To define the mechanism by which anagrelide normalizes the platelet count in essential thrombocythemia, we studied in vivo megakaryocytopoiesis in 10 newly diagnosed patients prior to and while on anagrelide therapy. Using flow cytometric analysis of aspirated marrow, megakaryocytopoiesis was quantified and correlated with the autologous platelet production rate. Megakaryocytes were identified by CD41a expression and enumerated in relation to the nucleated marrow erythroid precursors. Megakaryocyte diameters were directly measured by time-of-flight technique, and cell ploidy was measured by DNA staining. Two to 3 thousand megakaryocytes were analyzed in each sample. In the 10 patients, the platelet count was 1063 ± 419 × 109platelets/L (mean ± 1 SD) with markedly increased production (237 ± 74 × 109 platelets/L per day versus 43.1 ± 8.4 × 109 platelets/L per day in healthy individuals). The platelet survival was 8.2 ± 1.1 days versus 9.0 ± 0.5 days in healthy controls (P > .05). Megakaryocyte diameter was increased to 46 μm (versus 37 μm in controls; range, 21 μm for 2N to 56 μm for 64N cells). The volume increased to 48 × 103 μm3 versus 26 × 103 μm3 in controls, and the number increased to 14 × 106/kg (versus 7 × 106/kg in controls), resulting in 3.7-fold increase in megakaryocyte mass (66 × 1010 μm3/kg versus 18 × 1010 μm3/kg). Cell ploidy was enhanced showing a modal ploidy of 32N (versus 16N in healthy controls) with marked increase in 64N and 128N cells (P < .05). Anagrelide therapy reduced the platelet counts to 361 ± 53 × 109 platelets/L and the turnover rate to 81 × 109 platelets/L per day. The platelet survival was unchanged. Following therapy, megakaryocyte number decreased to 8 × 106/kg, diameter to 40 μm, and volume to 34 × 103 μm3 with a normalized modal ploidy of 16N, resulting in a megakaryocyte mass reduced by 60% (28 × 1010 μm3/kg;P < .05). This reduction in cell mass closely correlated with the reduction in platelet count and production rate by 66% (r = 0.96). The present data indicate that in essential thrombocythemia anagrelide therapy decreases circulating platelets by reducing both megakaryocyte hyperproliferation and differentiation.
Introduction
Essential thrombocythemia is a chronic myeloproliferative disorder characterized by marked megakaryocytic hyperplasia with sustained thrombocytosis. The clinical course may be complicated by thrombotic and hemorrhagic phenomena as a result of the marked increased platelet count and platelet dysfunction, respectively.1
The standard treatment options for this disorder include the use of alkylating agents and hydroxyurea, both of which cause nonselective suppression of bone marrow elements. If hemostatic complications are prevented, these patients may have a normal life span. Thus, the question of the leukemogenic side effects of both treatments poses a serious concern.2 Recently, anagrelide, a new noncytotoxic agent, has been introduced for the treatment of primary thrombocytosis with significant success.3-6
Anagrelide is an imidazo-quinazolin compound of the quinazolin series, which is known to inhibit platelet aggregation. Pharmacologic studies reveal that anagrelide exerts this effect through an inhibition of (1) the low Km platelet cyclic adenosine monophosphate phosphodiesterase (interfering with Ca++-mediated platelet stimulation), and (2) the enzyme phospholipase A2(inhibiting the release of arachidonic acid from membrane phospholipids). Anagrelide is 10 times more potent than theophylline in potentiating the effect of prostaglandin E1(PGE1).7
In vivo, anagrelide inhibits platelet production in a dose-dependent manner without affecting the production of other marrow elements.3,8 However, the mechanism by which anagrelide induces thrombocytopenia has not yet been defined.9 In vitro, anagrelide inhibits the formation of megakaryocyte (MK) colonies, but only at concentrations higher than those achieved in vivo.3,9,10 Recent data suggest that anagrelide may interfere with MK maturation. However, these data based on image analysis techniques on fixed marrow sections are limited by the number of patients studied (5 patients) and the number of cells analyzed (140/patient).9 10 Moreover, the changes in cell size were insufficient to explain the marked reduction in the platelet count. To further define the mechanism by which anagrelide normalizes platelet count in essential thrombocythemia, including the possibility that it acts by reducing cell number, we used a sensitive flow cytometric method to study 10 newly diagnosed, untreated patients at baseline and after normalization of their platelet counts by anagrelide therapy. The analysis (2500 MKs per patient) included the quantitative assessment of MK mass by measurement of marrow MK frequency, size, and ploidy. The flow cytometric assessment was correlated with the quantitative measurements of thrombocytopoiesis by studying autologous indium 111-platelet survival and turnover rate.
Patients, materials, and methods
Patients
Patient characteristics.
Newly diagnosed, untreated patients with essential thrombocythemia were selected according to the clinical criteria established by the Polycythemia Vera Study Group.1,11 12 The diagnosis was based primarily on peripheral blood smear examination and platelet count, assessment of bone marrow aspirate smears and biopsy specimens, and karyotype analysis and measurement of the red blood cell mass. Following screening tests, patients with evidence of potentially confounding conditions including inflammatory or malignant disorders, iron deficiency, blood loss, or thromboembolic phenomenon were excluded from the study. Subsequently, eligible patients were treated with anagrelide (see below).
Both before treatment and also after normalization of platelet counts by anagrelide therapy, all patients underwent flow cytometric analysis of marrow MKs, platelet kinetic studies to determine platelet life span and turnover rate, and an assessment of platelet function studies by aggregometry. All studies were approved by the institutional Human Investigations Committee.
Treatment with anagrelide.
Patients were initially administered anagrelide at a dose of 0.5 mg orally 4 times daily. Subsequently, the dose was gradually adjusted by 0.5 mg/d every week until the platelet count decreased below 500 × 109/L.4 Patients were restudied after reaching a steady-state normal platelet count for at least 4 consecutive weeks. The average maintenance dose was 3.0 mg/d (range, 2.0-5.0 mg/d). To preclude a possible inhibitory effect of anagrelide on platelet function,7 the aggregation studies were repeated after temporary discontinuation for 24 to 36 hours.
Analysis of marrow megakaryocytes
Marrow preparation for flow cytometry.
Preparation of marrow aspirates for flow cytometric analysis was performed as previously described.13-15 Briefly, 2 to 4 mL bone marrow from a posterior iliac crest site was collected into 20-mL plastic syringes containing 1/10 volume acid-citrate-dextrose National Institutes of Health formula A (ACD-A, Travenol, Deerfield, IL), 2.5 mM EDTA, and 2.2 μM PGE1 (Sigma Chemical, St Louis, MO) final concentrations. The marrow was gently pipetted, passed through a 120-μm monofilament nylon filter, and diluted with cold Ca++- and Mg++-free phosphate-buffered saline (PBS) containing 13.6 mM sodium citrate, 2.2 μM PGE1, 1 mM theophylline (Sigma), 3% bovine serum albumin (BSA fraction V, Calbiochem, La Jolla, CA), and 11 mM glucose. The pH was adjusted to 7.3 and the osmolarity to 290 mOsm/L. This buffer was designated MK medium.13
For size and ploidy determinations, megakaryocytes were analyzed both in unfractionated marrow (to preclude possible selective cell loss) and in fractionated marrow (enriched for MKs to hasten the analysis). Fractionation was performed by separating marrow cells over a discontinuous Percoll gradient of density 1.060 g/mL.15This procedure yields about a 15-fold enrichment with recovery of 98% or more of the MKs.14 For analysis of cell size using the “time-of-flight” (TOF) technique (see below), an aliquot of labeled marrow was fixed with 1% paraformaldehyde for 30 minutes at room temperature.14
MK labeling.
The MKs were directly labeled with a fluoresceinated, lineage-specific monoclonal antibody (mAb, fluorescein isothiocyanate [FITC]-LJ-P4, kindly supplied by Dr Z. M. Ruggeri, Scripps Research Institute, La Jolla, CA) directed to the membrane glycoprotein (GP) IIb-IIIa (CD41a) complex for cell identification, and stained with propidium iodide for DNA quantitation. The cell count was adjusted to 5 × 106/mL with MK medium and then incubated for 30 minutes on ice with a predetermined saturating concentration of LJ-P4 mAb. The saturating concentration of the mAb was determined by quantitative flow cytometric measurements of the fluorescence of large MKs. The mAbs were fluoresceinated to an fluorescein-protein (F/P) ratio of 3:1 using standard methodology.14 Aliquots of marrow cell suspension were also incubated under identical conditions with a fluoresceinated isotype-matched mAb and used as the control cell population. Using inhibitors and buffers as previously described, optimal dispersion and preservation of cells were obtained.13-15
To determine MK ploidy distributions, cellular DNA was stained by a modification of a previously described method.15 Briefly, the cell suspension was diluted 1:2 with 0.1% sodium citrate containing 20 μg/mL propidium iodide and 0.05% Triton X-100 (Sigma) for 15 minutes at 4°C, and then further diluted 1:2 with MK medium. This method resulted in highly efficient staining.14 15
Flow cytometric analysis.
Flow cytometric analysis was performed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with an adjustable logarithmic amplification system to improve resolution of the multiple ploidy peaks. An overlap in the emission spectra of green (FITC) and red (propidium iodide) was corrected using single-color labeled cells of the same preparation.15
The MK population was quantitatively analyzed for (1) cell size as estimated by forward light scatter; (2) cytoplasmic maturation as assessed by 90° light scatter or side scatter, which reflects the fine intracellular complexity, and (3) ploidy distribution as assessed by the intensity of the propidium iodide red fluorescence (Figure1). In addition, cell diameter was directly measured as the electronic pulse width representing the time required for a cell in suspension to pass through a focused light beam (TOF16). Preliminary studies showed this measurement to be directly related to the size of standard particles with a diameter ranging between 10 to 70 μm (r = 0.917).
Ploidy distribution of normal human megakaryocytes.
The distribution of the marrow cells according to the membrane-CD41a (GPIIb/IIIa) immunofluorescence (FL1) and the DNA content (FL3) is shown (A). Cells with background fluorescence (below the horizontal line) represent the major marrow cell population with ploidy classes of 2N and 4N. The highly fluorescent cells (above the horizontal line) represent the megakaryocyte population with polyploid subclasses, as demonstrated in the DNA histogram (B). The modal ploidy is 16N cells comprising about 50% of the total MKs. Three thousand MKs, consisting of 0.05% of the total nucleated marrow cells, were analyzed. To improve visualization, the presentation of the MK population was electronically enhanced in panel A.
Ploidy distribution of normal human megakaryocytes.
The distribution of the marrow cells according to the membrane-CD41a (GPIIb/IIIa) immunofluorescence (FL1) and the DNA content (FL3) is shown (A). Cells with background fluorescence (below the horizontal line) represent the major marrow cell population with ploidy classes of 2N and 4N. The highly fluorescent cells (above the horizontal line) represent the megakaryocyte population with polyploid subclasses, as demonstrated in the DNA histogram (B). The modal ploidy is 16N cells comprising about 50% of the total MKs. Three thousand MKs, consisting of 0.05% of the total nucleated marrow cells, were analyzed. To improve visualization, the presentation of the MK population was electronically enhanced in panel A.
The MKs were selected on the basis of their distinct immunofluorescence at levels above that of control cells labeled with an isotype-matched unrelated mAb. To achieve adequate statistical comparisons, 2000 to 3000 MKs were analyzed in each sample. Bivariate plots of green membrane fluorescence (FL1) versus forward-angle light scatter or the DNA red fluorescence (FL3) (Figure 1) give an easily distinguishable subset of the highly fluorescent large MKs and are used to establish the location of the desired cell population.13-15 The ploidy distribution was determined by setting markers at the nadirs between peaks using the 2N and 4N peaks of the whole marrow cells as internal reference standards.14
Enumeration of MKs in marrow.
The number of MKs relative to the total nucleated marrow cells was determined by flow cytometry and was corrected for dilution with peripheral blood cells by using the nucleated erythroid precursors (NEPs) as a reference for bone marrow cells. The NEP cells were identified using a modification of an established method.18 Briefly, bone marrow aspirates were separated over Percoll (1.087 g/mL density cut), and the light density fraction was then collected, washed, resuspended with MK medium, and adjusted to 1 × 107 cells/mL. To identify NEP cells, the marrow cell suspension was incubated with fluoresceinated mAb against human glycophorin A (mAb D2.10, Gen-Trak, Plymouth Meeting, PA), then washed, stained for DNA as described above, and analyzed by flow cytometry. NEPs were identified by the intense membrane immunofluorescence and the positive DNA staining (Figure 3B). Their identity was further confirmed by direct cell sorting using FACSort (Becton Dickinson; Figure 4). Another aliquot was simultaneously incubated with FITC-P4 mAb, stained with propidium iodide, and analyzed for MKs. Total nucleated marrow cells were identified by the propidium iodide–labeled DNA red fluorescence. Repeated cell count and microscopic examination of the marrow cell suspensions revealed no detectable cell loss or aggregation. The frequency of NEPs and MKs relative to the total nucleated marrow cells was determined and used to establish the NEP/MK ratio. This ratio was used to determine the increased MK frequency in patient marrows as compared to healthy controls and to determine the decreased MK frequency following treatment. A corresponding MK number was derived from the normal erythroid number (5.3 × 109cells/kg) as estimated by Harker and Finch19 based on ferrokinetic studies and analysis of bone marrow sections. For example, MK/total cells = 1/2000, NEPs/total cells = 1/2.6, ratio MKs/NEPs = 1.34 × 10−3; multiplied by 5.3 × 109 = 7.1 × 106cells /kg (Table 2, line 1).
Analysis of platelets
Platelet preparation.
Venous blood from patients and healthy volunteers was collected into syringes containing one-tenth volume of 3.2% wt/vol trisodium citrate, and platelet-rich plasma was prepared by low speed centrifugation (70g, 6 minutes) to obviate loss of large platelets. Platelet counts in the patient's blood and platelet-rich plasma samples were performed using a Baker System 9100 cell counter (Baker Instruments, Allentown, PA).
Platelet aggregometry.
Platelet-rich plasma was prepared from citrate-anticoagulated blood as described, and platelet aggregation in response to adenosine diphosphate (ADP), collage, epinephrine, and ristocetin was measured using a standard turbidimetric technique.20
Platelet kinetic studies.
Platelet kinetic studies were performed by labeling autologous platelets with 111In-oxine using a method we described, achieving high labeling efficiency (mean ≥ 95%).21 In brief, 42.5 mL blood was drawn into 7.5 mL ACD-A and platelet-rich plasma was prepared by centrifugation. The platelet-rich plasma was acidified to pH 6.5 using citric acid and platelets were concentrated by centrifugation, washed, and resuspended in Ringer buffer (Travenol) preadjusted to pH 6.5. Platelets were then incubated with111In-oxine (1 mCi/mL [37 MBq/mL], Amersham, Arlington Heights, IL). An aliquot of labeled platelet suspension containing 25 μCi (0.925 MBq) total radioactivity was diluted with the patient's platelet-poor plasma and reinfused as described.21 22
Venous blood samples were collected at 2 hours after injection and then twice daily for 6 days. The samples were counted and the small amount of radioactivity associated with cell-free plasma (5%-10%) was subtracted. The platelet survival time was determined by computer least-squares fitting of the raw data to a γ-function modeling program.23 Platelet recovery in the circulation at equilibrium was derived by extrapolating the survival curve to time zero and estimating the patient blood volume as described23using the formula: recovery in circulation = (total circulating platelet radioactivity)/(total platelet radioactivity injected). Platelet turnover, a measure of steady-state platelet production and destruction, was calculated by the formula: platelet turnover rate (platelets/L/d) = [(platelet count)/(blood platelet survival time in days)] × recovery.21 The recovery is expressed as a decimal.
Statistical analysis
Comparisons among the MK and platelet determinations were performed by standard least-square regression analysis as described.13 Deviation from the MK ploidy distribution was determined by comparison with the average distribution of healthy individuals (n = 18) using a multinomial distribution analysis as described.15 All values are expressed as mean ± SD.
Results
Thrombocytopoiesis
Prior to treatment, all patients showed a marked thrombocytosis with about a 4-fold increase as compared to a normal value (1063 ± 419 × 109 platelets/L versus 290 ± 110 × 109 platelets/L1; Table1). Platelet kinetic studies using autologous 111In platelets showed a mean platelet life span that was slightly decreased at baseline (8.2 ± 1.1 days versus 9.0 ± 0.5 days in controls, P > .0521,24). The increased platelet count with the slightly shortened platelet life span indicates that the actual platelet production rate is greater than that estimated by the peripheral platelet count. Accordingly, the platelet kinetic studies showed a concomitant increase in platelet turnover rate that averaged about 5.5 times the normal value (237 ± 74 × 109 platelets/L per day versus 43 × 109 platelets/L per day in healthy controls.24 The average recoveries at baseline (55.2% ± 11.3%) and following treatment (61.1% ± 15.5%) were within the normal range.21 24
Platelet count, survival, and turnover rate
| Patient . | Before treatment . | After treatment . | ||||
|---|---|---|---|---|---|---|
| Platelet count (× 109/L)* . | Platelet life span (d)† . | Platelet turnover (× 109/L/d)‡ . | Platelet count (× 109/L) . | Platelet life span (d) . | Platelet turnover (× 109/L/d) . | |
| 1 | 930 | 7.9 | 226 | 280 | 8.2 | 64 |
| 2 | 589 | 8.1 | 121 | 379 | 8.2 | 64 |
| 3 | 1194 | 9.4 | 284 | 380 | 6.6 | 117 |
| 4 | 740 | 6.8 | 200 | 340 | 7.5 | 70 |
| 5 | 770 | 6.0 | 285 | 409 | 7.6 | 111 |
| 6 | 1216 | 8.9 | 360 | 329 | 7.9 | 112 |
| 7 | 1412 | 8.5 | 210 | 431 | 8.3 | 62 |
| 8 | 523 | 7.6 | 126 | 405 | 7.6 | 67 |
| 9 | 1282 | 9.5 | 242 | 300 | 7.1 | 75 |
| 10 | 1970 | 9.1 | 318 | 350 | 9.6 | 68 |
| Mean ± SD | 1063 ± 419 | 8.2 ± 1.1 | 237 ± 74 | 361 ± 53 | 7.9 ± 0.8 | 81 ± 21 |
| Patient . | Before treatment . | After treatment . | ||||
|---|---|---|---|---|---|---|
| Platelet count (× 109/L)* . | Platelet life span (d)† . | Platelet turnover (× 109/L/d)‡ . | Platelet count (× 109/L) . | Platelet life span (d) . | Platelet turnover (× 109/L/d) . | |
| 1 | 930 | 7.9 | 226 | 280 | 8.2 | 64 |
| 2 | 589 | 8.1 | 121 | 379 | 8.2 | 64 |
| 3 | 1194 | 9.4 | 284 | 380 | 6.6 | 117 |
| 4 | 740 | 6.8 | 200 | 340 | 7.5 | 70 |
| 5 | 770 | 6.0 | 285 | 409 | 7.6 | 111 |
| 6 | 1216 | 8.9 | 360 | 329 | 7.9 | 112 |
| 7 | 1412 | 8.5 | 210 | 431 | 8.3 | 62 |
| 8 | 523 | 7.6 | 126 | 405 | 7.6 | 67 |
| 9 | 1282 | 9.5 | 242 | 300 | 7.1 | 75 |
| 10 | 1970 | 9.1 | 318 | 350 | 9.6 | 68 |
| Mean ± SD | 1063 ± 419 | 8.2 ± 1.1 | 237 ± 74 | 361 ± 53 | 7.9 ± 0.8 | 81 ± 21 |
Normal value 290 ± 110.(1)
Normal value 9.0 ± 0.5 days.
Normal value 43.1 ± 8.4 × 109platelets/L/d.
Treatment with anagrelide normalized the platelet count in all patients to an average of 361 ± 53 × 109 platelets/L with a marked decrease in the platelet turnover rate (81 ± 21 × 109 platelets/L per day,P < .05). The mean autologous platelet life span was unchanged (7.9 ± 0.8 days, P > .05) indicating that anagrelide reduced the platelet count by inhibiting platelet production rather than by increasing platelet destruction.
Platelet aggregation studies
Before treatment, platelet aggregation was invariably defective among patients with essential thrombocythemia, including the absent response to epinephrine and diminished aggregation with either ADP, collagen, or both (data are not shown). No spontaneous aggregation was observed. Patients were restudied following treatment with anagrelide and normalization of their platelet counts. To exclude a possible inhibitory effect of anagrelide on platelets function, the platelet functional evaluation was repeated after temporary discontinuation of treatment for 24 to 36 hours.7 8 The results showed the persistence of the platelet aggregation defect.
Megakaryocytopoiesis
The flow cytometric analysis of MKs is demonstrated in Figure 1. Panel A shows the distribution of marrow cells obtained from a healthy individual according to the membrane immunofluorescence (FL1) and the DNA content (FL3). Cells with background fluorescence represent the major marrow cell population consisting of 2N and 4N ploidy classes. The highly fluorescent cells represent the MKs with polyploid subclasses. The DNA histogram of the MK population is shown in panel B. In normal marrow, the modal ploidy is 16N comprising about 50% of the total MKs.
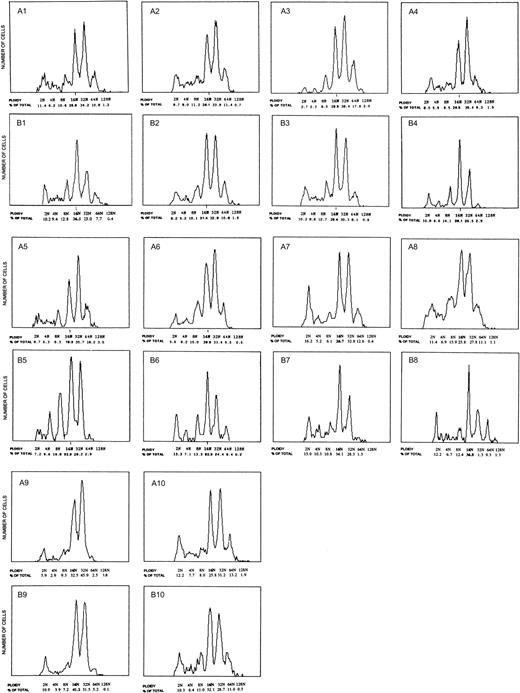
Prior to treatment, the flow cytometric analysis of patients' marrows revealed marked MK hyperproliferation with concurrent increases in both cell size and ploidy. Figure 2 demonstrates the ploidy distribution for each patient prior to therapy (Figure 2, A1-10) and the corresponding DNA histogram following treatment (Figure 2, B1-10). Whereas healthy individuals exhibit a modal ploidy of 16N with 24.7% of MKs being 32N or greater, patients with essential thrombocythemia demonstrate a marked increase in high ploidy cells with a modal ploidy of 32N and 51.4% of MKs being 32N or greater (Table 3, bolded numbers). The shift to the right in ploidy is further illustrated by comparing the relative frequency of the mature MK classes; the ratio of 32N or greater to 16N cells was increased by approximately 4-fold, from 0.54 in healthy to 2.0 in the patients' marrows.
Ploidy distribution of marrow megakaryocytes from patients with essential thrombocythemia.
Panels A1-10 show the ploidy distribution for each patient prior to anagrelide therapy. Increased MK ploidy was observed in all patients with a modal ploidy of 32N. Response to anagrelide was associated with decreased MK ploidy (corresponding panels B1-10). The modal ploidy was shifted from 32N to 16N as in healthy controls. The decreased ploidy correlated with a reduction in cell size and circulating platelet count.
Ploidy distribution of marrow megakaryocytes from patients with essential thrombocythemia.
Panels A1-10 show the ploidy distribution for each patient prior to anagrelide therapy. Increased MK ploidy was observed in all patients with a modal ploidy of 32N. Response to anagrelide was associated with decreased MK ploidy (corresponding panels B1-10). The modal ploidy was shifted from 32N to 16N as in healthy controls. The decreased ploidy correlated with a reduction in cell size and circulating platelet count.
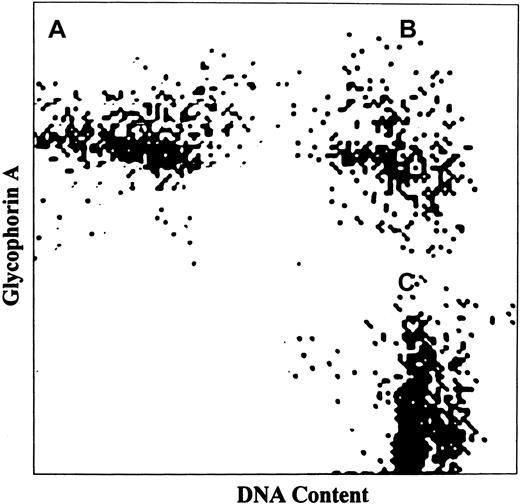
To correlate the increased platelet production rate with the effective megakaryocytopoiesis in essential thrombocythemia and to define the mechanism by which anagrelide affects platelet production, MK mass was measured based on MK frequency and average cell volume in patient marrows. To correct for variable dilution of marrow with peripheral blood, the frequency of MKs was related to that of NEPs because these cells are normally located only in marrow. All studied patients with essential thrombocythemia exhibited normal erythropoiesis based on peripheral blood examination, reticulocyte count, and red cell mass measurements. Erythropoiesis was not significantly altered by treatment with anagrelide (mean hematocrit 46.2% ± 7.5%, reticulocyte count 1.5% ± 0.7%, and red cell mass 30.0 ± 4.5 mL/kg before treatment versus mean hematocrit of 45.1% ± 7.2%, reticulocyte count 1.4% ± 0.8%, and red cell mass 29.2 ± 5.2 mL/kg after treatment, P > .05). Figure3 demonstrates the analysis of NEPs in the bone marrow following labeling of the cell surface for glycophorin A and staining of nuclear DNA with propidium iodide. Of the 3 cell populations shown in Figure 3, only NEPs (panel B) were both positive for glycophorin A and stained for DNA. The identity of NEPs was further confirmed by direct cell sorting and staining with Wright-Giemsa. The results showed pure population of nucleated red cell precursors (Figure4).
Analysis of NEPs in bone marrow aspirate.
NEPs were discriminated from other marrow cells by simultaneous labeling of the cell surface with mAb against human glycophorin A and staining of cell DNA with propidium iodide. Three cell populations are identified: (A) peripheral red blood cells (glycophorin A positive only), (B) NEPs (both glycophorin A and DNA positive) and (C) nonerythroid-nucleated marrow cells (DNA positive only).
Analysis of NEPs in bone marrow aspirate.
NEPs were discriminated from other marrow cells by simultaneous labeling of the cell surface with mAb against human glycophorin A and staining of cell DNA with propidium iodide. Three cell populations are identified: (A) peripheral red blood cells (glycophorin A positive only), (B) NEPs (both glycophorin A and DNA positive) and (C) nonerythroid-nucleated marrow cells (DNA positive only).
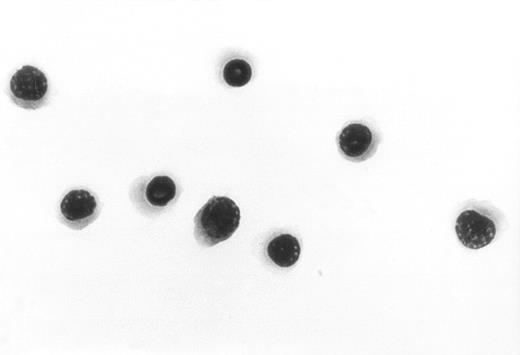
Sorted NEPs.
To further confirm the identity of the NEPs shown in Figure 3, the cell population marked (B) was directly sorted and stained with Wright-Giemsa. The results show pure nucleated red blood cell precursors. Original magnification, × 1000.
Sorted NEPs.
To further confirm the identity of the NEPs shown in Figure 3, the cell population marked (B) was directly sorted and stained with Wright-Giemsa. The results show pure nucleated red blood cell precursors. Original magnification, × 1000.
Cell size was directly measured in marrow suspension using the TOF technique. Preliminary studies showed that TOF measurement is directly related (r = 0.99) to the diameter of standard particles with a range of 10 to 70 μm.17 MK size measurement was directly related to cell DNA content (r = 0.91). The mean diameter of normal MKs (n = 14) was 37 μm compared to 14 ± 2 μm for the total bone marrow cells. The average diameter for the various ploidy classes was 21 μm for 2N, 28 μm for 4N, 33 μm for 8N, 38 μm for 16N, 46 μm for 32N, and 56 μm for 64N cells. In the patient group, for the same ploidy level, no significant difference in cell size was identified.
Prior to treatment, the average MK size was significantly increased with a mean diameter of 46 μm compared to 37 μm in healthy controls (P < .05) and was highly correlated with cell ploidy (r = 0.91). The corresponding cell volume was increased to approximately 1.8 times the normal value (48 μm3 versus 26 μm3; P < .05). MK frequency was also increased to twice the normal value (13.9 ± 2.6 × 106MK/kg versus 7.1 ± 2.4 × 106 MK/kg;P < .05; Table 2). The increased cell size and frequency resulted in a markedly increased MK mass (number × volume) to approximately 3.6 times the normal value (66.3 ± 13.2 × 1010 μm3/kg versus 18.4 ± 7.2 × 1010 μm3/kg;P < .05; Table 2).
Effect of anagrelide treatment on MK mass in essential thrombocythemia
| . | Platelet count (× 109/L) . | MK/NEP*(× 10−3) . | MK number (× 106/kg)† . | MK volume (× 103μm3) . | MK mass (× 1010μm3/kg) . |
|---|---|---|---|---|---|
| Controls (n = 14) | 240 ± 60‡ | 1.3 ± 0.8 | 7.1 ± 2.4 | 25.9 ± 4.7 | 18.4 ± 7.2 |
| Patients (n = 10) | |||||
| Before treatment | 1062 ± 419 | 2.6 ± 1.5 | 13.9 ± 2.6 | 47.7 ± 8.5 | 66.3 ± 13.2 |
| After treatment | 361 ± 53 | 1.6 ± 0.8 | 8.3 ± 2.5 | 33.8 ± 5.2 | 27.8 ± 8.3 |
| Ratio | |||||
| Before treatment/HC | 4.4 | 1.5 | 2 | 1.8 | 3.6 |
| After treatment/HC | 1.5 | 1.2 | 1.2 | 1.3 | 1.5 |
| Before/after treatment | 2.9 | 1.6 | 1.7 | 1.4 | 2.4 |
| . | Platelet count (× 109/L) . | MK/NEP*(× 10−3) . | MK number (× 106/kg)† . | MK volume (× 103μm3) . | MK mass (× 1010μm3/kg) . |
|---|---|---|---|---|---|
| Controls (n = 14) | 240 ± 60‡ | 1.3 ± 0.8 | 7.1 ± 2.4 | 25.9 ± 4.7 | 18.4 ± 7.2 |
| Patients (n = 10) | |||||
| Before treatment | 1062 ± 419 | 2.6 ± 1.5 | 13.9 ± 2.6 | 47.7 ± 8.5 | 66.3 ± 13.2 |
| After treatment | 361 ± 53 | 1.6 ± 0.8 | 8.3 ± 2.5 | 33.8 ± 5.2 | 27.8 ± 8.3 |
| Ratio | |||||
| Before treatment/HC | 4.4 | 1.5 | 2 | 1.8 | 3.6 |
| After treatment/HC | 1.5 | 1.2 | 1.2 | 1.3 | 1.5 |
| Before/after treatment | 2.9 | 1.6 | 1.7 | 1.4 | 2.4 |
Nucleated marrow erythroid precursor cells (MK/NEPs ratio of 1.3 = 1.3 × 10−3).
Derived from normal erythroid number (5.3 × 109 cells/kg, see “Patients, materials, and methods”).
Mean ± 1 SD.
HC indicates healthy controls.
Response to anagrelide was associated with decreased MK frequency from 13.9 ± 2.6 × 106 MK/kg to 8.3 ± 2.5 × 106 MK/kg. The reduction in cell number induced by anagrelide therapy was associated with concomitant decrease in MK size and ploidy. The modal ploidy shifted back from 32N to 16N as in healthy controls, with a decrease in the proportion of MKs 32N or greater from 51.4% to 35.0% (Figure 2 and Table 3, bolded numbers;P < .05). The ratio of 32N or greater to 16N cells decreased from 2.0 to 1.0. The average cell size also decreased from 46 μm to 40 μm diameter with a corresponding decrease in the average cell volume from 48 μm3 to 34 μm3 (Table2). The decrease in both cell size and number resulted in an overall decrease in MK mass from 66.3 ± 13.2 × 1010μm3/kg to 27.8 ± 8.3 × 1010μm3/kg (Table 2; P < .03). The decrease in MK mass by 58.1% closely correlated with a reduction of 66.0% in platelet count (r = 0.97), and of 65.8% in platelet production rate (r = 0.95), thus indicating the cohesive relationship between the marrow substrate and the circulating product.
Effect of anagrelide treatment on megakaryocyte ploidy in essential thrombocythemia
| . | 2N . | 4N . | 8N . | 16N . | 32N . | 64N . | 128N . |
|---|---|---|---|---|---|---|---|
| Controls (n = 18) | 10.7 ± 3.03-150 | 6.5 ± 1.4 | 12.5 ± 3.4 | 45.9 ± 3.4 | 23.7 ± 5.4 | < 1 | |
| Patients (n = 10) | |||||||
| Before treatment | 7.6 ± 3.0 | 5.3 ± 2.7 | 10.2 ± 3.3 | 25.5 ± 4.1 | 35.7 ± 5.4 | 13.4 ± 4.8 | 2.3 ± 0.7 |
| After treatment | 10.6 ± 2.2 | 7.5 ± 1.7 | 13.4 ± 2.7 | 33.5 ± 3.7 | 27.8 ± 2.7 | 6.9 ± 3.6 | 0.3 ± 0.3 |
| . | 2N . | 4N . | 8N . | 16N . | 32N . | 64N . | 128N . |
|---|---|---|---|---|---|---|---|
| Controls (n = 18) | 10.7 ± 3.03-150 | 6.5 ± 1.4 | 12.5 ± 3.4 | 45.9 ± 3.4 | 23.7 ± 5.4 | < 1 | |
| Patients (n = 10) | |||||||
| Before treatment | 7.6 ± 3.0 | 5.3 ± 2.7 | 10.2 ± 3.3 | 25.5 ± 4.1 | 35.7 ± 5.4 | 13.4 ± 4.8 | 2.3 ± 0.7 |
| After treatment | 10.6 ± 2.2 | 7.5 ± 1.7 | 13.4 ± 2.7 | 33.5 ± 3.7 | 27.8 ± 2.7 | 6.9 ± 3.6 | 0.3 ± 0.3 |
Percent of total.
Discussion
The present study demonstrates that anagrelide exerts its selective thrombocytopenic effect in essential thrombocythemia by decreasing both MK proliferation and size. This effect results in an overall decrease in MK mass thereby reducing the substrate available to form circulating platelets. The assessment of MK mass based on the flow cytometric measurements of cell size and frequency was corroborated by the independent assessment of autologous platelet production rate performed simultaneously.
Platelet life span measured directly with autologous platelets was slightly decreased at baseline (8.2 ± 1.1 day versus 9.0 ± 0.5 day in healthy controls, P > .05). The increased platelet count with the slightly shortened platelet life span indicates that the actual platelet production rate is greater than that estimated by the peripheral platelet count. Accordingly, the estimated platelet turnover rate was increased to an average of about 6-fold, whereas the average platelet count was increased only about 4-fold. Treatment with anagrelide normalized the platelet count in all patients and was associated with a marked decrease in platelet turnover rate. The mean autologous platelet life span, however, was unchanged (7.9 ± 0.8 days), indicating that anagrelide exerts its effect by inhibiting platelet production rather than by increasing platelet destruction. The finding that platelet survival was unaffected by anagrelide is in agreement with previous results obtained in healthy individuals8 and in a limited number of patients (n = 3).10 It is noteworthy that the platelet function studies revealed a persistent defect in platelet function as measured by aggregation after temporary discontinuation of the drug. This finding, consistent with previous studies,25 indicates that the thrombocytopenic effect of anagrelide is not accompanied by correction of the platelet qualitative abnormality.
To facilitate the quantitative analysis of the relatively rare marrow MK population (0.05% of total nucleated cells), we have developed quantitative flow cytometric methods for direct analysis of unfractionated marrow aspirates from individual patients. Marrow samples were analyzed by direct measurement of MK frequency and size, thereby permitting the estimation of MK mass. The high sensitivity and rapidity of the flow cytometric method, together with the use of lineage-specific markers allowed the study of statistically adequate numbers of MKs (2000-3000), a number not attainable when using microscopy or image analysis of fixed cells (not practical to analyze more than 200 cells). The TOF technique allowing more direct measurement of cell size and the sensitive DNA labeling method resulting in highly resolved ploidy classes (Figures 1 and 2) enabled the quantitative comparison of size and ploidy before and after therapy. Moreover, using the flow cytometric method it was possible to enumerate MKs in marrow by relating their frequency to that of NEPs (both found only in marrow) and to use the results to estimate the MK mass.
Before treatment, all newly diagnosed patients with essential thrombocythemia demonstrated both marked MK hyperproliferation with an elevated MK frequency that was twice the normal and a significantly increased cell size and ploidy. The finding that all newly diagnosed patients demonstrated a marked increase in ploidy with a shift to the right (51.4% are 32N or higher ploidy cells versus 24.7% cells in healthy controls) is consistent both with our previous findings based on flow cytometric studies of a smaller number of patients15 and other studies using microscopic techniques.26 27 Further analysis showed that the most significant change found in essential thrombocythemia was the increase in 32N cells and the presence of 64N and 128N cells (Table 3, bolded numbers). This finding suggests that the presence of very high ploidy cells may be unique to all chronic myeloproliferative disorders except chronic myelogenous leukemia (CML). It is of interest to note that the shift to the right was associated primarily with a reciprocal decrease in the 16N cells (26% versus 46% in healthy controls, Table 3), and with a much smaller change in the relative frequency of cells 8N or less. This finding may indicate that factors regulating thrombocytopoiesis could have a selective effect on more mature, high ploidy cells.
A response to anagrelide was characterized by a decrease in MK number (from 2.0-fold to 1.2-fold the normal value), as estimated by the MK/NEP ratio. MK ploidy was also decreased (from 51.4% being 32N or higher to 35.0% being 32N or higher ploidy cells) with a shift toward the normal ploidy mode of 16N. This change was also associated with a decrease in the average MK volume (from 48 μm3/L to 34 μm3/L), which was closely related to cell ploidy (r = 0.91), indicating the interference of anagrelide with the overall MK maturation process.10 As a result, MK mass (MK number × volume) decreased approximately by 2.4-fold (from 3.6-fold to 1.5-fold the normal value). The reduced MK mass correlated with a decrease, by approximately 2.9-fold, in platelet count (from 1063 ± 419 × 109 to 361 ± 53 × 109 platelets/L) and 2.9-fold in the platelet production (from 5.5-fold to 1.9-fold the normal value). Thus, it appeared that anagrelide treatment concordantly decreases the marrow substrate (MK mass) and circulating product (platelet turnover) by reducing the abnormally increased MK number along with size and ploidy in essential thrombocythemia.
Previous data regarding the mechanisms by which anagrelide induces thrombocytopenia are limited,3-9 especially with regard to the in vivo effect.3,8,10 In preliminary clinical studies there were no reported changes in MK numbers or morphology despite a substantial decrease in circulating platelets, a result probably due to insufficient sensitivity. In vitro, anagrelide has been shown to inhibit MK colony formation at concentrations in excess of the therapeutic levels.3,9 Thus, it is possible that the in vivo antiproliferative effect observed in this study is mediated by a metabolite of the drug. Nevertheless, using liquid (but not plasma clot) marrow culture and microscopic techniques, it was demonstrated9 that decreased maturation might occur at pharmacologic concentrations of anagrelide. Subsequently, a more recent study,10 using image analysis on fixed marrow sections from a limited number of patients (n = 5), reported a significant reduction in cell size, but did not demonstrate changes in MK number. Interestingly, in a patient who was refractory to therapy, anagrelide did not inhibit colony formation,10 thus implying that suppression of MK proliferation is required for the platelet-lowering effect. It is worth noting that in a study of patients with myelofibrosis with myeloid metaplasia treated with anagrelide,28 no patient had a clinically appreciable benefit. Bone marrow examination in these patients showed increased MK number, probably reflecting a progression of a refractory disease.
When analyzing both studies of patients with essential thrombocythemia, it is important to point out that the number of MKs analyzed in the previous study10 was restricted to a total of 700 cells using fixed marrow sections. In addition, lineage-specific markers were not used and no determination of cell ploidy was performed. In contrast, the present study analyzed approximately 25 000 MKs using the sensitive flow cytometric method, with direct correlation between size and ploidy measured simultaneously. It is also important to note that in the prior study there was no demonstration of increased MK frequency at baseline. Moreover, the reported decrease in cell size following therapy is insufficient by itself to explain the substantial reduction in platelet count (based on cell size measurements, an MK volume decrease of 1.7-fold contrasted with an average circulating platelet count decrease of 2.8-fold). In the present study, however, when the alterations in cell numbers and size were both considered, the measurements of MK mass, platelet counts, and platelet production rate were well correlated at both baseline and following therapy.
It is of interest to note that extended studies of all patients with chronic myeloproliferative disorders complicated by thrombocytosis were associated with increased MK size and ploidy except for patients with CML where a marked increase in small, low-ploidy MKs was noted (modal ploidy of 8N).29 30 Because essential thrombocythemia is also associated with a conspicuous increase in MK number, this observation may imply that reduction in cell size alone, while maintaining a high MK count, may not be sufficient to produce the substantial decrease in platelet counts observed in the patients treated with anagrelide. Indeed, in 2 patients with CML who were treated with anagrelide, the normalization of platelet counts was primarily related to a decrease in MK number without further reduction in cell size or ploidy (A.T., unpublished data, July 1999). This observation further supports our finding that in essential thrombocythemia, anagrelide treatment decreases the platelet count and turnover rate by suppressing both MK proliferation and differentiation.
We conclude that the combined flow cytometric method using routine bone marrow aspirates and conventional equipment provides a feasible approach for the study of MKs in individual marrows. This approach should be useful for studying megakaryocytopoiesis in both normal and disease states, for examining the recovery of platelets following stem cell transplantation, and for monitoring the effect of therapy including hematopoietic growth factors. The definition of the mechanism underlying the effect of anagrelide on platelet production provides insight into the process of megakaryocytopoiesis and may assist in the development of rationally based new therapies.
I am indebted to Dr Sidney F. Stein (Emory University, Winship Cancer Center, Atlanta, GA) for critical review of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Aaron Tomer, Soroka University Medical Center, Blood Bank and Transfusion Medicine, Beer-Sheva, PO Box 151, 84101 Israel; e-mail: atomer@bgumail.bgu.ac.il.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal