FAS germline mutations have been associated with the development of autoimmune lymphoproliferative syndrome (ALPS). Occurrence of Hodgkin lymphoma (HL) has been reported in 2 families with ALPS. In both families an uncle of the index patient developed HL. A 15-year-old boy with autoimmune thrombopenia, lymphadenopathy, and splenomegaly for 6 years was studied. In an axillary lymph node biopsy nodular lymphocyte predominant (NLP) HL was diagnosed; in the areas between the nodules a proliferation of double-negative blastic T cells were present, suggestive of ALPS. Analysis for the presence of aFAS gene mutation using the denaturing gradient gel electrophoresis technique indicated a mutation in exon 9. Direct sequence analysis revealed a mutation causing a substitution of arginine with glutamine at codon 234. Because ALPS and NLP HL are both highly infrequent conditions, the occurrence in at least 3 families suggests a causative relationship between germline FAS gene mutations and NLP HL.
Introduction
Fas (CD95) is a member of the tumor necrosis factor receptor family (TNFRSF6) and plays a role in the apoptotic pathway. Cross-linking to CD95 ligand (CD95L) results in a functional trimeric structure of the Fas protein. The intracellular domain of Fas, also known as the death domain, interacts with the Fas-associated death domain protein (FADD) to transduce a death signal. Fas is expressed on activated T and B cells and plays an important role in eliminating autoreactive T cells and in the maintenance of the peripheral lymphocyte homeostasis.
Germline mutations of the FAS gene are associated with the development of autoimmune lymphoproliferative syndrome (ALPS). Patients with ALPS usually have enlargement of the spleen and lymph nodes, various manifestations of autoimmunity, and elevation of CD4−CD8− T cells.1,2 Most patients with ALPS have dominant negative mutations in the death domain of the FAS gene.3
Hodgkin lymphoma (HL) has been previously reported in relatives of patients with ALPS in 2 families. In one family an uncle who carried the same mutation as the index patient developed nodular lymphocyte predominant (NLP) HL.4,5 In another family, the uncle of one of the affected children who carried the same Fas death domain mutation had HL. The histologic subtype of this case was not reported.6 Based on this, the question has arisen whether there is a relationship between FAS gene mutation and the occurrence of NLP HL. We report data on a 15-year-old boy with symptoms consistent with ALPS, a mutation in exon 9 of the FAS gene, and an axillary lymph node involved by NLP HL.
Study design
A 15-year-old boy presented with thrombocytopenia, splenomegaly, and generalized lymphadenopathy for 6 years. An axillary lymph node was excised and diagnosed as NLP HL. Approval for this study was obtained from the institutional review board. Immunohistochemical staining was performed with 3-step streptavidin-biotin procedures using polyclonal CD3, CD20 (L26), CD30 (Ber-H2), EMA (E29), and Mib-1 (Ki-67) (Dako, Glostrup, Denmark); CD4, CD8, CD15 (LeuM-1), CD21, and CD57 (Becton & Dickinson, Heidelberg, Germany); CD79a (Immunotech, Marseille, France); and CD95 (Upstate Biotechnology, Lake Placid, NY).
Amplification of the FAS gene and mutation detection
DNA isolation from frozen tissue sections was carried out according to standard laboratory protocols. Ten sets of primers were used to amplify all 9 exons of the FAS gene.7The polymerase chain reaction (PCR) products were denatured for 10 minutes at 94°C and heteroduplex molecules were formed for 50 minutes at 55°C. The resulting homo/heteroduplex molecules were analyzed on a denaturing gradient gel electrophoresis (DGGE).7 The gel was stained with ethidium bromide and aberrant homoduplex bands were excised from the gel and eluted in distilled water. A volume of 1 μL was reamplified using the same set of primers. Direct sequence analysis was performed using both PCR primers on a MegaBace fluorescence sequencer and a dye terminator sequence kit. Sequences were compared to the sequence present in the GenBank (APO1 sequence accession no.X63717). Codons 1-16 represent the signal peptide and the mature peptide consists of 319 amino acids (17-335).
Microdissection of cells for DGGE analysis
Preparation of tissue sections and isolation of DNA was carried out as described previously.8 The amplification was carried out as described above with the only exception that a nested PCR (30 cycles each) was performed using 2 new primers flanking the original primer set (F: 5′-aaacatggttttcactaatggg-3′, R: 5′-cactctagaccaagctttgg-3′).
Results and discussion
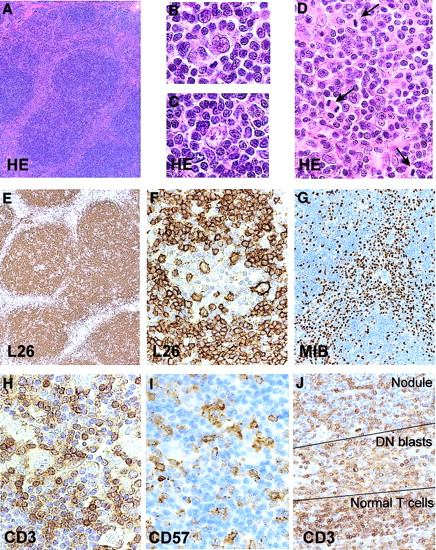
The affected lymph node showed effacement of the architecture and replacement by a nodular process (Figure1). In the nodules small lymphocytes predominated with scattered lymphocytic and histiocytic (L&H) type Reed-Sternberg (RS) cells. The majority of the lymphocytes were CD20+, CD79a+ and CD5−. The L&H cells were CD20+, CD79a+, EMA+, CD15−, and CD30−. The surrounding cells were usually CD3+ T cells with expression of CD57 for part of the cells. The histology and immunophenotype are consistent with a diagnosis of NLP HL.9 Between the nodules a population of CD3+ blasts was present with many mitotic figures (Figure1). These were CD4 and CD8 double-negative. Mib-1 (Ki-67) and CD57 were positive in a majority of the blasts. A proliferation of so-called double-negative T cells is frequently found in ALPS.10 11All cells stained positive for CD95.
Histology and immunohistochemistry of the lymph node.
Nodular architecture (A), presence of L&H RS cells in the nodules (B,C), internodular proliferation of blasts (D; mitotic figures are indicated with an arrow), predominance of CD20+ small B cells (E), presence of CD20+ L&H cells (F), Mib-1 positivity of the internodular blasts (G), presence of many CD3+ and a smaller number of CD57+ T cells surrounding the L&H cells (H,I), and presence of relatively weakly positive CD3 blasts between the nodules and the band of stronger staining CD3+ small T cells (J). Original magnifications × 28 (A,E), × 900 (B,C), × 500 (D,F,H.I), × 87 (G), and × 350 (J).
Histology and immunohistochemistry of the lymph node.
Nodular architecture (A), presence of L&H RS cells in the nodules (B,C), internodular proliferation of blasts (D; mitotic figures are indicated with an arrow), predominance of CD20+ small B cells (E), presence of CD20+ L&H cells (F), Mib-1 positivity of the internodular blasts (G), presence of many CD3+ and a smaller number of CD57+ T cells surrounding the L&H cells (H,I), and presence of relatively weakly positive CD3 blasts between the nodules and the band of stronger staining CD3+ small T cells (J). Original magnifications × 28 (A,E), × 900 (B,C), × 500 (D,F,H.I), × 87 (G), and × 350 (J).
The DGGE analysis revealed normal banding patterns for exons 1-8. An aberrant banding pattern was found for exon 9 (primer set 9.1) (Figure2). This was confirmed by a reamplification of exon 9.1 on a subsequent section of the same tissue sample. Direct sequence analysis revealed a transition (G>A) at position 943 of the FAS gene causing a substitution of arginine with glutamine at codon 234 of the mature protein. This mutation affects the BstBI restriction enzyme site present at bases 941-946 (TTCGAA to TTTGAA) in PCR amplicon 9.1. Reamplification of amplicon 9.1 followed by a BstB1 restriction enzyme digest indeed revealed a lack of thisBstB1 restriction site, which was not detected in 20 control samples. The disruption of the BstBI restriction enzyme recognition site confirmed the presence of the mutation in the involved tissue (Figure 2). The difference in strength of the 2 bands in the patient with ALPS suggests that the mutated allele is overrepresented as compared to the germline allele. This can most likely be explained by the formation of heteroduplex molecules, which resemble the homoduplex mutant PCR products and fail to be digested withBstB1. Analysis of microdissected cells from germinal center areas and from the regions in between the germinal centers containing the T-cell blasts revealed presence of the normal and the mutant allele in all samples. Analysis of 10 L&H cells in duplicate also revealed presence of the mutation. These analyses indicate that the mutation as detected in this case is present in all cells and most likely represents a germline mutation.
FAS mutation analysis.
(A) Image of the DGGE analysis, including a control sample, total tissue section of the ALPS patient, micromanipulated T-cell blasts (approximately 100 cells, nested PCR) and L&H cells (10 cells, nested PCR). (B) Sequence of the aberrant homoduplex band obtained after direct sequencing. (C) Confirmation of the mutation using aBstB1 restriction enzyme digest.BstB1 cuts the normal PCR product of 257 base pairs (bp) at nucleotide 130-136 resulting in 2 fragments of approximately 130 bp. Only part of the PCR product of the ALPS patient is digested with the BstB1 restriction enzyme indicating that theBstB1 restriction site is disrupted in one allele of the FAS gene of the ALPS patient. (D)BstB1 restriction site in the wild-type sequence and the disrupted BstB1 restriction site in the mutated sequence.
FAS mutation analysis.
(A) Image of the DGGE analysis, including a control sample, total tissue section of the ALPS patient, micromanipulated T-cell blasts (approximately 100 cells, nested PCR) and L&H cells (10 cells, nested PCR). (B) Sequence of the aberrant homoduplex band obtained after direct sequencing. (C) Confirmation of the mutation using aBstB1 restriction enzyme digest.BstB1 cuts the normal PCR product of 257 base pairs (bp) at nucleotide 130-136 resulting in 2 fragments of approximately 130 bp. Only part of the PCR product of the ALPS patient is digested with the BstB1 restriction enzyme indicating that theBstB1 restriction site is disrupted in one allele of the FAS gene of the ALPS patient. (D)BstB1 restriction site in the wild-type sequence and the disrupted BstB1 restriction site in the mutated sequence.
Mutations affecting the death domain of the FAS gene is the most common abnormality detected in patients with ALPS.3These mutations are dominant negative, which can be explained by the trimeric structure essential for a functional Fas receptor. A small number of patients with ALPS were found to have mutations of theCD95L gene12 or of the caspase 10 gene.13 The accumulation of double-negative T cells is caused by loss of function of wild-type Fas. This loss prevents the elimination of autoreactive T cells and the maintenance of normal lymphocyte homeostasis.
Development of a lymphoma has been found in 3% of ALPS cases, specifically in cases with mutations affecting the death domain of Fas.3 Relatives of affected kindred carrying death domainFAS gene mutations have also been reported to develop B-cell lymphomas.4,5,14 HL has been reported in 2 families. OneFAS gene mutation carrier developed a NLP HL,4,5 whereas a HL of unknown histologic subtype developed in a mutation carrier of another family.6 It is of interest that so-called progressively transformed germinal centers (PTGCs) are frequently present in the enlarged lymph nodes of patients with ALPS.10 PTGCs indeed are considered precursor lesions of NLP HL.9
Germinal center B cells normally express FAS and negative selection of B cells within the germinal center is regulated via FAS-mediated apoptosis.15 Somatic mutations of theFAS gene are frequently acquired during the normal germinal center reaction.16 RS cells in the majority of cases of HL have highly mutated immunoglobulin genes and most likely are derived from crippled germinal center B cells that have escaped apoptosis in the germinal center.17 RS cells frequently express Fas.18 Recently, nonclonal FAS gene somatic mutations were demonstrated in isolated single RS cells from 2 of 10 classical HL cases.19 The absence of clonality indicates that mutation of the FAS gene may play a role but was not the primary event in these cases. Combination of these data suggests that mutations of the FAS gene might contribute to the persistence of crippled germinal center B cells developing into RS (precursor) cells.
In summary, the patient described in this report has symptoms of ALPS and shows features diagnostic of NLP HL in an axillary lymph node. Because ALPS and NLP HL are both highly infrequent conditions, the occurrence in at least 3 families suggests a causative relation between germline FAS gene mutations and NLP HL. Mutation analysis of the FAS gene indicated a germline mutation with a substitution of arginine with glutamine at codon 234 affecting the death domain. This mutation affects the L&H type RS cells as well as the reactive CD57+ T cells. It is not clear whether the mutation in the L&H cells, in the reactive T cells or in both cell types is of pathogenetic relevance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sibrand Poppema, Pathology and Laboratory Medicine, PO Box 30001, 9700 RB, Groningen, The Netherlands; e-mail: s.poppema@med.rug.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal