Determination of the reticulocyte hemoglobin content (CHr) provides an early measure of functional iron deficiency because reticulocytes are the earliest erythrocytes released into blood and circulate for only 1 to 2 days. The CHr in 78 patients undergoing bone marrow examination was measured to assess its clinical utility for the diagnosis of iron deficiency. Twenty-eight patients were iron deficient, based on the lack of stainable iron in the aspirate. The diagnostic power of CHr is limited in patients with high mean cellular volume (MCV) or red cell disorders such as thalassemia. However, when patients with MCV more than 100 fL are excluded, receiver operator curve analysis of CHr, ferritin, transferrin saturation, and MCV demonstrates that CHr has the highest overall sensitivity and specificity of these peripheral blood tests for predicting the absence of bone marrow iron stores.
Introduction
Accurate, early diagnosis of iron deficiency is essential because it may be the presenting sign of a gastrointestinal malignancy.1 Numerous peripheral blood tests are performed to diagnose iron deficiency, including ferritin, transferrin saturation, serum iron, and mean cellular volume (MCV). Serum ferritin of 12 μg/L or less is the most specific indicator of iron deficiency.2 However, because it is an acute-phase reactant, ferritin may be normal or increased in iron-deficient patients with other medical problems. Because reticulocytes are the earliest erythrocytes released into blood and circulate for only 1 to 2 days, the reticulocyte hemoglobin content (CHr) provides a measure of iron available to red cells recently produced by the bone marrow. CHr has been shown to be an early indicator of iron-restricted erythropoiesis in patients receiving erythropoietin therapy3-7 and is a strong predictor of iron deficiency in children.8 However, a study evaluating the ability of CHr to predict bone marrow iron stores has not been performed.
Study design
Subjects
Seventy-eight patients undergoing bone marrow examination were enrolled in this study after informed consent. Peripheral blood samples from 34 medical students were obtained anonymously during a pathology course to establish reference limits. No student was anemic as defined by a hematocrit less than 36% for women and less than 39% for men.
Analytical methods
Peripheral blood samples were obtained on the day of the bone marrow examination and were submitted for routine analysis at the Memphis Veterans Affairs Medical Center clinical laboratories. A complete blood count and reticulocyte analysis, including CHr, were measured in whole blood collected in K3EDTA with the use of an Advia 120 hematology analyzer (Bayer Diagnostics, Tarrytown, NY). The CHr is determined from measurements of light scatter at 2 different angles after isovolumetric sphering of oxazine 750-stained reticulocytes. From the amount of light scattered at the 2 different angles, the hemoglobin concentration and cellular volume of individual reticulocytes are independently determined.9,10 As a reticulocyte matures into an erythrocyte, the hemoglobin concentration increases as the cell volume decreases. Thus, CHr (the product of the hemoglobin concentration and the cell volume) is a more stable parameter than reticulocyte hemoglobin concentration. Ferritin was measured in serum by using an Access Immunoanalyzer (Beckman-Coulter, Brea, CA). The percentage of transferrin saturation was calculated from serum iron, and the serum total iron binding capacity was measured by using a Dimension chemistry analyzer (Dade-Behring, Newark, DE). Bone marrow aspirates were collected in K3EDTA and stained for iron with Prussian blue. Stainable iron was determined to be absent or present in aspirates containing marrow spicules by 2 pathologists who were kept blinded to the peripheral blood test results. Discrepant samples were referred to a third pathologist for interpretation. Interobserver variability occurs in histopathologic diagnosis; thus, the bone marrow aspirate is not a perfect gold standard assay for iron deficiency. The Cohen coefficient Κ for the 2 pathologists initially interpreting the specimens was 0.78, indicating substantial agreement.11
Results and discussion
The average CHr in samples from 34 medical students was 30.8 ± 0.90 pg with a range of 28.8 to 32.9 pg. No significant difference between male and female values was present. To determine its efficacy in predicting bone marrow iron stores, CHr was measured in 78 patients undergoing bone marrow biopsy. Reasons for biopsy included anemia work-up (34%), benign hematologic disorders such as thrombocytopenia or monoclonal gammopathy (22%), hematologic malignancy (19%), lymphoma or multiple myeloma (19%), and other malignancies (6%). As determined by the absence of stainable iron in the bone marrow aspirate, 28 (36%) of the 78 patients were classified as iron deficient. The average CHr in the iron-deficient patients was 28.3 ± 5.2 pg with a range of 21.0 to 38.6 pg, whereas in iron-replete patients the average CHr was 30.2 ± 3.4 pg with a range of 22.8 to 43.7 pg. Of the 28 iron-deficient patients, 17 had a CHr of 28.0 pg or less, giving an overall sensitivity for the assay of 60.7% in this patient population (Table 1). Because the CHr is the product of the cellular volume and cellular hemoglobin concentration, patients with combined iron deficiency and megaloblastic anemia may have a falsely elevated CHr because of the high MCV associated with megaloblastosis. The MCV among iron-deficient patients ranged from 61.9 to 120.9 fL, and 7 patients, all with CHr less than 28 pg, had an MCV less than 81 fL. Of the iron-deficient patients with CHr more than 28.0 pg, 5 had MCV of 100 fL or more. One patient had a CHr of 30.5 pg, and the other 4 patients had CHr values between 36 and 38 pg. An iron-replete patient who developed pancytopenia and megaloblastic anemia (MCV, 105 fL) secondary to vitamin B12 deficiency had a CHr of 43.7 pg, 5 pg more than any other patient in this study, further emphasizing the effects of megaloblastic changes on CHr. When patients with MCV of 100 fL or more (5 iron deficient and 5 iron replete) are excluded from analysis, the average CHr is 26.7 ± 3.9 pg in iron-deficient patients and 29.6 ± 2.6 pg in iron-replete patients, and the sensitivity for the assay increases to 73.9%. Of the 6 remaining iron-deficient patients with CHr more than 28.0 pg, 3 had been clinically diagnosed with iron deficiency and were receiving oral iron therapy. The CHr is an early indicator of response to iron therapy, increasing within 2 to 4 days of the initiation of intravenous iron therapy.12Thus, in patients receiving iron, the CHr may normalize before bone marrow iron stores return, resulting in false-negative test values. The 3 other patients with chronic lymphocytic leukemia, B-cell lymphoma and diabetes, arthritis and anemia, respectively, had no obvious reason for normal CHr values. Of the iron-replete patients, 38 of 50 had CHr more than 28.0 pg/cell, giving an overall specificity of 76.0% for the assay (Table 1). Among the 12 patients with CHr of 28.0 pg or less, 2 had thalassemia, which is a recognized cause of low CHr,102 had a clinical diagnosis of iron deficiency, and 3 had hematologic malignancy. The remaining 5 patients had various diseases such as diabetes or arthritis and were examined for anemia evaluation.
Test characteristics based on optimal cutoff values determined by ROC analysis
| . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | ROC area* . |
|---|---|---|---|---|---|
| CHr (no more than 28.0 pg) | 60.7 | 76.0 | 58.6 | 77.6 | 0.642 ± 0.15 |
| CHr† | 73.9 | 73.3 | 58.6 | 84.6 | 0.735 ± 0.14 |
| Ferritin (no more than 50 mg/L) | 42.3 | 93.6 | 78.6 | 74.6 | 0.660 ± 0.14 |
| Ferritin† | 52.4 | 92.9 | 78.6 | 79.6 | 0.690 ± 0.15 |
| Tf sat (no more than 13%) | 62.5 | 73.8 | 57.7 | 77.5 | 0.660 ± 0.15 |
| Tf sat† | 65.0 | 70.3 | 54.2 | 78.8 | 0.637 ± 0.16 |
| MCV (no more than 81 fL) | 25.9 | 94.0 | 70.0 | 70.1 | 0.505 ± 0.15 |
| MCV† | 31.8 | 93.3 | 70.0 | 73.7 | 0.570 ± 0.15 |
| . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | ROC area* . |
|---|---|---|---|---|---|
| CHr (no more than 28.0 pg) | 60.7 | 76.0 | 58.6 | 77.6 | 0.642 ± 0.15 |
| CHr† | 73.9 | 73.3 | 58.6 | 84.6 | 0.735 ± 0.14 |
| Ferritin (no more than 50 mg/L) | 42.3 | 93.6 | 78.6 | 74.6 | 0.660 ± 0.14 |
| Ferritin† | 52.4 | 92.9 | 78.6 | 79.6 | 0.690 ± 0.15 |
| Tf sat (no more than 13%) | 62.5 | 73.8 | 57.7 | 77.5 | 0.660 ± 0.15 |
| Tf sat† | 65.0 | 70.3 | 54.2 | 78.8 | 0.637 ± 0.16 |
| MCV (no more than 81 fL) | 25.9 | 94.0 | 70.0 | 70.1 | 0.505 ± 0.15 |
| MCV† | 31.8 | 93.3 | 70.0 | 73.7 | 0.570 ± 0.15 |
PPV, positive predictive value; NPV, negative predictive value; ROC, receiver operator curve; CHr, reticulocyte hemoglobin content; Tf sat, transferrin saturation; MCV, mean cellular volume.
The ± 95% confidence interval was calculated by using the Wilcoxon statistic according to Hanley and McNeil.13 14
Values determined after excluding patients with MCV of at least 100 fL.
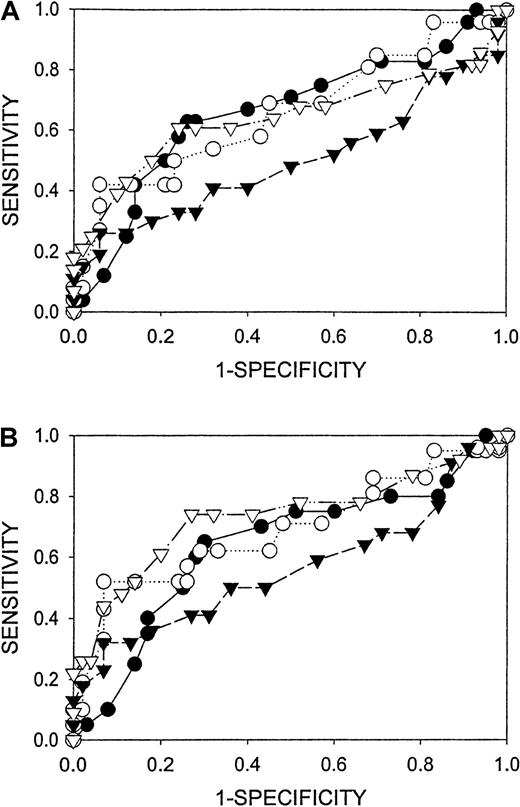
The ability of CHr to predict bone marrow iron stores was compared with that of ferritin, percentage of transferrin saturation, and MCV by receiver operator curve (ROC) analysis (Figure1). When patients with MCV 100 fL or more are excluded from analysis, the area under the ROC curve is greater for CHr than for the other 3 tests, indicating that it has the best overall sensitivity and specificity for iron deficiency in the population of patients tested.13 14 The optimal cutoff values for the different assays were determined by ROC analysis and are presented along with sensitivity, specificity, and positive and negative predictive values in Table 1.
ROC comparison of peripheral blood tests (▿, CHr; ▾, MCV; ●, transferrin saturation; O, Ferritin) for the detection of absent bone marrow iron stores.
(A) All patients. (B) Patients with MCV more than 100 are excluded from analysis. Diagnostic cutoff concentrations spanned the full range of sensitivity and specificity for all analytes. Areas under the curves (± 95% confidence interval) are presented in Table 1.
ROC comparison of peripheral blood tests (▿, CHr; ▾, MCV; ●, transferrin saturation; O, Ferritin) for the detection of absent bone marrow iron stores.
(A) All patients. (B) Patients with MCV more than 100 are excluded from analysis. Diagnostic cutoff concentrations spanned the full range of sensitivity and specificity for all analytes. Areas under the curves (± 95% confidence interval) are presented in Table 1.
The clinical utility of CHr for the diagnosis of the anemia of chronic disease has not been carefully studied. The soluble transferrin receptor is another peripheral blood test that appears to predict iron deficiency more accurately than traditionally used tests15-17 and is particularly useful in differentiating iron deficiency anemia from the anemia of chronic disease.18-20 Both CHr and soluble transferrin receptor can be affected by other erythrocyte disorders that produce false-positive or false-negative test results for iron deficiency. Therefore, the correct diagnosis of iron deficiency requires that these tests are interpreted in the context of the patient's overall erythrocyte physiology, including knowledge of recent transfusions, iron therapy, vitamin B12 or folate deficiency, and the results of hemoglobin electrophoresis.
We thank the medical technologists in the Memphis VA Hospital Clinical Laboratories for assistance in obtaining the bone marrow aspirates and performance of the peripheral blood tests.
Supported by the Office of Research and Development, Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan Mast, Research Service-151, VA Hospital, 1030 Jefferson Ave, Memphis, TN 38104; e-mail: alan.mast3@med.va.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal