Regeneration of hematopoiesis after allogeneic hematopoietic cell transplantation (HCT) involves conversion of the recipient's immune system to donor type. It is likely that distinct cell lineages in the recipient reconstitute at different rates. Dendritic cells (DCs) are a subset of hematopoietic cells that function as a critical component of antigen-specific immune responses because they modulate T-cell activation, as well as induction of tolerance. Mature DCs are transferred with hematopoietic grafts and subsequently arise de novo. Little information exists about engraftment kinetics and turnover of this cell population in patients after allogeneic HCT. This study examined the kinetics of DC chimerism in patients who underwent matched sibling allogeneic HCT. T-cell, B-cell, and myelocytic and monocytic chimerism were also studied. Peripheral blood cells were analyzed at defined intervals after transplantation from 19 patients with various hematologic malignancies after treatment with myeloablative or nonmyeloablative preparatory regimens. Cell subsets were isolated before analysis of chimerism. Despite the heterogeneity of the patient population and preparatory regimens, all showed rapid and consistent development of DC chimerism. By day +14 after transplantation approximately 80% of DCs were of donor origin with steady increase to more than 95% by day +56. Earlier time points were examined in a subgroup of patients who had undergone nonmyeloablative conditioning and transplantation. These data suggest that a major proportion of blood DCs early after transplantation is donor-derived and that donor chimerism develops rapidly. This information has potential implications for manipulation of immune responses after allogeneic HCT.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is routinely performed with the intent to cure patients with hematologic malignancies. One major consequence of allogeneic HCT is the replacement of the recipient's immune system with that of the donor. The change from host to donor immune type is complex and not completely understood. Appropriate immune regulation is of central importance after allogeneic HCT because aberrations in the immune response can lead to the induction of graft-versus-host disease (GVHD), the inability to resist environmental pathogens and/or loss of graft-versus-tumor activity. After allogeneic HCT, immune recovery is determined by both the mature cells transferred with the graft as well as by de novo production of immune populations that arise from committed progenitors and hematopoietic stem cells. Variability in graft composition as well as heterogeneity among patients can also affect this regenerative process. A patient's prior clinical history and conditioning regimen are thought to influence the level and time course to donor hematopoietic cell engraftment.
One key population of immune-modulating cells that must be integrated into the new developing immune system is dendritic cells (DCs). DCs are a heterogeneous subset of cells with diverse functional capabilities. They belong to the family of professional antigen-presenting cells that also include macrophages and B lymphocytes. However, among antigen-presenting cells, DCs are considered to be both the most potent stimulators of T-cell responses (for review see Banchereau & Steinman1) and capable of induction of T-cell tolerance.2,3 DCs arise from bone marrow and migrate through the blood to peripheral tissues where they search for antigens to be taken up and processed. They efficiently present both endogenous and exogenous antigens to T cells. DCs produce an array of cytokines and variably express costimulatory molecules. It has been shown that subtypes of DCs exist that can skew T-cell responses toward T helper 1 versus T helper 2 type.4 Thus, DCs can polarize T-cell responses, depending on the profile of cytokines produced and/or expression of costimulatory molecules.
Little is known about donor DC engraftment or function after clinical allogeneic HCT. Given their central role in mediating antigen-specific immune responses and a report suggesting DC turnover effects development of GVHD,5 we were interested in examining the time course of DC turnover after allogeneic HCT. Furthermore, we wanted to investigate DC chimerism in the context of T-cell, B-cell, and myelocyte and monocyte chimerism. We hypothesized that the intensity of the conditioning regimen would significantly affect the time course to donor DC chimerism. Therefore, patients treated with myeloablative therapies were compared with those conditioned with a nonmyeloablative preparatory regimen. Additionally, the patients enrolled were heterogeneous with regard to underlying disease and prior courses of chemotherapy.
Our study demonstrates rapid turnover of DCs in the blood compartment after allogeneic HCT and might have implications for therapeutic manipulations of immune responses in this patient population.
Patients, materials, and methods
Patients
Between March 1999 and June 2001, 19 patients, aged 27 to 71 years (median, 58), underwent allogeneic HCT from HLA-matched siblings at Stanford University Medical Center (Table1). After informed consent was obtained, all patients were treated on clinical research protocols approved by the institutional review board of our medical center. Blood samples were obtained from 13 patients before transplantation and after transplantation on days +14, +28, +42, +56, +100, and +180 if the patients had a total white cell count more than 1000/μL. Five patients underwent earlier chimerism analysis on day +1, +3, +5, and +7.
Patient characteristics
| Patient SPN . | Age, y . | Sex . | Disease . | Prior treatment . | Type of preparative regimen . | Preparative regimen . | Graft composition . | GVHD . | Status at d + 180 . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC (× 109) . | CD34+/kg (× 106) . | CD3+/kg (× 108) . | |||||||||
| 2240 | 45 | F | CML | HU | Myeloablative | Bu/Cy | 22 | NA | NA | 0 | Cytog rel |
| 2242 | 27 | F | AML | Ida-Ara/Ara | Myeloablative | FTBI/Etop | 177 | 23 | 3 | 0 | Alive/CR |
| 2246 | 52 | F | NHL | CHOP/ESHAP | Myeloablative | BCNU/Etop/Cy | 88 | 9 | 3 | 0 | Alive/CR |
| 2250 | 31 | M | CML-BP | HU/IFN/VINC/Pred | Myeloablative | FTBI/Etop/Cy | 145 | 15 | 3 | SkinAc2 | Alive/CR |
| 2252 | 43 | F | CML | HU/IFN | Myeloablative | Bu/Cy | 24 | NA | NA | Mouth Ch | Alive/CR |
| 2443 | 33 | M | CML | HU | Myeloablative | Bu/Cy | 32 | 2 | 0.4 | 0 | NA |
| 2444 | 46 | M | AML | Ida-Ara/Ara | Myeloablative | FTBI/Etop | 291 | 9 | 3 | 0 | NA |
| 2446 | 28 | F | AML | Ida-Ara/Ara | Myeloablative | FTBI/Etop | 95 | 6 | 2 | 0 | NA |
| 2185 | 61 | M | MM | XRT/VAD/Thal/AutoTX | Nonmyeloablative | Flu/TBI | 47 | 6 | 2 | Mouth Ch | Alive/PR |
| 2270 | 62 | M | MM | VAD/AutoTX | Nonmyeloablative | Flu/TBI | 176 | 8 | 4 | 0 | Alive/PR |
| 2320 | 52 | M | CLL | Flu/CVPB/Campath | Nonmyeloablative | Flu/TBI | 134 | 5 | 3 | 0 | Alive/PR |
| 2331 | 62 | F | MDS | None | Nonmyeloablative | Flu/TBI | 67 | 8 | 3 | GiAc2 | Alive/CR |
| 2238 | 58 | M | MM | VAD/Cy/AutoTX | Nonmyeloablative | Flu/TBI | 125 | 12 | 2 | 0 | Alive/PR |
| 2251 | 61 | F | MM | MP/VAD/AutoTX | Nonmyeloablative | Flu/TBI | 179 | 10 | 4 | 0 | Died sepsis |
| 2307 | 71 | M | MM | MP/XRT/AutoTX | Nonmyeloablative | Flu/TBI | 147 | 2 | 5 | 0 | Alive/PR |
| 2337 | 64 | M | MM | VAD/XRT/AutoTX | Nonmyeloablative | Flu/TBI | 156 | 7 | 4 | 0 | Alive/PR |
| 2410 | 61 | M | MF | None | Nonmyeloablative | Flu/TBI | 106 | 7 | 4 | 0 | NA |
| 2452 | 60 | F | MM | VAD/AutoTX | Nonmyeloablative | Flu/TBI | 135 | 12 | 7 | 0 | NA |
| 2492 | 61 | M | NHL | CHOP, Flu/Cy/rituxmab | Nonmyeloablative | Flu/TBI | 120 | 13 | 4 | 0 | NA |
| Patient SPN . | Age, y . | Sex . | Disease . | Prior treatment . | Type of preparative regimen . | Preparative regimen . | Graft composition . | GVHD . | Status at d + 180 . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNC (× 109) . | CD34+/kg (× 106) . | CD3+/kg (× 108) . | |||||||||
| 2240 | 45 | F | CML | HU | Myeloablative | Bu/Cy | 22 | NA | NA | 0 | Cytog rel |
| 2242 | 27 | F | AML | Ida-Ara/Ara | Myeloablative | FTBI/Etop | 177 | 23 | 3 | 0 | Alive/CR |
| 2246 | 52 | F | NHL | CHOP/ESHAP | Myeloablative | BCNU/Etop/Cy | 88 | 9 | 3 | 0 | Alive/CR |
| 2250 | 31 | M | CML-BP | HU/IFN/VINC/Pred | Myeloablative | FTBI/Etop/Cy | 145 | 15 | 3 | SkinAc2 | Alive/CR |
| 2252 | 43 | F | CML | HU/IFN | Myeloablative | Bu/Cy | 24 | NA | NA | Mouth Ch | Alive/CR |
| 2443 | 33 | M | CML | HU | Myeloablative | Bu/Cy | 32 | 2 | 0.4 | 0 | NA |
| 2444 | 46 | M | AML | Ida-Ara/Ara | Myeloablative | FTBI/Etop | 291 | 9 | 3 | 0 | NA |
| 2446 | 28 | F | AML | Ida-Ara/Ara | Myeloablative | FTBI/Etop | 95 | 6 | 2 | 0 | NA |
| 2185 | 61 | M | MM | XRT/VAD/Thal/AutoTX | Nonmyeloablative | Flu/TBI | 47 | 6 | 2 | Mouth Ch | Alive/PR |
| 2270 | 62 | M | MM | VAD/AutoTX | Nonmyeloablative | Flu/TBI | 176 | 8 | 4 | 0 | Alive/PR |
| 2320 | 52 | M | CLL | Flu/CVPB/Campath | Nonmyeloablative | Flu/TBI | 134 | 5 | 3 | 0 | Alive/PR |
| 2331 | 62 | F | MDS | None | Nonmyeloablative | Flu/TBI | 67 | 8 | 3 | GiAc2 | Alive/CR |
| 2238 | 58 | M | MM | VAD/Cy/AutoTX | Nonmyeloablative | Flu/TBI | 125 | 12 | 2 | 0 | Alive/PR |
| 2251 | 61 | F | MM | MP/VAD/AutoTX | Nonmyeloablative | Flu/TBI | 179 | 10 | 4 | 0 | Died sepsis |
| 2307 | 71 | M | MM | MP/XRT/AutoTX | Nonmyeloablative | Flu/TBI | 147 | 2 | 5 | 0 | Alive/PR |
| 2337 | 64 | M | MM | VAD/XRT/AutoTX | Nonmyeloablative | Flu/TBI | 156 | 7 | 4 | 0 | Alive/PR |
| 2410 | 61 | M | MF | None | Nonmyeloablative | Flu/TBI | 106 | 7 | 4 | 0 | NA |
| 2452 | 60 | F | MM | VAD/AutoTX | Nonmyeloablative | Flu/TBI | 135 | 12 | 7 | 0 | NA |
| 2492 | 61 | M | NHL | CHOP, Flu/Cy/rituxmab | Nonmyeloablative | Flu/TBI | 120 | 13 | 4 | 0 | NA |
SPN indicates Stanford patient number; TNC, total nucleated cells; GVHD, graft-versus-host disease; CML, chronic myeloid leukemia; BP, blast phase; HU, hydroxyurea; Bu, busulfan; Cy, cyclophosphamide; Vinc, vincristine; Pred, prednisone; NA, not available; Cytog rel, cytogenetic relapse; AML, acute myeloid leukemia; Ida, idarubicin; Ara, cytarabinoside; FTBI, fractionated total body irradiation; Etop, etoposide; CR, complete remission; NHL, non-Hodgkin lymphoma; CHOP, cyclophosphamide, adriamycin, vincristine, and prednisone; ESHAP, etoposide, cytarabinoside, prednisone, and cisplatinum; BCNU, carmustine; Flu, fludarabine; IFN, interferon; Mouth Ch, chronic GVHD of the oral cavity; MM, multiple myeloma; XRT, radiotherapy; VAD, vincristine, Adriamycin, and dexamethasone; Thal, thalidomide; AutoTX, high-dose chemotherapy with autologous hematopoietic rescue; TBI, total body irradiation; PR, partial remission; CLL, chronic lymphatic leukemia; CVBP, cyclophosphamide, vincristine, bleomycin, and prednisone; Campath, antibody against CD52; MDS, myelodysplastic syndrome; GiAc2, acute GVHD grade 2 of the gastrointestinal system; MP, melphalan, prednisone; MF, myelofibrosis; and rituximab, antibody against CD20.
Eight patients were treated with myeloablative preparatory regimens for chronic myeloid leukemia (CML) (n = 4), acute myeloid leukemia (n = 3), and diffuse large cell–non-Hodgkin lymphoma (NHL) (n = 1). For CML in chronic phase, the conditioning regimens consisted of busulfan and cyclophosphamide6; for CML in accelerated phase, the conditioning regimes was fractionated total body irradiation and etoposide and cyclophosphamide.7 The regimen for patients with acute myeloid leukemia contained fractionated total body irradiation and etoposide,8 and the patient with NHL was conditioned with carmustine, etoposide, and cyclophosphamide.9 For GVHD prophylaxis patients received cyclosporine (CSP) and methotrexate; CSP and prednisone; or CSP, methotrexate, and prednisone; or CSP and mycophenolate mofetil.
Eleven patients underwent nonmyeloablative transplantation (Table 1) for multiple myeloma (n = 7), chronic lymphocytic leukemia (n = 1), NHL (n = 1), or myelodysplastic syndromes (n = 2). All of these patients received the same conditioning regimen that consisted of total body irradiation and fludarabine.10 After transplantation, patients received immunosuppression with mycophenolate mofetil and CSP to suppress host-versus-graft reactions and to prevent GVHD.
Cell purification
Chimerism analysis was performed on distinct cell subsets isolated by immunomagnetic separation techniques. Peripheral blood DCs were purified by a multistep procedure according to an earlier reported method11,12 and used by several other groups of investigators13-16 with a commercially available kit (Miltenyi, Auburn, CA). Briefly, peripheral blood mononuclear cells (PBMCs) were first isolated by Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) or Hypaque-Ficoll (Sigma, St Louis, MO) density gradient centrifugation. The DC fraction was then enriched by a 2-step immunomagnetic bead selection process. First, B cells (CD19+), T cells (CD3+), natural killer cells (CD16+), and monocytes (CD11b+) were depleted from PBMCs by monoclonal antibodies (mAbs) coupled to immunomagnetic beads followed by a separation over a magnetic column. The depleted fraction was then further enriched for DCs by repeated rounds of positive selection for CD4+ cells by using anti-CD4–conjugated magnetic beads.
The purity of the isolated cellular population was assessed by fluorescence-activated cell sorting (FACS) analysis. Two-color analysis was performed by using a cocktail of fluorescein isothiocyanate–conjugated mouse antihuman mAbs directed against lineage markers (T-cell receptor-α/β, CD3, CD14, CD19, CD56; Becton Dickinson, San Jose, CA) versus phycoerythrin-conjugated mAbs directed against HLA-DR (Becton Dickinson). Irrelevant mouse isotype controls (Becton Dickinson) were included in the analysis. Data acquisition and analysis was performed on a FACS Scan flow cytometer (Becton Dickinson) with the use of Cellquest software.
Myelomonocytic cells were isolated directly from the peripheral blood by using immunomagnetic-positive selection with CD15 Beads (Dynal, Lake Success, NY) according to the manufacturer's protocol. T cells and B cells were similarly isolated from the Ficoll density gradient-enriched PBMC fraction by immunomagnetic beads coupled to CD3 or CD19 mAbs, respectively (Dynal).
Allogeneic mixed leukocyte reaction
The stimulatory ability of DCs was assessed in an allogeneic mixed leukocyte reaction. For all assays, PBMCs from the same healthy donor served as the source of responder cells (1 × 105responder cells per well were plated in RPMI + 10% fetal calf serum). Patients' PBMCs were enriched by Ficoll-Hypaque density gradient centrifugation, and DCs were immunomagnetically separated as previously described. DCs isolated from the peripheral blood are immature and were, therefore, cultured and matured overnight in RPMI + 10% fetal calf serum and tumor necrosis factor α (TNF-α; 10μg/mL; R&D Systems, Minneapolis, MN). DCs were irradiated (25 Gy), and decreasing numbers were mixed with responder PBMCs (as indicated in the legend to Figure 1) for 5 days and pulsed with 0.037 MBq (1 μCi) [3H] thymidine during the last 16 hours of culture. [3H] thymidine incorporation was measured by using a liquid scintillation counter (Wallac-Perkin-Elmer, Wallac, Finland).
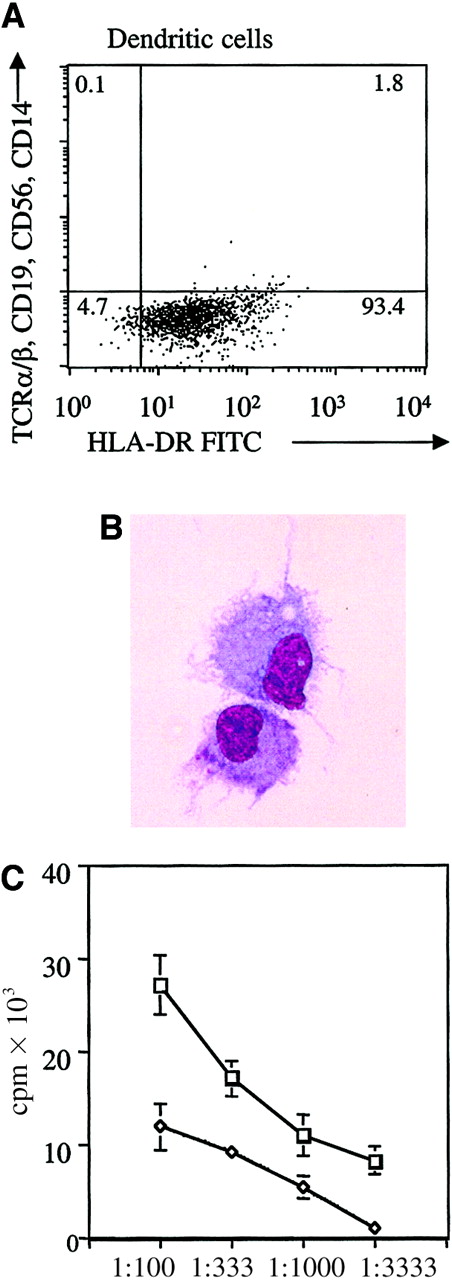
Characterization of purified DC population.
(A) FACS profile of the enriched DC population. DCs were purified by a depletion of lineage-positive cells followed by repeated positive selection for CD4low cells. This figure shows the resultant purified DC population obtained from an allogeneic transplantation patient. As shown, the DC population as characterized by DR+, lineage− population constitutes approximately 93.4% of the cells. (B) Morphologic analysis of peripheral blood DC from a patient after allogeneic transplantation. Peripheral blood DCs derived from a patient after allogeneic transplantation were immunomagnetically purified and terminally matured overnight with TNF-α. DCs were then cytocentrifuged and stained with Giemsa. The studies were visualized by light microscopy at × 60 magnification. (C) Allogeneic mixed leukocyte reaction stimulation by DCs from a patient after allogeneic transplantation compared with PBMCs from a healthy donor. Proliferative response of third-party PBMCs to DCs from a patient after allogeneic hematopoietic cell transplantation (■) or to PBMCs from a healthy donor (◊). The mean of triplicates is shown. Vertical bars represent the SD. Counts per minute (cpm) reflect cell proliferation, shown at different stimulator-to-responder ratios (1:100, 1:333, 1:1000, and 1:3333).
Characterization of purified DC population.
(A) FACS profile of the enriched DC population. DCs were purified by a depletion of lineage-positive cells followed by repeated positive selection for CD4low cells. This figure shows the resultant purified DC population obtained from an allogeneic transplantation patient. As shown, the DC population as characterized by DR+, lineage− population constitutes approximately 93.4% of the cells. (B) Morphologic analysis of peripheral blood DC from a patient after allogeneic transplantation. Peripheral blood DCs derived from a patient after allogeneic transplantation were immunomagnetically purified and terminally matured overnight with TNF-α. DCs were then cytocentrifuged and stained with Giemsa. The studies were visualized by light microscopy at × 60 magnification. (C) Allogeneic mixed leukocyte reaction stimulation by DCs from a patient after allogeneic transplantation compared with PBMCs from a healthy donor. Proliferative response of third-party PBMCs to DCs from a patient after allogeneic hematopoietic cell transplantation (■) or to PBMCs from a healthy donor (◊). The mean of triplicates is shown. Vertical bars represent the SD. Counts per minute (cpm) reflect cell proliferation, shown at different stimulator-to-responder ratios (1:100, 1:333, 1:1000, and 1:3333).
DC morphology
Peripheral blood DCs were isolated and matured overnight as described above. On harvest 1 to 2 × 103 DCs were resuspended in phosphate-buffered saline and spun onto slides and stained with Giemsa. Images to assess morphology were taken with an objective × 60 magnification (oil).
DNA isolation and short tandem repeat polymerase chain reaction
Genomic DNA from purified cells was isolated with a DNA extraction kit following the manufacturer's instructions (PUREGENE DNA Isolation Kit; Gentra Systems, Minneapolis, MN). The relative contribution of donor and recipient in each cell population was determined by quantitating, in the DNA preparation, informative microsatellite short tandem repeat (STR) alleles by using a modification of a previously described method.17 Briefly, for each donor/recipient pair, amplification of STR loci in donor DNA and in recipient pretransplantation DNA was performed in 25 μL polymerase chain reactions (PCR) with the primers shown in Table2. One member of each primer pair was synthesized (Operon, Alameda, CA) with a Carbocyanin 5 label to permit detection and quantification of fluorescent amplicon in gel electrophoresis on an automated sequencing apparatus. Each PCR reaction contained approximately 50 ng genomic DNA, forward and reverse primer pairs (Table 2), 2 U Taq polymerase (Perkin Elmer Amplitaq, Foster City, CA) mixed 1:1 with anti-Taq antibody (Clontech, Palo Alto, CA), 2.5 μL GeneAmp 10 × PCR buffer (Perkin Elmer), and 2.5 mM each of dNTPs (Perkin Elmer). The PTC-100 thermal cycler (MJ Research, Watertown, MA) program consisted of an initial 3-minute denaturation at 94°C; 30 cycles of 30 seconds at 94°C, 1 minute at 55°C, 30 seconds at 72°C; and a final 10-minute extension at 72°C. PCR products (0.5 to 2 μL added to formamide [Sigma] to yield a total volume of 12 μL) were electrophoresed at 40°C in a denaturing 8% (wt/vol) acrylamide/bisacrylamide monomers gel (ReproGel High Resolution Gel; Amersham Pharmacia Biotech, Pisctaway, NJ) on an ALF or ALF Express (Amersham Pharmacia Biotech). ALF Fragment Manager software (Amersham Pharmacia Biotech) was used to size and quantify each fluorescent amplicon and to calculate the relative abundance of each STR allele present in the template DNA. A STR locus was considered informative for a donor/recipient pair if alleles of the donor differed in size from the respective recipient. Informative loci were subsequently PCR amplified in DNA samples collected after transplantation. For each such sample, the quantity of donor-specific amplicon relative to the quantity of total unique amplicons at that locus yielded the percentage of DNA of donor origin present in the sample. This assay is linear over a range of 5% to 95% for artificial mixes of donor DNA into recipient DNA (data not shown). Each posttransplantation sample was run in parallel with 5% and 95% standards consisting of 5:95 and 95:5 mixes of donor-recipient pretransplantation DNA. By using the ALF Manager program, the standard mixtures yielded measured values (mean ± 2 SD) of 6% ± 4% and 94% ± 4% donor DNA, respectively.
Short tandem repeat primer
| STR locus* . | Chromosome . | Repeat sequence . | Amplicon size . | Primers† (5′ → 3′) . | pM per reaction . |
|---|---|---|---|---|---|
| SE33 | 9 | AAAG | 234 -318 | Fwr: AAT CTG GGC GAC AAG AGT GA | 3 |
| (V00481) | Rev: ACA TCT CCC CTA CCG CTA TA | 3 | |||
| HUMTHO1 | 11p15.5-p15 | AATG | 152 -180 | Fwr: GTG ATT CCC ATT GGC CTG TTC CTC | 3 |
| (D00269) | Rev: GTG GGC TGA AAA GCT CCC GAT TAT | 3 | |||
| HUMFES/FPS | 15q25-qter | AAAT ATTT (7 or 15) | 152 -180 | Fwr: GGG ATT TCC CTA TGG ATT GG | 3 |
| (X06292) | Rev: GCG AAA GAA TGA GAC TAC AT | 3 | |||
| HUMvWA31/A | 12p12-12pter | AGAT TCTA with | 102 -154 | Fwr: CCC TAG TGG ATG ATA AGA ATA ATC | 3 |
| (M25858) | TCTG and TCCA inserts | Rev: GGA CAG ATG ATA AAT ACA TAG GAT GGA TGG | 3 | ||
| D21S11 | 21 | TCTA with TCTG, TA, | 209 -243 | Fwr: GTG AGT CAA TTC CCC AAG | 10 |
| (M84567) | TCA, TC inserts | Rev: GTT GTA TTA GTC AAT GTT CTC | 10 | ||
| CSF1PO | 5q33.5-34 | AGAT | 295 -327 | Fwr: AAC CTG AGT CTG CCA AGG ACT AGC | 4 |
| (X14720) | Rev: TTC CAC ACA CCA CTG GCC ATC TTC | 4 | |||
| D7S796 | 7 | CTAT | 162 -198 | Fwr: TTT TGG TAT TGG CCA TCC TA | 6 |
| (G20015) | Rev: GAA AGG AAC AGA GAG ACA GGG | 6 | |||
| D1S533 | 1 | AGxAGATxAGAT | 193 -225 | Fwr: CAT CCC CCC CAA AAA ATA TA | 2 |
| (G07788) | Rev: TTG CTA ATC AAA TAA CAA TGG G | 2 |
| STR locus* . | Chromosome . | Repeat sequence . | Amplicon size . | Primers† (5′ → 3′) . | pM per reaction . |
|---|---|---|---|---|---|
| SE33 | 9 | AAAG | 234 -318 | Fwr: AAT CTG GGC GAC AAG AGT GA | 3 |
| (V00481) | Rev: ACA TCT CCC CTA CCG CTA TA | 3 | |||
| HUMTHO1 | 11p15.5-p15 | AATG | 152 -180 | Fwr: GTG ATT CCC ATT GGC CTG TTC CTC | 3 |
| (D00269) | Rev: GTG GGC TGA AAA GCT CCC GAT TAT | 3 | |||
| HUMFES/FPS | 15q25-qter | AAAT ATTT (7 or 15) | 152 -180 | Fwr: GGG ATT TCC CTA TGG ATT GG | 3 |
| (X06292) | Rev: GCG AAA GAA TGA GAC TAC AT | 3 | |||
| HUMvWA31/A | 12p12-12pter | AGAT TCTA with | 102 -154 | Fwr: CCC TAG TGG ATG ATA AGA ATA ATC | 3 |
| (M25858) | TCTG and TCCA inserts | Rev: GGA CAG ATG ATA AAT ACA TAG GAT GGA TGG | 3 | ||
| D21S11 | 21 | TCTA with TCTG, TA, | 209 -243 | Fwr: GTG AGT CAA TTC CCC AAG | 10 |
| (M84567) | TCA, TC inserts | Rev: GTT GTA TTA GTC AAT GTT CTC | 10 | ||
| CSF1PO | 5q33.5-34 | AGAT | 295 -327 | Fwr: AAC CTG AGT CTG CCA AGG ACT AGC | 4 |
| (X14720) | Rev: TTC CAC ACA CCA CTG GCC ATC TTC | 4 | |||
| D7S796 | 7 | CTAT | 162 -198 | Fwr: TTT TGG TAT TGG CCA TCC TA | 6 |
| (G20015) | Rev: GAA AGG AAC AGA GAG ACA GGG | 6 | |||
| D1S533 | 1 | AGxAGATxAGAT | 193 -225 | Fwr: CAT CCC CCC CAA AAA ATA TA | 2 |
| (G07788) | Rev: TTG CTA ATC AAA TAA CAA TGG G | 2 |
STR indicates short tandem repeat; Fwr, forward; and Rev, reverse.
GenBank accession no. is given in parentheses.
Forward primers are 5′ labeled with Carbocyanin 5, reverse primers with fluorescein.
Results
Clinical course
Nineteen patients were enrolled in our study. Table 1 lists the patient characteristics, including age, sex, underlying hematologic disorder, prior chemotherapy, preparative regimen, graft composition, and clinical outcome. All patients in the myeloablative treatment group demonstrated complete engraftment, including erythrocytes, white cells, and platelets. Median time to absolute neutrophil count more than 500/μL was 13 days, to absolute lymphocyte count more than 500/μL was 28 days, and to absolute lymphocyte count more than 1000/μL was 119 days. The incidence and manifestation of clinically evident GVHD was low and mild, respectively (Table 1). Patient SPN 2250 experienced acute GVHD grade II of the skin (macular-papular eruption of 25% to 50% of body surface) that was controlled by CSP and prednisone treatment. Patient SPN 2252 developed limited chronic GVHD of the oral cavity. All patients achieved a complete hematologic remission of their diseases. Patient SPN 2240 was diagnosed with a cytogenetic relapse of the underlying CML on day +180 after the transplantation (Table 1).
Eleven patients were treated with the nonmyeloablative regimen developed in a canine model (for review see McSweeney and Storb18) and extended to human HCT recipients.10 All 11 patients had prompt engraftment of myelocytic, monocytic, and lymphoid lineages (ie, more than 50% donor origin at more than 2 time points tested). In this group of patients the median time to absolute neutrophil count more than 500/μL was 18 days, to absolute lymphocyte count more than 500/μL was 28 days, and to absolute lymphocyte count more than 1000/μL was 81 days. Patient SPN 2251 died of clinical sepsis on day +102 after transplantation, after having attained a complete hematologic remission (Table 1). The incidence of GVHD in the nonmyeloablative treatment group was low, and the affected patients had only mild GVHD. Grade II acute GVHD of the gastrointestinal tract was observed in patient SPN 2331, and patient SPN 2185 is showing signs of limited chronic GVHD of the oral cavity. In both patients GVHD was controlled by the addition of prednisone to the prophylactic treatment regimen.
Purification of DCs
DCs constitute approximately 0.5% PBMC in healthy donors.11,19 Because of this low frequency and to accurately assess the contribution of donor versus host DCs in the transplantation patients, a multistep protocol was followed to obtain purified DCs from peripheral blood. A minimum of 2 × 103DCs were obtained from 60 mL blood. Circulating human DCs express HLA DRhigh and CD4low on their surface but do not express the lineage markers CD3, CD19, CD16, or CD11b found on T, B, and natural killer cells and monocytes, respectively. Thus, the latter cell populations were first negatively selected by using magnetic bead sorting directed against lineage markers. The depleted fraction was then positively enriched for DCs by 2 sequential rounds of selection for CD4+ cells. Selected cells were analyzed by FACS to assess DC purity. Figure 1A is a representative FACS profile of the enriched DC population obtained from an allogeneic transplantation patient. As shown, the DR-positive, lineage-negative (T-cell receptorα/β, CD19, CD56, and CD14) population constitutes approximately 93% of the cells. This HLA DRhigh and lineageneg cell population has been shown to represent DCs that are capable of priming and stimulating T cells in vitro and are fully functional1,11 and has been widely used to isolate peripheral blood DCs.13-16 By using this methodology it was possible to isolate DCs from the peripheral blood of patients treated with nonmyeloablative preparatory therapy as early as day +1 and for patients treated with myeloablative pretransplantation therapy on day +14.
DC morphology and function
To confirm that the cell population isolated from the patients' peripheral blood was DCs as previously shown by other investigators,11,13-16 morphologic and functional studies were performed. It has been demonstrated that DCs directly isolated from peripheral blood are immature.11 Therefore, the isolated cells were matured overnight in the presence of TNF-α. Evaluation of the resultant cell population demonstrated that the isolated cells developed features characteristic of DCs, including nonadherence to plastic and a typical veiled morphology (Figure 1B). Furthermore, the antigen-presenting and stimulatory capacities of these cells were evaluated by mixed leukocyte reaction. The DCs isolated from allogeneic transplantation patients were efficient in their capacity to stimulate healthy allogeneic third-party PBMC responder cells. Their stimulatory capacity was significantly better than PBMCs obtained from healthy control individuals (Figure 1C).
DC chimerism after myeloablative allogeneic HCT
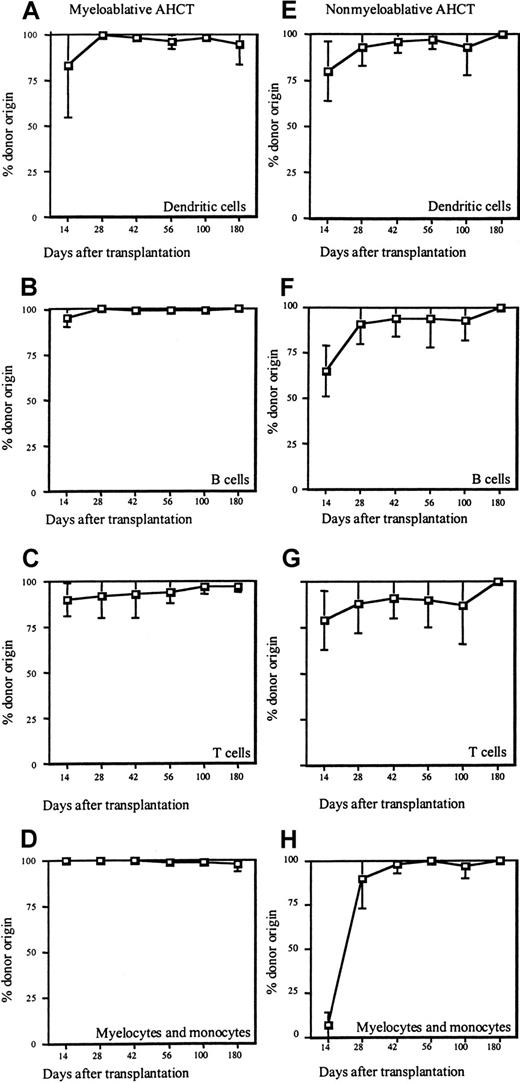
Given that patients undergoing myeloablative pretransplantation therapy experience profound pancytopenia in the days immediately after allogeneic HCT, the earliest time point that DC chimerism could be assessed was at day +14 after transplantation. Figure 2A-D summarizes the percentage of donor chimerism after allogeneic HCT for the DC lineage, as well as for myelomonocytes and T and B lymphocytes in the patients that underwent myeloablative preparative regimens. As shown, 14 days after allogeneic HCT 83% ± 28% DCs in the peripheral blood originated from the donor (Figure 2A), in contrast to 100% ± 0% of myelomonocytoid cells, 95% ± 5% of B cells, and 90% ± 9% of T cells (Figure 2B-D). By day +28 after transplantation, DCs, myelocytes, monocytes, and B cells were exclusively of donor origin (≥ 99% ± 1%), whereas T-cell chimerism developed at a slower pace as shown by 92% ± 12% donor T cells at that time point. Throughout the remaining observation period the donor contribution to DCs, myelocytes, monocytes, and B cells remained stable at 99% or more, and T-cell chimerism progressively increased to 97% ± 3%. The only exception to this pattern of chimerism was patient SPN 2240, whose CML recurred on day +180. The relapse of her disease was associated with a decrease in DC chimerism to 75% and myelocyte and monocyte chimerism to 92% at day +180.
Donor chimerism after myeloablative and nonmyeloablative transplantation.
Peripheral blood was obtained from patients on days +14, +28, +42, +56, +100, and +180 after transplantation. Subpopulations were purified by magnetic bead sorting and analyzed for percentage of donor origin by STR analysis. The graphs show the mean percentage of donor chimerism and SD for each cell population studied. (A-D) Mean donor chimerism for 5 patients that underwent myeloablative allogeneic HCT. (A-E) Mean donor chimerism for 8 patients that underwent nonmyeloablative allogeneic HCT. Cell subsets analyzed were dendritic cells (A,E), B lymphocytes (B,F), T lymphocytes (C,G), and myelocytes and monocytes (D,H).
Donor chimerism after myeloablative and nonmyeloablative transplantation.
Peripheral blood was obtained from patients on days +14, +28, +42, +56, +100, and +180 after transplantation. Subpopulations were purified by magnetic bead sorting and analyzed for percentage of donor origin by STR analysis. The graphs show the mean percentage of donor chimerism and SD for each cell population studied. (A-D) Mean donor chimerism for 5 patients that underwent myeloablative allogeneic HCT. (A-E) Mean donor chimerism for 8 patients that underwent nonmyeloablative allogeneic HCT. Cell subsets analyzed were dendritic cells (A,E), B lymphocytes (B,F), T lymphocytes (C,G), and myelocytes and monocytes (D,H).
DC chimerism after nonmyeloablative allogeneic HCT
Studies on DC chimerism in the nonmyeloablative treatment group initially focused on the same time points examined for the myeloablated patients. Figure 2E demonstrates that development of DC chimerism was similar in pace and pattern to that in patients with myeloablative treatment. By day +14 after transplantation 80% ± 16% DCs were of donor origin, followed by further increases to 93% ± 10% on day +28 and 96% ± 6% on day +42, and nearly all DCs of donor origin by day +56 (98% ± 5%; Figure 2E). Establishment of T-cell chimerism was also similar to that observed for the myeloablated patients, 79% ± 16% of T cells derived from the donor on day +14, 88% ± 16% on day +28, 91% ± 11% on day +56, and nearly complete donor origin on day +100 (99% ± 2%; Figure2G). The appearance of donor B cells and myelocyte and monocyte lineages was slightly delayed as compared with patients who received myeloablative regimens for the engraftment in the nonmyeloablative group. However, by day +100 all cell types tested were exclusively of donor origin (100% ± 1%; Figure 2F,H).
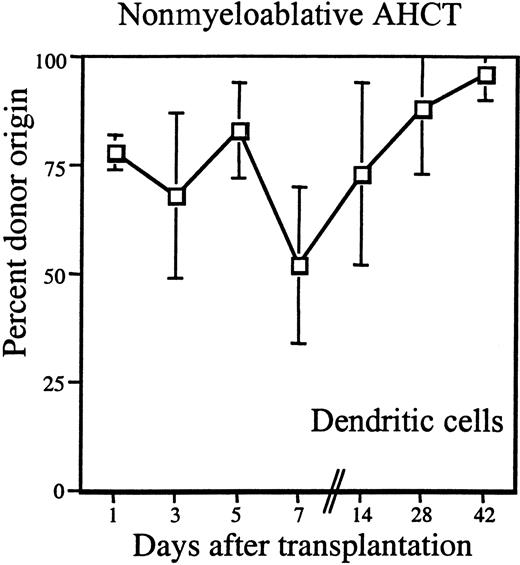
The high percentage of donor DCs observed in the peripheral blood of both patient groups as early as day +14 was unexpected. Because it was technically possible to isolate DCs from nonmyeloablated patients before day +14, we examined DC chimerism in this group of patients in the first week after allogeneic HCT.
During the first week after HCT, blood samples were obtained from 5 patients every other day beginning on day +1. Figure3 shows the percentage of donor DCs in these patients. As early as day +1 after transplantation, donor DCs constituted 78% ± 4% and remained more than 68% on average until day +7 when a transient decrease to 50% was noted (68% ± 19% on day +3, 83% ± 11% on day +5, and 52% ± 18% on day +7). There followed a steady increase of donor DCs to 73% ± 21% isolated on day +14, 88% ± 15% on day +28, 96% ± 6% on day + 42, and 97% ± 6% on day +56 (Figure 3).
Donor chimerism of DCs early after nonmyeloablative transplantation.
Peripheral blood DCs were purified by magnetic bead sorting and analyzed for percentage of donor origin by STR analysis. Peripheral blood was obtained from patients on days +1, +3, +5, +7, +14, +28, and +42 after transplantation. The mean of 5 studied patients is shown (■); the vertical lines represent the SD.
Donor chimerism of DCs early after nonmyeloablative transplantation.
Peripheral blood DCs were purified by magnetic bead sorting and analyzed for percentage of donor origin by STR analysis. Peripheral blood was obtained from patients on days +1, +3, +5, +7, +14, +28, and +42 after transplantation. The mean of 5 studied patients is shown (■); the vertical lines represent the SD.
Discussion
It was the purpose of this study to investigate the kinetics of chimerism of DCs in patients after allogeneic HCT. The patients studied were heterogeneous with regard to their underlying diagnoses, pretransplantation cytoreductive therapy, types of grafts, and conditioning regimens. Surprisingly, we found that the kinetics of appearance of donor DCs in the peripheral blood was rapid and highly consistent among all patients evaluated beginning on day +14. For patients undergoing nonmyeloablative conditioning treatment, we were able to isolate DCs from the peripheral blood as early as day +1 and found that even at this early time point approximately 78% of the DCs originated from the donors. Fluctuations in donor DC chimerism were observed, but donor chimerism remained more than 50% until day +14, when a steady increase in donor DC levels was observed. This kinetics led us to conclude that most donor DCs are initially transfused with the hematopoietic graft. Then, beginning around 2 weeks after hematopoietic cell infusion DCs arose de novo from donor progenitors and stem cell source. This time frame of DC reconstitution observed in vivo correlates well with prior in vitro studies, wherein the time course to DC generation from CD34+ hematopoietic progenitors cells under optimized conditions takes approximately 14 days.20-22
The implications of rapid donor DC generation and repopulation after allogeneic HCT are broad. It is now known that DCs have multiple functional roles in the activation and tolerance induction of T cells.1,23 On the one hand, DCs are considered to be the most potent antigen-presenting cells capable of stimulating T-cell responses (for review see Bell et al24). On the other hand, DCs have been shown to be capable of inducing intrathymic deletion of alloreactive cells2,3 as well as mediating the establishment of peripheral tolerance.25,26 DCs process and present both endogenously and exogenously derived antigens. Therefore, in the setting of a major histocompatibility complex–matched allogeneic HCT procedure (the most common clinical scenario), DCs arising from the donor graft and DCs persisting from recipients are expected to present endogenous minor donor antigen peptides that will influence T-cell activation and/or induction of tolerance. The clinical implications of DC turnover in the postallogeneic HCT setting were demonstrated by investigators5 who reported that GVHD in a major histocompatibility complex–matched allogeneic murine bone marrow transplantation model was driven by host antigen-presenting cells. Thus, understanding the time course of donor DC turnover may be useful for determining an appropriate length of immunosuppression for GVHD prophylaxis. In a more limited study of 6 patients with only a single data point each (between days 24 and 205 after related or unrelated HCT), full donor DC chimerism was associated with the absence of GVHD.27 In our study a low incidence and severity of GVHD was observed throughout the full 6-month observation period after transplantation. Thus, it was not possible to correlate GVHD to the establishment of donor DC status.
It has been demonstrated that DCs are a heterogeneous cell population. In humans at least 2 subsets of DCs (DC-1 and DC-2) have been identified in peripheral blood and secondary lymphoid organs28 that can be phenotypically differentiated by expression of myeloid markers (eg, CD11c).12 These DC subsets demonstrate functional differences in their capacities to stimulate and polarize various T-cell subsets.29,30 This phenomenon appears to depend on both the type and kinetics of cytokines produced as well as the repertoire of costimulatory molecules expressed. Two recent reports presented data on the characteristics of DCs derived from peripheral blood19 and spleen31 after granulocyte colony-stimulating factor (G-CSF) treatment. The investigators19 found that human G-CSF–mobilized peripheral blood contained relatively high numbers of DC-2 and their precursors and further demonstrated that activated DC-2 skews T-cell responses toward the production of T helper 2-type cytokines in vitro. These investigators suggested that such DC-2 predominance may partially explain why, despite significantly increased numbers of T cells contained in mobilized peripheral blood as compared with bone marrow grafts, increased incidences of acute GVHD have not been observed.32 Studies in mice31demonstrated that cotransfer of splenocytes plus bone marrow from G-CSF–treated donors resulted in better survival and lower GVHD as compared with untreated donors. Splenic DCs from G-CSF–treated donors demonstrated decreased production of TNF-α and interleukin-12 in response to lipopolysaccharide, again suggesting that after G-CSF treatment there is an emergence and predominance of “tolerizing” DC population(s) in the peripheral blood and lymphoid organs. Interestingly, it was found that, in tumor-bearing mice that underwent syngeneic transplantation with non-G-CSF–primed bone marrow, there was massive expansion and activation of tumor-specific T cells in the immediate posttransplantation period.33 The researchers had also noted a rapid decline after that time period in the immune response in association with tumor progression. These studies imply that DCs present in the early posttransplantation phase are functionally more capable of inducing antitumor responses as compared with DC population(s) that arise more than 6 weeks after transplantation.
In conclusion, we found that after allogeneic HCT of a highly heterogeneous patient group there is rapid establishment of donor DC chimerism that appears to be independent of the underlying disease and the intensity of the conditioning regimen. Given the central role that DCs play in the activation and tolerance induction of antigen-specific cellular immune responses and the emerging understanding of multiple functional DC subsets, further studies on DC biology in the posttransplantation setting will likely lead to new therapeutic concepts for recipients of allogeneic HCT.
We thank D. Kathryn Tierney, RN, MS, and the nurses of the Stanford University Bone Marrow Transplant Unit for their assistance with blood sample collection and Drs Irving L. Weissman, Markus Manz, and Michael R. Verneris for critically reviewing the manuscript.
Supported by grants from the Dr. Mildred Scheel Stiftung fuer Krebsforschung, Germany (S.A.-G.), the National Institutes of Health (PPG PO1 CA49605), the Multiple Myeloma Research Foundation, and the McCarty Cancer Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Judith A. Shizuru, Bone Marrow Transplantation Program, Stanford University Medical Center, Stanford, CA 94305; e-mail: jshizuru@stanford.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal