n | 6 | 20 | 26 | 37 |
| P2-153 | .1188 | .4871 | .1890 | |
| P2-155 | .2726 | |||
| CD34+cell fraction | ||||
| % Fas+cells | 15.78 ± 6.39 | 14.48 ± 10.33 | 15.02 ± 9.62 | 8.38 ± 3.68 |
| Median (range) | 14.48 (8.90-25.50) | 9.88 (5.60-44.70) | 12.0 (5.60-44.70) | 9.2 (1.59-14.52) |
| n | 6 | 14 | 20 | 25 |
| P2-153 | .0166 | .040 | .0210 | |
| P2-155 | .2482 | |||
| % 7AADdim cells | 15.61 ± 8.69 | 10.38 ± 8.12 | 11.70 ± 9.32 | 5.93 ± 4.37 |
| Median (range) | 14.28 (8.24-32.04) | 7.70 (2.30-32.07) | 8.70 (2.30-32.07) | 3.94 (1.20-44.70) |
| n | 6 | 17 | 23 | 24 |
| P2-153 | .004 | .047 | .0136 | |
| P2-155 | .123 | |||
| CD34+/Fas+cell fraction | ||||
| % 7AADdimcells | 40.29 ± 7.63 | 40.21 ± 23.24 | 39.21 ± 21.06 | 25.71 ± 17.73 |
| Median (range) | 38.89 (30.51-51.20) | 37.57 (8.70-83.30) | 37.56 (8.70-83.30) | 23.86 (1.05-66.67) |
| n | 6 | 14 | 20 | 24 |
| P2-153 | .0406 | .0468 | .0310 | |
| P2-155 | .9015 | |||
| CD34+/Fas−cell fraction | ||||
| % 7AADdimcells | 11.98 ± 10.64 | 6.21 ± 5.74 | 7.94 ± 7.73 | 6.78 ± 7.42 |
| Median (range) | 8.80 (2.60-32.51) | 4.50 (1.50-24.08) | 5.41 (1.50-32.51) | 4.41 (0.92-32.00) |
| n | 6 | 14 | 20 | 24 |
| P2-153 | .0737 | .7737 | .2940 | |
| P2-155 | .1171 |
| . | Untreated RA patients* . | Treated RA patients† . | Total RA patients* . | Healthy controls . |
|---|---|---|---|---|
| BMMC fraction | ||||
| % CD34+cells | 1.18 ± 0.44‡ | 1.49 ± 1.06 | 1.42 ± 0.94 | 2.13 ± 1.02 |
| Median (range) | 1.30 (0.55-1.80) | 1.20 (0.40-4.10) | 1.20 (0.40-4.10) | 1.90 (0.70-4.80) |
| n | 6 | 20 | 26 | 37 |
| P2-153 | .0142 | .0091 | .0016 | |
| P2-155 | .9519 | |||
| % CD34+/CD38+ cells | 0.92 ± 0.32 | 1.04 ± 0.76 | 1.03 ± 0.69 | 1.69 ± 0.89 |
| Median (range) | 1.00 (0.46-1.30) | 1.84 (0.20-3.00) | 0.92 (0.20-3.00) | 1.60 (0.30-4.50) |
| n | 6 | 20 | 26 | 37 |
| P2-153 | .0439 | .0038 | .0017 | |
| P2-155 | .8550 | |||
| % CD34+/CD38− cells | 0.26 ± 0.14 | 0.41 ± 0.34 | 0.36 ± 0.31 | 0.44 ± 0.28 |
| Median (range) | 0.25 (0.09-0.50) | 0.30 (0.10-1.40) | 0.25 (0.09-1.40) | 0.38 (0.10-1.18) |
| n | 6 | 20 | 26 | 37 |
| P2-153 | .1188 | .4871 | .1890 | |
| P2-155 | .2726 | |||
| CD34+cell fraction | ||||
| % Fas+cells | 15.78 ± 6.39 | 14.48 ± 10.33 | 15.02 ± 9.62 | 8.38 ± 3.68 |
| Median (range) | 14.48 (8.90-25.50) | 9.88 (5.60-44.70) | 12.0 (5.60-44.70) | 9.2 (1.59-14.52) |
| n | 6 | 14 | 20 | 25 |
| P2-153 | .0166 | .040 | .0210 | |
| P2-155 | .2482 | |||
| % 7AADdim cells | 15.61 ± 8.69 | 10.38 ± 8.12 | 11.70 ± 9.32 | 5.93 ± 4.37 |
| Median (range) | 14.28 (8.24-32.04) | 7.70 (2.30-32.07) | 8.70 (2.30-32.07) | 3.94 (1.20-44.70) |
| n | 6 | 17 | 23 | 24 |
| P2-153 | .004 | .047 | .0136 | |
| P2-155 | .123 | |||
| CD34+/Fas+cell fraction | ||||
| % 7AADdimcells | 40.29 ± 7.63 | 40.21 ± 23.24 | 39.21 ± 21.06 | 25.71 ± 17.73 |
| Median (range) | 38.89 (30.51-51.20) | 37.57 (8.70-83.30) | 37.56 (8.70-83.30) | 23.86 (1.05-66.67) |
| n | 6 | 14 | 20 | 24 |
| P2-153 | .0406 | .0468 | .0310 | |
| P2-155 | .9015 | |||
| CD34+/Fas−cell fraction | ||||
| % 7AADdimcells | 11.98 ± 10.64 | 6.21 ± 5.74 | 7.94 ± 7.73 | 6.78 ± 7.42 |
| Median (range) | 8.80 (2.60-32.51) | 4.50 (1.50-24.08) | 5.41 (1.50-32.51) | 4.41 (0.92-32.00) |
| n | 6 | 14 | 20 | 24 |
| P2-153 | .0737 | .7737 | .2940 | |
| P2-155 | .1171 |
Statistical analysis was performed using the nonparametric Mann-Whitney test. P value ≤ .05 was considered statistically significant.
Patients previously treated or not with methotrexate, corticosteroids, or other disease-modifying antirheumatic drugs.
Treated and untreated patients.
Values are expressed as mean ± SD.
Comparison between patient groups and healthy controls.
Comparison between the groups of untreated and treated patients with RA.
To explore whether the decrease of CD34+ cells in patients with RA was due to increased apoptotic cell death, we evaluated the proportion of apoptotic cells within the CD34+ cell fraction using 7AAD staining (Table 2). We found that patient CD34+ cells contained significantly higher numbers of apoptotic (7AADdim) cells compared with the controls (P = .0136). In contrast, no statistically significant difference was found between patients (6.96% ± 5.86%, n = 23) and control subjects (6.69% ± 5.61%, n = 24) in the percentage of apoptotic cells detected in the non-CD34+ BMMC fraction (P = .6630).
Because Fas antigen expression has been associated with apoptosis of BM hematopoietic progenitor cells,27 we evaluated the expression of this molecule on patients' CD34+ cells (Table 2). We found that the proportion of Fas+ cells in the gate of CD34+ cells was significantly higher in the group of patients than in the group of controls (P = .0210). A highly significant positive correlation was noted between the proportion of Fas+ cells and the percentage of apoptotic cells within the CD34+ cell population in the entire group of subjects studied (r = 0.611,P < .0001). Moreover, the increased apoptosis found in patients' CD34+ cell compartments was due mainly to the increased proportion of apoptotic cells within the CD34+/Fas+ cell fraction (P = .0310), because no statistically significant difference could be demonstrated between patients and healthy controls in the proportion of apoptotic cells within the CD34+/Fas− cell fraction (P = .2940). Notably, the percentage of apoptotic cells was significantly higher among the CD34+/Fas+cells than among the CD34+/Fas− cells in both the patient (P < .0001) and control (P < .0001) groups. Taken together, these data suggest that Fas antigen expression may account for the increased apoptosis detected in patients' CD34+ cell compartments. Moreover, a highly significant inverse correlation was noted between the percentage of CD34+ cells and the proportion of both Fas+cells (r = −0.462, P = .0018) and apoptotic cells (r = −0.326, P = .0375), indicating that Fas antigen expression and increased apoptosis are probably involved in the aforementioned reduction of CD34+ cells in patients with RA.
Because the majority of the patients had been previously treated with cytotoxic and/or immune suppressant agents, a subset analysis in the number of CD34+, Fas+, and apoptotic cells in the groups of previously treated (n = 20) and untreated (n = 6) patients was performed, to exclude the possibility of drug-induced damage. Statistical analysis was similar in treated and untreated patients (Table 2), suggesting that drug-induced damage of patient progenitor cells is unlikely.
Colony-forming cells
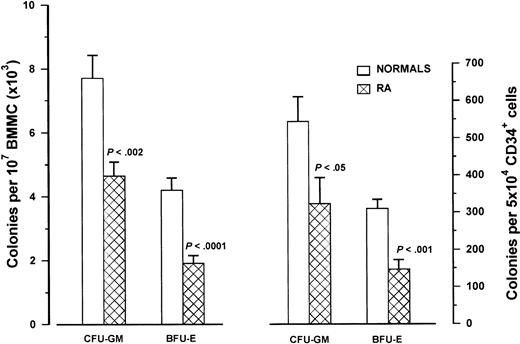
The frequency of clonogenic progenitor cells in the BMMC fraction of patients with RA (n = 25) and healthy controls (n = 25) is depicted in Figure 2. The mean number of CFU-GM and BFU-E obtained by 107 BMMCs was significantly lower in the patients (4648 ± 2197 and 1923 ± 1286, respectively) compared with the controls (7680 ± 3603 and 4196 ± 1859, respectively; P < .002 andP < .0001 respectively). Further analysis showed that compared with the healthy controls, the numbers of CFU-GM and BFU-E were significantly lower in both the previously treated (n = 19) (5284 ± 2079 per 107 BMMCs; P = .0007 and 2169 ± 1368 per 107 BMMCs;P = .0005, respectively) and untreated (n = 6) (2633 ± 1097 per 107 BMMCs; P = .0209 and 1150 ± 769 per 107 BMMCs; P = .0002, respectively) groups of patients, suggesting further that drug-induced damage concerning the number and/or the clonogenic potential of patient progenitor cells is unlikely.
Clonogenic progenitor cells in RA patients.
The left bars represent the mean number (± SD) of CFU-GM and BFU-E obtained by 107 BMMCs in 25 patients with RA and 25 healthy controls using clonogenic progenitor cell assays. The right bars represent the mean colony values (± SD) obtained by 5 × 104 highly purified immunomagnetically sorted CD34+ cells in 12 patients with RA and 21 healthy controls. Comparison between patient and control values was performed by means of the nonparametric Mann-Whitney test.
Clonogenic progenitor cells in RA patients.
The left bars represent the mean number (± SD) of CFU-GM and BFU-E obtained by 107 BMMCs in 25 patients with RA and 25 healthy controls using clonogenic progenitor cell assays. The right bars represent the mean colony values (± SD) obtained by 5 × 104 highly purified immunomagnetically sorted CD34+ cells in 12 patients with RA and 21 healthy controls. Comparison between patient and control values was performed by means of the nonparametric Mann-Whitney test.
Individual CFC values correlated with the proportion of CD34+ cells in the group of controls (r = .508,P < .01) but not in the group of patients with RA (r = .196, P = .359), suggesting a possible defect in the clonogenic potential of patient progenitor cells. To further investigate this hypothesis, we evaluated the colony-forming potential of immunomagnetically purified CD34+ cells from patients with RA (n = 12) (Figure 2). Indeed, CFU-GM and BFU-E colony formation by 5 × 104 CD34+ cells was significantly lower in the patient group (324 ± 241 and mean 147 ± 88, respectively) than in the group of controls (n = 21) (544 ± 308 and 310 ± 107, respectively;P = .036 and P = .0005, respectively).
Standard LTBMCs
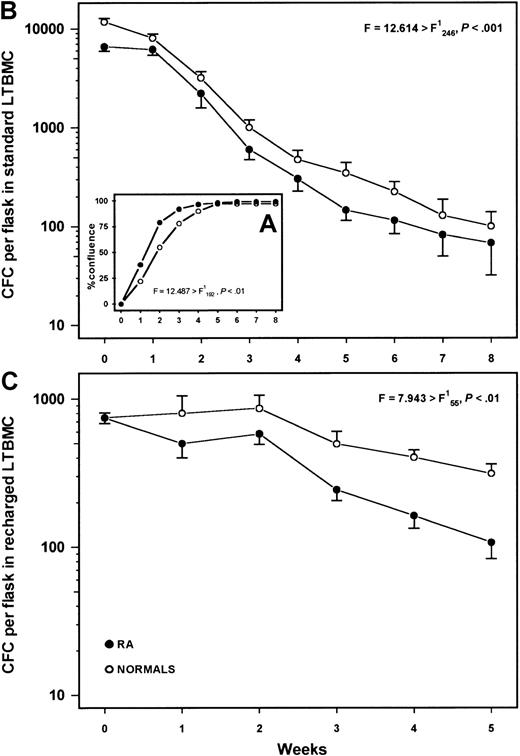
Typical stromal layers consisting of fibroblastlike cells, macrophages, and cobblestone areas were formed over the first 3 weeks in both patient (n = 25) and normal (n = 25) LTBMCs. However, stromal layers from patients with RA reached confluence earlier than those from the healthy controls (F = 12.487 > F,P < .01) (Figure 3A) indicating increased number and/or enhanced proliferating capacity of patient adherent cells. The average nonadherent cell recovery did not differ statistically between patient and normal LTBMCs (F = 1.439 < F;P > .05) and the mean duration of colony production by nonadherent cells was similar in both patients (9 weeks ± 1.6 weeks) and healthy controls (9 weeks ± 1.9 weeks;P = .915) (data not shown). However, the committed progenitor cell content (CFC) in the nonadherent cell fraction was significantly lower in patients with RA compared with the controls (F = 12.614 > F;P < .001) (Figure 3B), suggesting a defect in the clonogenic potential of patient progenitor cells and/or a defect in the hematopoiesis supporting capacity of patient stromal cells. A subset analysis showed that CFC numbers in both the previously treated (n = 19) and untreated (n = 6) patients were lower than those in the healthy controls all over the period of culture (F = 10.901 > F;P < .001 and F = 73.408 > F; P < .001, respectively).
Long-term bone marrow cultures.
(A) Percentage of confluence in the adherent layer of standard LTBMCs in patients with RA (n = 25) and healthy controls (n = 25). (B) Mean frequency of CFCs (± SEM) in the nonadherent cell fraction throughout 8 weeks of culture. (C) Mean number of CFCs (± SEM) in the nonadherent cell fraction of irradiated and recharged LTBMCs from 8 patients with RA and 5 healthy controls over a period of 5 weeks following the CD34+ cell inoculum. Comparison between patient and healthy cultures was performed using the 2-way analysis of variance test.
Long-term bone marrow cultures.
(A) Percentage of confluence in the adherent layer of standard LTBMCs in patients with RA (n = 25) and healthy controls (n = 25). (B) Mean frequency of CFCs (± SEM) in the nonadherent cell fraction throughout 8 weeks of culture. (C) Mean number of CFCs (± SEM) in the nonadherent cell fraction of irradiated and recharged LTBMCs from 8 patients with RA and 5 healthy controls over a period of 5 weeks following the CD34+ cell inoculum. Comparison between patient and healthy cultures was performed using the 2-way analysis of variance test.
Recharged LTBMCs
To further evaluate the hematopoiesis-supporting capacity of patient stromal cells independently of stem cell function, confluent stromal layers from 8 patients with RA and 5 healthy controls were recharged with normal CD34+ cells. Patient LTBMC stromal layers failed to support normal hematopoiesis as indicated by the average total number of nonadherent cells (F = 10.908 > F; P < .01) (data not shown) and the mean CFC frequency in the nonadherent cell fraction (F = 7.943 > F;P < .01) over a period of 5 weeks after the CD34+ cell inoculum (Figure 3C).
TNFα in supernatants
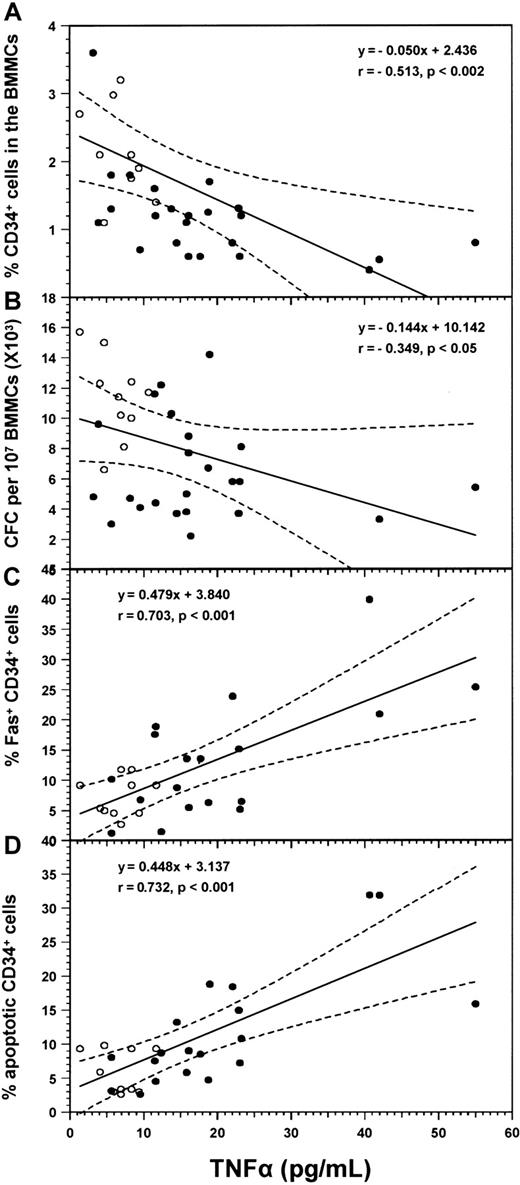
The levels of TNFα in the supernatants of confluent LTBMCs (weeks 3-4) were determined in all patients and 11 healthy controls (Figure 4). Patients with RA displayed significantly higher cytokine concentrations (17.87 ± 12.01 pg/mL, median 15.98 pg/mL, range 3.21 to 55.00 pg/mL) compared with the healthy subjects (6.28 pg/mL ± 2.41 pg/mL, median 6.93 pg/mL, range 1.37 to 9.40 pg/mL; P = .0005). Individual TNFα values inversely correlated with the proportion of CD34+ cells (r = −0.513; P = .0016) and the total number of CFCs (r = −0.349; P = .040), and positively with the proportion of CD34+/Fas+cells (r = 0.703; P < .001) and the proportion of CD34+/7AADdim cells (r = .732;P < .001) in the entire group of subjects studied (Figure5). These data suggest that the increased TNFα production by patient BM stromal cells probably accounts for the quantitative and functional abnormalities found in patients' progenitor cell compartments. However, experiments in which 1 × 106 normal BMMCs were incubated for 48 hours in 1 mL IMDM 20% FCS in the absence or presence of human rhTNFα (R&D Systems) at a concentration of 6.28 pg/mL and 17.87 pg/mL, representing the mean TNFα value found in LTBMC supernatants of healthy subjects and patients with RA respectively, did not show any difference in the expression of Fas within the CD34+ cell population (data not shown). These findings, however, do not reverse our suggestion regarding the role of TNFα on hematopoiesis in RA since the in vivo conditions are different and yet unknown. In fact, we do not know the real concentration of TNFα within the BM microenvironment in soluble and membrane-bound form, or the interactions of the cytokine with other factors locally produced.11 It is probable that higher concentrations of TNFα are required for the in vitro demonstration of the cytokine effect on the expression of Fas on CD34+progenitor cells.28
Levels of TNFα in long-term bone marrow culture supernatants.
Dots represent individual values of TNFα in LTBMC supernatants harvested on confluence and determined by means of ELISA in 26 patients with RA and 11 healthy controls. The mean concentration of the cytokine in patients and control subjects and the 95% confidence limits are indicated by horizontal lines and dotted rectangles, respectively. Comparison was performed using the nonparametric Mann-Whitney test (P = .0005).
Levels of TNFα in long-term bone marrow culture supernatants.
Dots represent individual values of TNFα in LTBMC supernatants harvested on confluence and determined by means of ELISA in 26 patients with RA and 11 healthy controls. The mean concentration of the cytokine in patients and control subjects and the 95% confidence limits are indicated by horizontal lines and dotted rectangles, respectively. Comparison was performed using the nonparametric Mann-Whitney test (P = .0005).
Correlations between the levels of TNFα in culture supernatants and the numbers of CD34+ cell subpopulations and clonogenic progenitor cells.
Diagrams show linear regression analysis for the correlation between the values of TNFα in LTBMC supernatants and the proportion of CD34+ cells (A), the number of CFCs (B), the percentages of CD34+/Fas+ cells (C), and the proportion of CD34+/7AADdim cells (D) in the entire group of subjects (26 patients with RA and 11 healthy controls). Coefficient of correlation (r) and degree of significance (P) are indicated. Regression lines are shown as solid lines and the 95% confidence limits as dotted lines. Patients with RA, (●); healthy controls, (○).
Correlations between the levels of TNFα in culture supernatants and the numbers of CD34+ cell subpopulations and clonogenic progenitor cells.
Diagrams show linear regression analysis for the correlation between the values of TNFα in LTBMC supernatants and the proportion of CD34+ cells (A), the number of CFCs (B), the percentages of CD34+/Fas+ cells (C), and the proportion of CD34+/7AADdim cells (D) in the entire group of subjects (26 patients with RA and 11 healthy controls). Coefficient of correlation (r) and degree of significance (P) are indicated. Regression lines are shown as solid lines and the 95% confidence limits as dotted lines. Patients with RA, (●); healthy controls, (○).
Effect of anti-TNFα treatment on BM progenitor cell reserve and on stromal cell function in patients with RA
BMMCs from 10 patients with RA were analyzed for CD34/CD38, Fas, and 7AAD expression 6 to 8 months after initiation of anti-TNFα treatment and results were compared to pretreatment values (Table3). A significant increase was found in the percentage of CD34+ cells following treatment (P = .003), probably reflecting the increase of the committed CD34+/CD38+ cells (P = .004), because no significant changes were noted in the proportion of early CD34+/CD38− cells (P = .696). The proportion of apoptotic cells within the CD34+ cell compartment was found to be significantly reduced after anti-TNFα treatment (P = .016), and this reduction was associated with a significant decrease in the proportion of CD34+ cells expressing Fas antigen (P = .029). Further analysis showed a significant decrease in the percentage of apoptotic cells detected in the CD34+/Fas+ cell fraction (P = .009) but not in the CD34+/Fas− cell fraction (P = .972) following treatment.
Flow-cytometric data in patients with RA after anti-TNFα treatment
| . | Before treatment (n = 10) . | After treatment (n = 10) . | P . |
|---|---|---|---|
| BMMC fraction | |||
| % CD34+cells | 1.21 ± 0.403-150 | 2.03 ± 0.73 | .003 |
| (1.20, 0.60-1.80) | (2.00, 0.70-3.50) | ||
| % CD34+/CD38+cells | 0.86 ± 0.39 | 1.72 ± 0.66 | .004 |
| (0.86, 0.20-1.40) | (1.75, 0.60-3.20) | ||
| % CD34+/CD38−cells | 0.34 ± 0.18 | 0.31 ± 0.24 | .696 |
| (0.32, 0.10-0.70) | (0.25, 0.10-0.90) | ||
| CD34+ cell fraction | |||
| % Fas+ cells | 16.20 ± 7.26 | 10.87 ± 6.25 | .029 |
| (15.48, 5.60-25.70) | (10.15, 2.60-19.60) | ||
| % 7AADdimcells | 16.09 ± 10.57 | 8.31 ± 5.65 | .016 |
| (13.85, 3.30-32.07) | (6.30, 2.80-18.60) | ||
| CD34+/Fas+ cell fraction | |||
| % 7AADdimcells | 43.94 ± 21.07 | 24.84 ± 16.87 | .009 |
| (39.87, 12.30-73.70) | (19.95, 8.70-58.10) | ||
| CD34+/Fas− cell fraction | |||
| % 7AADdimcells | 7.69 ± 6.15 | 7.79 ± 5.30 | .972 |
| (6.40, 2.90-24.08) | (7.25, 2.30, 17.0) |
| . | Before treatment (n = 10) . | After treatment (n = 10) . | P . |
|---|---|---|---|
| BMMC fraction | |||
| % CD34+cells | 1.21 ± 0.403-150 | 2.03 ± 0.73 | .003 |
| (1.20, 0.60-1.80) | (2.00, 0.70-3.50) | ||
| % CD34+/CD38+cells | 0.86 ± 0.39 | 1.72 ± 0.66 | .004 |
| (0.86, 0.20-1.40) | (1.75, 0.60-3.20) | ||
| % CD34+/CD38−cells | 0.34 ± 0.18 | 0.31 ± 0.24 | .696 |
| (0.32, 0.10-0.70) | (0.25, 0.10-0.90) | ||
| CD34+ cell fraction | |||
| % Fas+ cells | 16.20 ± 7.26 | 10.87 ± 6.25 | .029 |
| (15.48, 5.60-25.70) | (10.15, 2.60-19.60) | ||
| % 7AADdimcells | 16.09 ± 10.57 | 8.31 ± 5.65 | .016 |
| (13.85, 3.30-32.07) | (6.30, 2.80-18.60) | ||
| CD34+/Fas+ cell fraction | |||
| % 7AADdimcells | 43.94 ± 21.07 | 24.84 ± 16.87 | .009 |
| (39.87, 12.30-73.70) | (19.95, 8.70-58.10) | ||
| CD34+/Fas− cell fraction | |||
| % 7AADdimcells | 7.69 ± 6.15 | 7.79 ± 5.30 | .972 |
| (6.40, 2.90-24.08) | (7.25, 2.30, 17.0) |
Median and range values are indicated in parentheses. Statistical analysis was performed using the Student t test for paired samples. P value ≤ .05 was considered statistically significant.
BMMC indicates bone marrow mononuclear cells.
All values are expressed as means ± SD.
Furthermore, the number of CFCs obtained by 107 BMMCs in the clonogenic assays after anti-TNFα treatment was significantly higher compared with pretreatment values (11 370 ± 5138 versus 7650 ± 3469, respectively; P = .033). Similarly, the number of CFCs in the nonadherent cell fraction of standard LTBMCs over a period of 5 weeks was significantly higher following treatment compared with pretreatment values (F = 11.16 > F; P < .001), and TNFα levels in the supernatants on confluence were found to be significantly reduced following treatment (P = .0010, n = 12) (Figure 6).
TNFα levels in LTBMC supernatants before and after in vivo anti-TNFα treatment.
Dots represent individual TNFα levels in LTBMC supernatants harvested on confluence (weeks 3-4) in 12 patients with RA studied before and after in vivo anti-TNFα treatment. Comparison was performed using the Student t test for paired samples.
TNFα levels in LTBMC supernatants before and after in vivo anti-TNFα treatment.
Dots represent individual TNFα levels in LTBMC supernatants harvested on confluence (weeks 3-4) in 12 patients with RA studied before and after in vivo anti-TNFα treatment. Comparison was performed using the Student t test for paired samples.
Finally, to investigate the possible changes in the capacity of BM stroma to support hematopoiesis after anti-TNFα treatment, confluent stromal layers from 3 patients with RA with previously abnormal hematopoiesis-supporting capacity and 2 healthy controls were irradiated and recharged with the same allogeneic normal CD34+ cells as described above. We found that LTBMC stromal layers from the patients with RA displayed hematopoiesis-supporting capacities similar to those of the healthy controls, because no statistically significant difference was found in the number of nonadherent cells (F = 4.33 < F; P > .05) and the frequency of CFCs detected weekly over a period of 5 weeks in culture (F = 3.00 < FP > .05).
Effect of anti-TNFα neutralizing antibody in LTBMCs
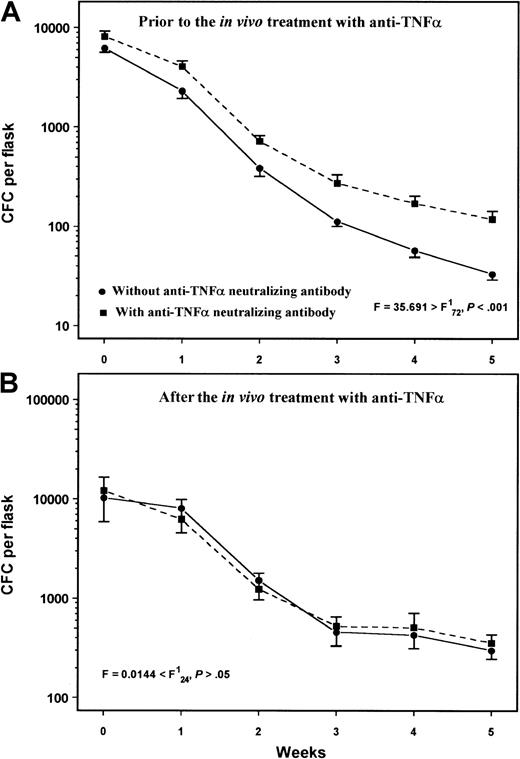
To determine whether the increased TNFα production by BM stromal cells accounts for the impaired hematopoiesis in RA, we also tested the effect of the exogenous addition of an anti-TNFα neutralizing antibody on the colony formation in LTBMCs. We used the antibody at a concentration 100-fold higher than the highest TNFα value found in patient LTBMC supernatants (55 pg/mL). In patients with RA studied prior to in vivo treatment with anti-TNFα (n = 7), the exogenous weekly addition of anti-TNFα in LTBMCs significantly increased the number of nonadherent cells and the CFCs compared to their untreated LTBMCs (F = 4.845 > F;P < .05 and F = 35.691 > F; P < .001, respectively) (Figure 7A). In contrast, in patients studied after in vivo anti-TNFα treatment (n = 3), no statistically significant difference was found in the number of nonadherent cells or the CFC recovery in LTBMCs treated with anti-TNFα compared with their untreated cultures (F = .574 < F;P > .05 and F = .0144 < F; P > .05, respectively) (Figure 7B). Similar results were obtained when Remicade was added to the cultures at a dose 100-fold higher than the concentration of 55 pg/mL (data not shown). Taken together these data further indicate the inhibitory effect of TNFα in hematopoiesis in patients with RA.
Effect of anti-TNFα neutralizing antibody in LTBMCs.
Exogenously added mouse antihuman TNFα neutralizing antibody in LTBMCs at a dose of 1.8 μg/mL per week significantly increased the number of CFCs in those patients with RA studied prior to in vivo anti-TNFα treatment (A) but not in patients studied after treatment (B). Data points represent the mean CFC number (± SEM) in the nonadherent cell fraction of LTBMCs from patients with RA before (n = 7) or after (n = 3) in vivo anti-TNFα treatment. Comparison between treated and untreated with neutralizing antibody cultures was performed using the 2-way analysis of variance test.
Effect of anti-TNFα neutralizing antibody in LTBMCs.
Exogenously added mouse antihuman TNFα neutralizing antibody in LTBMCs at a dose of 1.8 μg/mL per week significantly increased the number of CFCs in those patients with RA studied prior to in vivo anti-TNFα treatment (A) but not in patients studied after treatment (B). Data points represent the mean CFC number (± SEM) in the nonadherent cell fraction of LTBMCs from patients with RA before (n = 7) or after (n = 3) in vivo anti-TNFα treatment. Comparison between treated and untreated with neutralizing antibody cultures was performed using the 2-way analysis of variance test.
Discussion
Regulation of hematopoiesis and maintenance of homeostasis in BM requires a well-balanced interaction between the hematopoietic cells and the immune system. It has been shown that immune dysregulation in autoimmune and chronic inflammatory disorders may modulate the function of BM hematopoietic progenitor cells and/or their microenvironment, either by inflammatory cytokine production or by intricate cell-to-cell interactions.29 RA is a chronic systemic inflammatory disease of autoimmune origin, which has previously been associated with a variety of functional and immunophenotypic abnormalities in BM attributable mainly to augmented local production of inflammatory cytokines30,31 or increased T-cell activation,32,33 or even to an intrinsic or drug-induced34 hematopoietic or stroma abnormality.6 7 This study describes a significant quantitative and functional hematopoietic progenitor cell defect in patients with active RA indicated by the low number of CD34+ cells, the impaired clonogenic potential of BM progenitor cells, and the decreased progenitor cell recovery in LTBMCs. These abnormalities may represent either an intrinsic progenitor cell defect or secondary progenitor cell damage in response to an underlying inflammatory process within the BM microenvironment. Indeed, data from this study showed that patient BM stromal cells produced abnormally high amounts of TNFα which might affect the compartment of progenitor cells.
Using flow cytometry we found that the proportion of apoptotic cells within patients' CD34+ cell compartments was significantly increased and was associated with a significant up-regulation of Fas antigen. Although Fas antigen expression does not always lead to apoptotic cell death,35 it seems likely that the Fas pathway is actively involved in the apoptotic cell depletion of patient CD34+ cells since a highly significant correlation was found between the proportions of Fas+ and apoptotic CD34+ cells and a negative correlation was found between the percentage of CD34+ cells and the proportion of Fas+ progenitor cells in our patients. In keeping with this aspect is the observation that the percentage of apoptotic cells was significantly higher among the Fas+ rather than among the Fas− cells in the subjects studied. Fas antigen is normally expressed on various hematopoietic cell subsets, however under steady-state conditions normal human marrow CD34+ cells do not express Fas or express the antigen at low levels.36Fas up-regulation on marrow CD34+ cells has been reported to be induced by cytokines such as TNFα and interferon-γ and has been associated with bone marrow failure syndromes due to accelerated stem cell apoptosis.36 37 Our patients displayed increased TNFα production by BM stromal cells, and levels of cytokine correlated strongly with the proportions of Fas+ and apoptotic CD34+ cells and inversely with the percentage of total BM CD34+ cells, suggesting that TNFα was critically involved in mediating the apoptotic depletion of patient progenitor cells. The reduction in the proportion of Fas+ and 7AADdim cells within the CD34+ cell compartment and the increase in the number of CD34+ cells following anti-TNFα treatment further corroborate this assumption.
Colony formation by BMMCs was defective in our patients in both short-term and long-term cultures. Although one could postulate that this defect might simply reflect the lower proportion of CD34+ cells in patients' BMMC fractions, this hypothesis seems unlikely since the colony-producing cell number did not correlate with the proportion of CD34+ cells in the group of patients, suggesting a possible intrinsic defect in the clonogenic potential of patient progenitor cells. The reduced colony formation by highly purified patient CD34+ cells is consistent with this assumption. The underlying mechanism(s) responsible for this abnormality is unclear; however, since CFC numbers correlated inversely with TNFα levels, a TNFα-induced growth inhibition of patient progenitor cells cannot be excluded. On the other hand, the suppressive effect of TNFα on marrow progenitor cells has long been demonstrated and is mediated by affecting cell viability or by modulating the expression of numerous cytokine receptors on the cell surface.38
The long-term exposure to antirheumatic and cytotoxic agents such a methotrexate might be a contributing factor affecting BM progenitor cell reserve and function in patients with RA. However, it has been shown that the myelosuppression occasionally seen during treatment with methotrexate affects exclusively late stages of hematopoietic development but not the early myeloid and progenitor cells.39,40 Immunosuppressive agents including glucocorticoids have been reported to induce apoptosis in mitogen-induced peripheral blood mononuclear cells41; however, there is no available evidence that they may affect the BM stem cell compartment. In contrast, there are reports suggesting a rather positive effect of glucocorticoids in the proliferation and differentiation of BM progenitor cells.42-44 On the other hand, 6 of our patients, suggesting a proportion of 23.1%, were studied prior to exposure to cytotoxic agents or immune suppressants, indicating that drug-induced damage is not the major factor affecting hematopoiesis in patients with RA. In keeping with this assumption is the low number of CD34+ cells, the increased apoptosis, and expression of Fas antigen in the CD34+ compartment and the low progenitor cell recovery in short-term and long-term BM cultures in the subgroup of patients who had never been previously treated with immune suppressants compared with healthy subjects.
This study also reports impaired BM stroma function in RA, indicated by the reduced capacity of patient stromal layers to support the growth of autologous or allogeneic normal hematopoietic progenitors in an LTBMC system. In addition to the increase of CD34+ cell number, the anti-TNFα treatment restored stromal cell function, suggesting that increased TNFα production by BM stroma probably accounts for the impaired ability of patient stromal cells to sustain hematopoiesis. The increase of CFC numbers observed following the exogenous addition of the anti-TNFα neutralizing antibody in LTBMCs from patients with RA prior to the in vivo anti-TNFα treatment but not from patients after treatment, and the significantly reduced TNFα levels in LTBMC supernatants following treatment compared with pretreatment values further corroborate this assumption.
Patient LTBMC stromal layers reached confluence earlier than did those of the healthy controls. This finding is in agreement with previous reports, suggesting increased proportion and proliferative capacity of cells of monocyte-macrophage lineage in the BMMC fraction of patients with RA.10,45 These cells primarily participate in the adherent layer formation in LTBMC assays and their synovial counterparts are considered to be the central inflammatory cells producing TNFα in RA. Interestingly, recent studies suggest that fibroblastlike cells in the synovial tissue and the BM microenvironment share a common origin in RA and may contribute to the disease pathogenesis by producing inflammatory cytokines including TNFα.5 46
In conclusion, data from this study suggest that patients with active RA exhibit low frequency and accelerated apoptosis of BM CD34+ cells, defective clonogenic potential of marrow progenitor cells, and impaired hematopoiesis-supporting capacity of BM stroma. We suggest that these abnormalities may be due, at least in part, to the increased TNFα production by inflammatory cells in BM microenvironment. The effectiveness of anti-TNFα treatment in BM progenitor cell and stromal cell reconstitution further supports the concept of a TNFα-mediated suppression of hematopoiesis in patients with RA. Furthermore, consistent with previous reports,47data from this study provide additional evidence for potential difficulties in the stem cell transplantation procedure in patients with RA undergoing ASCT, since BM progenitor or stromal cell abnormalities might impact on the harvesting and engraftment potential of stem cells in these patients.
We would like to thank Dr Maria Psyllaki and Dr Charis Ponticoglou for their technical assistance and the rheumatology, internal medicine, and orthopedics clinical staff of the University Hospital of Heraklion for providing BM samples from patients with RA and from healthy controls.
Supported by University Hospital of Heraklion, Greece.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Helen A. Papadaki, Department of Hematology, University Hospital of Heraklion, PO Box 1352, Heraklion, Crete, Greece; e-mail: epapadak@med.uoc.gr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal