Exogenous C2-ceramide has been shown to inhibit polymorphonuclear leukocyte (PMN) phagocytosis through inhibition of phospholipase D (PLD) and downstream events, including activation of extracellular signal–regulated kinases 1 and 2, leading to the hyphothesis that the sphingomyelinase pathway is involved in termination of phagocytosis. Here it is postulated that increased PLD activity generating phosphatidic acid and diacylglycerol (DAG) is essential for superoxide release and degranulation and that ceramide, previously shown to be generated during PMN activation, inhibits PLD activation, thereby leading to inhibition of PMN function. When PMNs were primed with granulocyte colony-stimulating factor (G-CSF) and then activated with N-formyl-methionyl-leucyl-phenylalanine (FMLP), C2-ceramide (10 μM) completely inhibited release of superoxide, lactoferrin, and gelatinase; the DAG analog sn-1,2-didecanoylglycerol (DiC10) (10 μM) restored oxidase activation and degranulation in the ceramide-treated cells. Similarly, C2-ceramide inhibited oxidase activity and degranulation of PMNs treated with cytochalasin B followed by FMLP, and DiC10 restored function. In contrast, C2-ceramide did not inhibit phosphorylation of p47phox or p38 mitogen-activated protein kinase, or translocation of p47phox, PLD-containing organelles, adenosine diphosphate–ribosylation factor 1, RhoA, protein kinase C (PKC)–β or PKC-α to the plasma membrane in G-CSF or cytochalasin B–treated, FMLP-activated PMNs. PLD activity increased by 3-fold in G-CSF–primed PMNs stimulated by FMLP and by 30-fold in cytochalasin B–treated PMNs stimulated by FMLP. Both PLD activities were completely inhibited by 10 μM C2-ceramide. In conclusion, superoxide, gelatinase, and lactoferrin release require activation of the PLD pathway in primed PMNs and cytochalasin B–treated PMNs. Ceramide may affect protein interactions with PLD in the plasma membrane, thereby attenuating PMN activation.
Introduction
Polymorphonuclear leukocytes (PMNs) ingest microbes, then destroy them by release of reactive oxygen intermediates and granule enzymes to the phagosome. These processes must be closely regulated temporally and spatially to effectively destroy microbes without damage to surrounding tissue, suggesting a signaling linkage in which release of cytotoxins occurs after phagocytosis. Several signaling and effector processes are similar or shared among the PMN functions of phagocytosis, oxidant production, and degranulation, including activation of mitogen-activated protein (MAP) kinases,1-3 some protein kinase C (PKC) isoforms,4,5 phospholipase D (PLD),6-8 and cytoskeletal reorganization.9-11 Specifically, phagocytosis is dependent upon activation of PLD, extracellular signal–regulated kinase (ERK)–1, ERK2, and myosin light chain kinase, and involves translocation of PKC-δ and the small guanosine 5′-triphosphatase (GTPase) Raf-1.1,4,7,9 The MAP kinases and PLD have also been implicated in degranulation. The p38 MAP kinase inhibitor SB203580 partially inhibits secondary granule release in PMNs,12 while degranulation in several cases is related to p38 or ERK1/2.12-15 Release of both specific and primary granules is inhibited by ethanol, indicating PLD activity is required for degranulation.6,8,16N-formyl-methionyl-leucyl-phenylalanine (FMLP) alone activates phospholipase C, but cytochalasin B pretreatment results in PLD activation by FMLP.17 Similarly, a stimulus in addition to FMLP is required for significant degranulation or oxidant production, suggesting phospholipase C activation is not sufficient to initiate these functions. HL-60 cells differentiated with dimethyl sulfoxide to PMN-like cells lack specific granules and are unable to produce superoxide when adherent to fibrinogen, suggesting a link between degranulation and oxidant production.11
Activation of the nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidase is dependent on translocation of the p47phox-p67phox-p40phox cytosolic complex and small GTPase Rac2 to the plasma or phagosomal membrane where they associate with cytochrome b558 and Rap1A.18,19Phosphorylation of p47phox, p67phox, and p40phox occurs, although only p47phox phosphorylation may be required for oxidase activation. P47phox is phosphorylated on several residues, mediated by PKA, PKC, a phosphatidic acid–activated kinase, p21-activated kinases, and/or MAP kinases.2,18,20,21 The MAP kinase p38 may be involved in phosphorylation of p47phox, as evidenced by in vitro phosphorylation2 and inhibition of oxidase by the p38 inhibitor SB203580.22 There is also evidence that ERKs 1 and 2 participate in phosphorylation of p47phox.2 3
We have previously shown that endogenous ceramide levels increase with activation in adherent PMNs stimulated with FMLP and in PMNs undergoing phagocytosis of antibody-opsonized particles. Ceramide is the product of neutral sphingomyelinase, and plasma membrane–associated neutral sphingomyelinase activity increases during FMLP stimulation of PMNs.23 Once maximal levels of endogenous ceramide are achieved, PMN functional responses are terminated.7,23,24 Since neutral sphingomyelinase activity also increases concurrently with ceramide accumulation during phagocytosis,7 we concluded that the neutral sphingomyelinase pathway may be involved in terminating both phagocytosis and oxidant production. Exogenous C2-ceramide inhibits oxidant production in both suspended and adherent PMNs24-27 and inhibits the increase in PLD activity occurring during oxidant production in adherent PMNs24 and phagocytosis of antibody-coated particles by PMNs in suspension.7 C2-ceramide also inhibits PLD activity in cell-free systems28; in HL-60 cells, ceramide inhibits by preventing activation of PLD by the small GTPases adenosine diphosphate–ribosylation factor (ARF)–1 and RhoA.29Following inhibition of PLD activity, both phosphatidic acid (PA) and diacylglycerol (DAG) fail to accumulate. The lack of DAG theoretically could lead to decreased activation of PKC isoforms α, βI, βII, γ, and δ. The deficiency of DAG may also result in impaired granule membrane fusion.30 31
We hypothesized that the sphingomyelinase pathway is involved in terminating oxidant production, degranulation, and phagocytosis by leading to ceramide inhibition of PLD activity. To examine this possibility, we used suspended PMNs to measure oxidant release and degranulation of secondary and tertiary granules in the presence of exogenous C2-ceramide. PLD involvement was tested in 2 ways: by using a DAG analog to attempt restoration of oxidant and granule release following ceramide treatment and by directly measuring C2-ceramide inhibition of PLD activity under conditions that result in oxidant production and degranulation. In an effort to determine the mechanism of PLD inhibition by ceramide, translocation of ARF1, RhoA, PKC-α, and PLD-containing secretory vesicles were investigated. Because C2-ceramide treatment inhibits phosphorylation of several kinases involved in PMN activation, we investigated ceramide's effect on other components of NADPH oxidase activation: phosphorylation and translocation of p47phox and PKC-β, and activation of p38 MAP kinase.
Materials and methods
Materials
D-erythro-C2-ceramide and D-erythro-dihydro-C2-ceramide were purchased from Matreya (Pleasant Gap, PA). The DAG analog sn-1,2-didecanoylglycerol (DiC10) was obtained from Avanti Polar Lipids (Alabaster, AL). Granulocyte colony-stimulating factor (G-CSF) was a gift from Amgen (Thousand Oaks, CA). Antibodies against lactoferrin and gelatinase were a gift from Dr Niels Borregaard, State University Hospital (Copenhagen, Denmark). Antibody against p47phox (NIH G1588) was a gift from Dr Harry Malech, National Institutes of Health (Bethesda, MD). Antibody against the phosphorylated, active form of p38 MAP kinase was purchased from Promega (Madison, WI). Antibodies against RhoA and ARF1 were obtained from Santa Cruz Biotechnology (CA). Antibodies against PKC-β and PKC-α were purchased from BD Transduction Laboratories (San Diego, CA). H3[32P]O4 was obtained from ICN Pharmaceuticals (Irvine, CA).
Cells
PMNs were isolated from peripheral venous blood from healthy volunteers as previously described.7 Approval was obtained from the institutional review board at the University of Michigan for these studies. Informed consent was provided according to the Declaration of Helsinki. For experiments in which cells were disrupted, PMNs were pretreated with 5 mM diisopropyl fluorophosphate on ice for 5 minutes, then washed 3 times with phosphate-buffered saline (PBS).
Degranulation
Incubation with C2-ceramide or dihydro-C2-ceramide was performed before stimulation as follows: PMNs were suspended at 2 × 106/mL (final) in PBS containing 1 mM Ca++, 1 mM Mg++, and 5 mM glucose; then lipids were added and cells incubated for 30 minutes at 22°C. Where indicated, DiC10 was added, and incubation proceeded for another 15 minutes at 22°C. PMNs were then stimulated at 37°C with G-CSF (50 ng/mL) for 10 minutes, followed by FMLP (100 nM) for 5 minutes. Alternatively, PMNs were stimulated with cytochalasin B (5 μg/mL) for 3 minutes, then FMLP (100 nM) for 5 minutes. Cells were placed on ice for 5 minutes, then centrifuged. Lactoferrin and gelatinase content in the supernatants was measured by enzyme-linked immunosorbent assay (ELISA) as previously described.32Unstimulated controls were incubated at the same temperatures and times as the stimulated samples.
Superoxide
PMNs were treated with lipids as described above. Before stimulation, cytochrome C and superoxide dismutase or buffer were added to give final concentrations of 75 μM/mL and 60 μg/mL, respectively, and 1 × 106 PMNs per milliliter. PMNs were stimulated as described above, and the reduction of cytochrome C in supernatants was read spectroscopically as an end-point assay.33 Unstimulated controls were incubated at the same temperatures and times as the stimulated samples.
Phospholipase D
PMNs were labeled with 1-O-[3H]-octadecyl-sn-glycero-3 phosphocholine (Amersham, Arlington Heights, IL) as previously described.7 Cells were treated with lipids and stimulated at 2 × 106/mL as described above, except that before stimulation cells were incubated with 1% ethanol for 5 minutes at 37°C. Cell pellets were extracted according to the method of Van Veldhoven and Bell.34 Lipid products were separated with the use of thin-layer chromatography (TLC) as previously described,6 and the [3H]-phosphatidylethanol (PEt) was removed and counted in a scintillation counter.
Subcellular fractionation
PMNs (2 × 106/mL) were incubated with C2-ceramide and stimulated with cytochalasin B and FMLP as described above. PMNs were then centrifuged and suspended at 25 to 40 × 106/mL in disruption buffer containing 100 mM KCl, 3 mM NaCl, 10 mM piperazine diethanesulfonic acid (pH 7.0), and 3.5 mM MgCl2. PMNs were nitrogen cavitated, layered over a 3-layer Percoll gradient, and fractionated as described by Kjeldsen et al.35 Gelatinase was determined by ELISA and latent alkaline phosphatase by the difference in activity in the presence and absence of Triton X-100.35
The p47phox phosphorylation
PMNs were labeled at 108/mL with H3[32P]O4, 2 mCi (74 × 106 Bq)/108 PMNs, in 30 mM Hepes (pH 7.4), 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 10 mM glucose. Cells were washed twice and suspended (2 × 106/mL) in PBS containing 1 mM Ca++ and 1 mM Mg++, with each sample containing 107 PMNs. PMNs were exposed to lipids and stimulated as described above, except that FMLP stimulation was for 1 minute. A positive control was made by stimulating one sample with 100 ng/mL phorbol myristate acetate (PMA) for 3 minutes at 37°C. PMNs were centrifuged, then lysed 30 minutes on ice in 0.8 mL buffer containing 10 mM Tris (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% sodium deoxycholate, 1% Igepal CA-630, 40 mMP-nitrophenylphosphate, 1 mM phenymethyl sulfonyl fluoride (PMSF), 1 mM Na3VO4, 50 mM NaF, and 10 μg/mL each of aprotinin, leupeptin, pepstatin, and soybean trypsin inhibitor. Samples were microfuged for 5 minutes; then goat anti-p47phox was added to supernatants and incubated, rotating for 1 hour at 4°C. Rabbit antigoat antibody was added and samples were incubated overnight. Protein A-sepharose beads were added and samples incubated 1 hour. Beads were washed and boiled in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) buffer; then supernatants were subjected to 10% SDS-PAGE. Gels were stained, dried, and exposed to x-ray film. Samples were also processed on a 25-cm-tall 10% SDS-PAGE gel to separate p47phox from the 55-kd immunoglobulin (Ig)–G band. Proteins were transferred to polyvinylidene fluoride membranes, then probed with antibody to p47phox to show equal amounts of protein per sample.
The p47phox, PKC-α, and PKC-β translocation
PMNs were exposed to lipids in PBS (2 × 106/mL) as described above, with the use of 0.8 to 1 × 108 per sample. Samples were centrifuged and resuspended at 5 × 107 PMNs per milliliter in Hanks balanced salt solution with the addition of 10 mM Hepes, and lipids were added again. Samples were placed in a 37°C water bath for 1 to 2 minutes, then stimulated with G-CSF and/or FMLP as described above except that FMLP stimulation was carried out for 2 minutes. Samples were placed in an ice-water bath for 5 minutes, then centrifuged, and the pellets were suspended in 50 mM Tris (pH 7.5), 2 mM ethyleneglycotetraacetic acid, 1 mM PMSF, 1 μg/mL leupeptin, 10 μM benzamidine, 10 μM pepstatin, and 0.2 μg/mL aprotinin. A probe sonicator was used to disrupt the PMNs for two 12-second bursts on ice. Samples were centrifuged at 400g for 10 minutes to remove unbroken cells and nuclei. The supernatants were layered over discontinuous sucrose gradients of 15% and 35%, then centrifuged for 30 minutes at 150 000g. Cytosol was collected from the top of each gradient, and plasma membrane was taken from the 15%-to-35% interface. Membranes were pelleted for 30 minutes at 200 000g, then homogenized by means of a 1-mL glass tissue grinder with polytetrafluoroethylene pestle. Protein concentration was determined by means of a bicinchoninic acid (BCA) assay. Western blotting was performed essentially as described in Raeder et al.36 Briefly, equal amounts of protein were separated on 12% SDS-PAGE, then transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were probed with goat anti-p47phox or mouse anti–PKC-β or α, followed by horseradish peroxidase–conjugated antigoat or antimouse respectively, and chemiluminescence detection.
The p38 MAP kinase activation
PMN samples (2 × 106) were treated with C2-ceramide and stimulated with G-CSF and FMLP as described above. PMNs were lysed in 1% Triton X-100, 50 mM Tris (pH 8.0), 100 mM NaCl, 1 mM PMSF, 1 mM Na3VO4, and 10 μg/mL each of aprotinin, leupeptin, pepstatin, and soybean trypsin inhibitor. Lysates were microfuged for 5 minutes and supernatants combined with SDS-PAGE buffer. Western blots were performed as described above and membranes probed with antibody against active (phosphorylated) p38. Blots were stripped and reprobed with antibody recognizing both active and inactive p38 to demonstrate equal loading.
Results
C2-ceramide inhibition of function
We hypothesized that the sphingomyelinase pathway, particularly the sphingomyelinase product ceramide, is involved in termination of oxidant production and degranulation as well as phagocytosis because of the functional linkage of these processes and previous studies showing common elements of signaling. To address this hypothesis, we first assayed oxidant production and degranulation in the presence of exogenous cell-permeable C2-ceramide to simulate accumulation of endogenous ceramide. PMNs were incubated with C2-ceramide or its inactive analog dihydro-C2-ceramide, then stimulated with G-CSF (or, in some experiments, cytochalasin B), followed by FMLP; then supernatants were assayed for superoxide, gelatinase (tertiary granule marker), or lactoferrin (specific granule marker). G-CSF–primed PMNs were used because pilot experiments showed that FMLP alone yielded low levels of oxidant release and degranulation (data not shown), and PLD activation represented by PA and DAG accumulation is low without an additional stimulus.17 37
Superoxide production in G-CSF–primed, FMLP-stimulated PMNs was inhibited in dose-dependent fashion by C2-ceramide. Approximately 35% of superoxide release was inhibited by 3 μM C2-ceramide, and 74% by 10 μM, reducing superoxide to levels not significantly different from those of unstimulated PMNs (Figure 1A). These concentrations are similar to those reported by others as inhibiting PMN superoxide release.25-27 Superoxide was not significantly inhibited by 10 μM dihydro-C2-ceramide. G-CSF alone did not stimulate oxidant release, while FMLP alone stimulated little (approximately 3 nmol/106 PMNs per 5 minutes) oxidase activity (data not shown). Superoxide release stimulated by PMA was not significantly inhibited by C2-ceramide (data not shown), probably because PMA bypasses receptor-mediated signaling to directly stimulate PKC. Similarly to the superoxide results, the release of gelatinase and lactoferrin was inhibited by C2-ceramide but not by dihydro-C2-ceramide, and 10 μM C2-ceramide reduced release of both granule sets to unstimulated levels (Figure 1B-C). To demonstrate that C2-ceramide did not directly affect the oxidase or measurement of granule markers, C2-ceramide was added immediately after FMLP, and no inhibition was observed (data not shown). Notably, the dose response was different for gelatinase and lactoferrin. Lactoferrin release declined at low C2-ceramide concentrations and was significantly inhibited at concentrations as low as 1 μM (Figure 1C), while 5 μM was necessary to significantly inhibit gelatinase release (Figure 1B). This is consistent with observations by others that tertiary granules degranulate more readily than specific granules38 and suggests that tertiary granules may also be more refractory to inhibition. When activating PMNs with cytochalasin B and FMLP, superoxide and lactoferrin release were increased 2- to 3-fold, and gelatinase release increased about 60% compared with G-CSF–primed, FMLP-stimulated cells. However, C2-ceramide incubation resulted in essentially the same inhibition and dose response (data not shown). Previously, we determined that the time of ceramide formation occurred together with the termination of the respiratory burst24 and phagocytosis.7 Inhibition by C2-ceramide suggests that these functions, like phagocytosis, may be terminated by intracellular ceramide accumulation.
Inhibition of PMN superoxide production and degranulation by C2-ceramide.
PMNs were suspended in buffer containing cytochrome C with or without superoxide dismutase (panel A only). PMNs were incubated with C2-ceramide, dihydro-C2-ceramide (control lipid), or buffer for 30 minutes at 22°C. PMNs were then primed with 50 ng/mL G-CSF for 10 minutes at 37°C. FMLP (1 μM) was added, and samples were incubated at 37°C for an additional 5 minutes, then placed on ice. PMNs were removed by centrifugation. (A) Superoxide present in the supernatants was determined by spectroscopic measurement of cytochrome C reduction. (B-C) The markers of specific and tertiary granule release, lactoferrin and gelatinase respectively, were measured in the supernatants by ELISA. Data represent the mean ± SEM of at least 3 experiments. *Significantly different from control;P < .05.
Inhibition of PMN superoxide production and degranulation by C2-ceramide.
PMNs were suspended in buffer containing cytochrome C with or without superoxide dismutase (panel A only). PMNs were incubated with C2-ceramide, dihydro-C2-ceramide (control lipid), or buffer for 30 minutes at 22°C. PMNs were then primed with 50 ng/mL G-CSF for 10 minutes at 37°C. FMLP (1 μM) was added, and samples were incubated at 37°C for an additional 5 minutes, then placed on ice. PMNs were removed by centrifugation. (A) Superoxide present in the supernatants was determined by spectroscopic measurement of cytochrome C reduction. (B-C) The markers of specific and tertiary granule release, lactoferrin and gelatinase respectively, were measured in the supernatants by ELISA. Data represent the mean ± SEM of at least 3 experiments. *Significantly different from control;P < .05.
DAG restoration of function
We previously showed that C2-ceramide inhibits PLD activity during phagocytosis in suspended PMNs7 and during oxidant production in adherent PMNs.24 We surmised that the mechanism of ceramide inhibition of suspended PMN oxidant production and degranulation was also through its inhibition of PLD and resulting decrease in DAG production. To address this proposal, we used the DAG analog DiC10 in an attempt to reverse ceramide inhibition of functional responses. Treating PMNs with DiC10 prior to C2-ceramide treatment restored superoxide production and degranulation stimulated by G-CSF and FMLP (Figure2). In the presence of 10 μM C2-ceramide, 10 μM DiC10 restored superoxide, gelatinase, and lactoferrin release to levels observed when no ceramide was present, and 20 to 30 μM DiC10 increased release to an even greater degree. When no C2-ceramide was present, DiC10 also increased superoxide production and degranulation in G-CSF–primed, FMLP-stimulated PMNs (Figure 2). The results with and without C2-ceramide converged at 20 to 30 μM, suggesting that a limit to the effect of DiC10 was reached. High concentrations (exceeding 30 μM) of DiC10 stimulated PMNs to release superoxide in the absence of other stimulation. However, 10 μM DiC10, a level at which function is fully restored in stimulated PMNs (Figure 2), did not significantly increase oxidant release or degranulation by itself (data not shown). DiC10 also completely restored oxidant production and degranulation in PMNs activated with cytochalasin B and FMLP (data not shown). DiC10 reversal of C2-ceramide inhibition indicates that the PLD pathway is likely to be involved in oxidant production and degranulation and that C2-ceramide inhibition of these functions is a result of its effect on PLD.
Restoration of PMN oxidant production and degranulation by DAG analog DiC10.
PMNs were treated as in Figure 1, with the exception that after ceramide incubation DiC10 was added and the cells were incubated for an additional 15 minutes at 22°C, followed by stimulation with G-CSF and FMLP. Data represent the mean ± SEM of at least 3 experiments.
Restoration of PMN oxidant production and degranulation by DAG analog DiC10.
PMNs were treated as in Figure 1, with the exception that after ceramide incubation DiC10 was added and the cells were incubated for an additional 15 minutes at 22°C, followed by stimulation with G-CSF and FMLP. Data represent the mean ± SEM of at least 3 experiments.
C2-ceramide inhibition of PLD activity
We measured PLD activity under conditions that stimulate degranulation and oxidant production in the presence of C2-ceramide to further investigate the involvement of the sphingomyelinase pathway in the activation of PLD. PMNs were labeled with 1-O-[3H]-octadecyl-sn-glycero-3 phosphocholine and stimulated in the presence of ethanol; the accumulation of PEt, a product of PLD under these conditions, was measured. Stimulation of PMNs with G-CSF and FMLP increased PLD activity 3-fold, and activity was inhibited in a dose-dependent fashion by C2-ceramide (Figure 3). Similarly, the PLD activity of PMNs stimulated with cytochalasin B and FMLP increased about 30-fold and was inhibited to unstimulated levels by 10 μM C2-ceramide (Figure 3). These observations are consistent with the hypothesis that oxidant production and degranulation involve the sphingomyelinase and PLD pathways.
C2-ceramide inhibitition of PLD activity in PMNs stimulated for superoxide production and degranulation.
PMNs were labeled with 1-O-[3H]-octadecyl-sn-glycero-3 phosphocholine. PMNs were treated with lipids as in Figure 1, then incubated with 1% ethanol for 5 minutes at 37°C. Cells were then stimulated at 37°C with G-CSF (50 ng/mL, 10 minutes) (panel A) or cytochalasin B (5 μg/mL, 3 minutes) (panel B) followed by FMLP (1 μM, 5 minutes). Cell pellets were extracted with chloroform and methanol. Lipid products were separated by means of TLC, and [3H]-labeled PEt was removed and counted in a scintillation counter. Data represent the mean ± SEM of 4 experiments. *Significantly different from control;P < .05.
C2-ceramide inhibitition of PLD activity in PMNs stimulated for superoxide production and degranulation.
PMNs were labeled with 1-O-[3H]-octadecyl-sn-glycero-3 phosphocholine. PMNs were treated with lipids as in Figure 1, then incubated with 1% ethanol for 5 minutes at 37°C. Cells were then stimulated at 37°C with G-CSF (50 ng/mL, 10 minutes) (panel A) or cytochalasin B (5 μg/mL, 3 minutes) (panel B) followed by FMLP (1 μM, 5 minutes). Cell pellets were extracted with chloroform and methanol. Lipid products were separated by means of TLC, and [3H]-labeled PEt was removed and counted in a scintillation counter. Data represent the mean ± SEM of 4 experiments. *Significantly different from control;P < .05.
Translocation of PLD
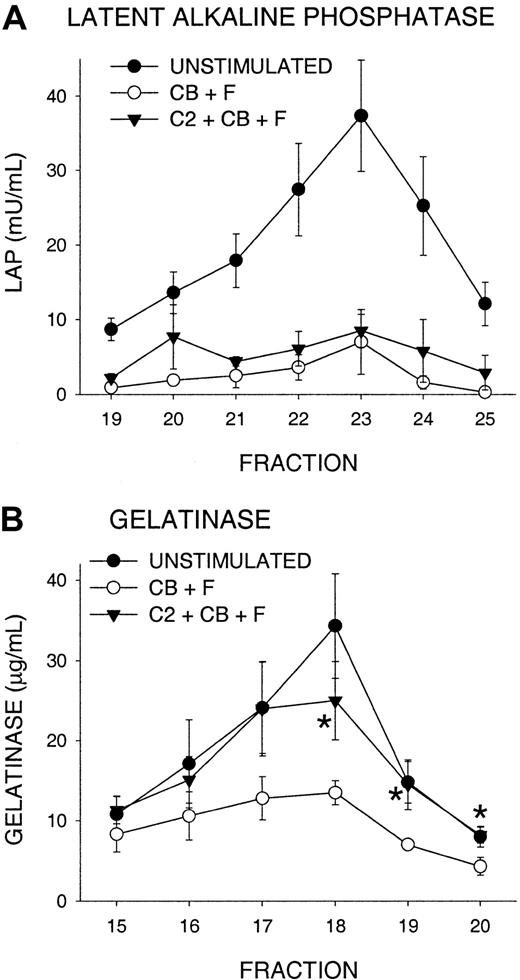
In resting PMNs, PLD is found primarily in secretory vesicles and is mobilized to the plasma membrane upon FMLP stimulation.39 We performed subcellular fractionation and examined release of secretory vesicles to determine whether C2-ceramide inhibited PLD translocation to the plasma membrane. Latent alkaline phosphatase (LAP) was used as a marker, as it is distributed mostly in the secretory vesicles with a smaller proportion in tertiary granules.32 LAP is depleted (becomes nonlatent) during FMLP activation as secretory vesicles fuse with the plasma membrane.35 Pretreatment with C2-ceramide did not significantly affect this mobilization of LAP from secretory vesicles (Figure4A). Thus, PLD translocation was unlikely to have been impaired by ceramide. In the same experiments, C2-ceramide inhibited oxidant production (data not shown) and depletion of gelatinase from tertiary granules (Figure 4B), consistent with ceramide inhibition of gelatinase release.
Failure of C2-ceramide to inhibit mobilization of secretory vesicles.
PMNs were treated with 10 μM C2-ceramide (C2), then activated with 5 μg/mL cytochalasin B (CB) and 100 nM FMLP (F) (triangles) as described in Figure 3. Controls were unstimulated (closed circles) or activated in the same way without C2-ceramide (open circles) and were incubated in parallel. Samples were N2-cavitated, loaded on a 3-layer Percoll gradient, and fractionated. Alkaline phosphatase activity was determined in the presence and absence of Triton X-100, and gelatinase by ELISA. (A). Latent alkaline phosphatase in fractions representing secretory vesicles and plasma membrane. (B) Gelatinase in tertiary granule fractions. Data represent the mean ± SEM of 6 experiments. *C2-ceramide–treated sample (C2 + CB + F) significantly different from stimulated control (CB + F); P < .05.
Failure of C2-ceramide to inhibit mobilization of secretory vesicles.
PMNs were treated with 10 μM C2-ceramide (C2), then activated with 5 μg/mL cytochalasin B (CB) and 100 nM FMLP (F) (triangles) as described in Figure 3. Controls were unstimulated (closed circles) or activated in the same way without C2-ceramide (open circles) and were incubated in parallel. Samples were N2-cavitated, loaded on a 3-layer Percoll gradient, and fractionated. Alkaline phosphatase activity was determined in the presence and absence of Triton X-100, and gelatinase by ELISA. (A). Latent alkaline phosphatase in fractions representing secretory vesicles and plasma membrane. (B) Gelatinase in tertiary granule fractions. Data represent the mean ± SEM of 6 experiments. *C2-ceramide–treated sample (C2 + CB + F) significantly different from stimulated control (CB + F); P < .05.
Effect of phosphatase inhibitor on superoxide production
Ceramide activates a serine-threonine phosphatase whose effects can be inhibited by 0.1 to 10 nM okadaic acid, an inhibitor of serine-threonine phosphatases.40 We used okadaic acid to attempt to restore superoxide production in C2-ceramide–treated PMNs. No significant reversal of ceramide inhibition was obtained with 0.1 to 300 nM okadaic acid (data not shown), leading us to conclude that an indirect effect of ceramide on protein phosphorylation was not responsible for inhibition of PLD.
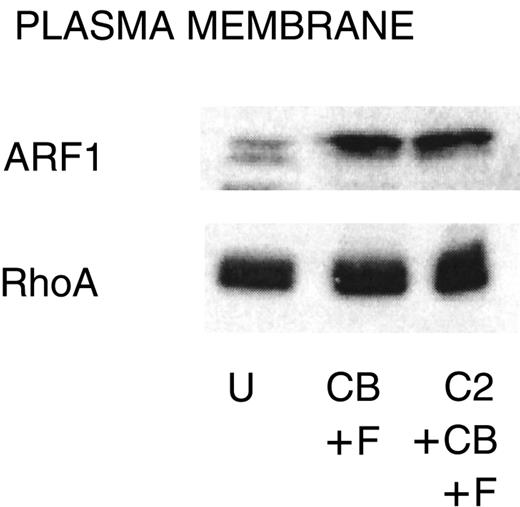
Translocation of ARF1 and RhoA
The small GTPases ARF1 and RhoA are regulators of PLD,41 and the amounts of these proteins associated with the plasma membrane increase with stimulation in PMNs and HL-60 cells.29,42 C2-ceramide (50 μM) inhibits this translocation in HL-60 cells29; therefore, we determined whether the same mechanism operates in PMNs. A concentration of C2-ceramide (10 μM) that completely inhibits function in PMNs (Figure 1) had no effect on the amount of ARF1 and RhoA associated with the membrane (Figure 5).
Failure of C2-ceramide to inhibit ARF1 or RhoA association with the plasma membrane.
PMNs were treated with C2-ceramide and stimulated as indicated for Figure 4. Cells were probe sonicated and fractionated on 15% to 35% discontinuous sucrose gradients as described in “Materials and methods” to isolate plasma membrane. Equal amounts of protein were run on 15% SDS-PAGE, and Western blots were probed with antibody against ARF1 or RhoA. U indicates unstimulated; CB, cytochalasin B; F, FMLP, and C2, C2-ceramide. One representative experiment of 3.
Failure of C2-ceramide to inhibit ARF1 or RhoA association with the plasma membrane.
PMNs were treated with C2-ceramide and stimulated as indicated for Figure 4. Cells were probe sonicated and fractionated on 15% to 35% discontinuous sucrose gradients as described in “Materials and methods” to isolate plasma membrane. Equal amounts of protein were run on 15% SDS-PAGE, and Western blots were probed with antibody against ARF1 or RhoA. U indicates unstimulated; CB, cytochalasin B; F, FMLP, and C2, C2-ceramide. One representative experiment of 3.
Translocation of PKC
DAG, the downstream product of PLD, is an activator of PKC; therefore, we tested the effect of C2-ceramide on PKC-β, an isomer associated with oxidant production and p47phox phosphorylation.43 During PMN activation, PKC-β translocates from the cytosol to the plasma membrane.43 In addition, there is a small amount of PKC-α in PMNs, and this kinase regulates PLD in some systems.28 Therefore, we also looked for a ceramide effect on translocation of PKC-α. The association of either protein kinase with the plasma membrane was not affected by C2-ceramide treatment (data not shown). This observation was consistent with the lack of effect by C2-ceramide on oxidant production stimulated by directly activating PKC with phorbol ester.
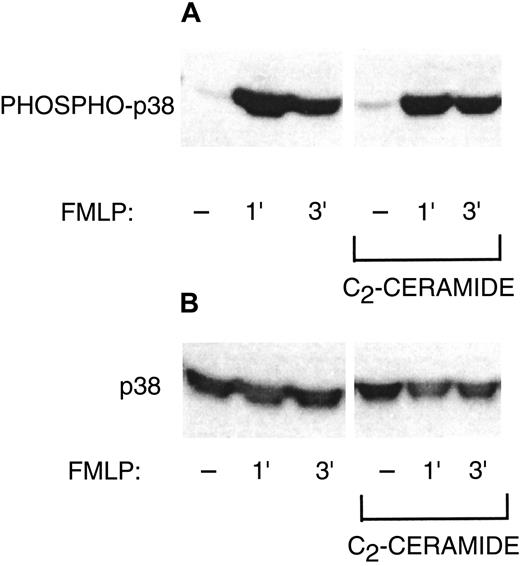
Phosphorylation of p47phox and p38 MAP kinase
Although ceramide inhibition of PLD may be a sufficient explanation for inhibition of the oxidase, we also wished to investigate other possible targets of ceramide. C2-ceramide inhibits phosphorylation and activation of ERK1 and ERK2 during PMN FMLP activation or phagocytosis,1 while sphingosine inhibits translocation of PKC-δ and Raf-1.4 Therefore, we studied phosphorylation of 2 proteins known to be a part of NADPH oxidase activation: p47phox and p38 MAP kinase. Phosphorylation of p47phox occurs during oxidant production.18 The p47phox phosphorylation was enhanced by PMN activation with G-CSF and FMLP, and C2-ceramide did not inhibit this phosphorylation (Figure 6A). Figure 6B demonstrates equal amounts of p47phox among the samples in Figure 6A. Translocation of p47phox to the plasma membrane is also associated with oxidase activation. Similarly, C2-ceramide had no inhibitory effect on the amount of p47phox in the plasma membrane (Figure 6C). The p38 MAP kinase, which is capable of in vitro phosphorylation of p47phox, was phosphorylated, indicating activation during G-CSF and FMLP stimulation (Figure 7). The p38 phosphorylation was not inhibited by C2-ceramide. Therefore, NADPH oxidase inhibition was not a direct result of ceramide effects on p47phox or p38 MAP kinase, but could be attributed to inhibition of PLD activity.
Failure of C2-ceramide to inhibit p47phox phosphorylation or translocation.
(A) PMNs were labeled with H3[32P]O4, washed, and exposed to 10 μM C2-ceramide or buffer for 30 minutes at 22°C. Cells were then stimulated at 37°C with 50 ng/mL G-CSF (10 minutes) and then 1 μM FMLP (5 minutes) or 100 ng/mL PMA (5 minutes) as indicated. PMNs were lysed with 1% Igepal CA-630, and p47phox was immunoprecipitated. Samples (equal volumes) were run on 10% SDS-PAGE and exposed to x-ray film. One representative experiment of 3. (B) Samples (equal volumes) from panel A were loaded on a 25-cm-tall 10% SDS-PAGE gel to separate p47phox from the 55-kd IgG band. Proteins were transferred to PVDF, and the membrane was probed with anti-p47phox to show equivalent loading. (C) PMNs were treated with C2-ceramide and stimulated as indicated for panel A. Cells were probe sonicated and fractionated on 15% to 35% discontinuous sucrose gradients as described in “Materials and methods” to isolate plasma membrane. Equal amounts of protein were run on 10% SDS-PAGE, and Western blots were probed with antibody against p47phox. One representative experiment of 3. U indicates unstimulated; G, G-CSF; F, FMLP; C2, C2-ceramide; and P, PMA.
Failure of C2-ceramide to inhibit p47phox phosphorylation or translocation.
(A) PMNs were labeled with H3[32P]O4, washed, and exposed to 10 μM C2-ceramide or buffer for 30 minutes at 22°C. Cells were then stimulated at 37°C with 50 ng/mL G-CSF (10 minutes) and then 1 μM FMLP (5 minutes) or 100 ng/mL PMA (5 minutes) as indicated. PMNs were lysed with 1% Igepal CA-630, and p47phox was immunoprecipitated. Samples (equal volumes) were run on 10% SDS-PAGE and exposed to x-ray film. One representative experiment of 3. (B) Samples (equal volumes) from panel A were loaded on a 25-cm-tall 10% SDS-PAGE gel to separate p47phox from the 55-kd IgG band. Proteins were transferred to PVDF, and the membrane was probed with anti-p47phox to show equivalent loading. (C) PMNs were treated with C2-ceramide and stimulated as indicated for panel A. Cells were probe sonicated and fractionated on 15% to 35% discontinuous sucrose gradients as described in “Materials and methods” to isolate plasma membrane. Equal amounts of protein were run on 10% SDS-PAGE, and Western blots were probed with antibody against p47phox. One representative experiment of 3. U indicates unstimulated; G, G-CSF; F, FMLP; C2, C2-ceramide; and P, PMA.
Failure of C2-ceramide to inhibit p38 MAP kinase phosphorylation.
(A) PMNs were treated with 10 μM C2-ceramide or buffer for 30 minutes at 22°C. Cells were then stimulated with 50 ng/mL G-CSF for 10 minutes at 37°C, alone or followed by 1 μM FMLP for 1 or 3 minutes. Cells were lysed with Triton X-100 and samples run on 10% SDS-PAGE. Western blots were probed with antibody against phosphorylated p38 MAP kinase. One representative experiment of 3. (B) The Western blot from panel A was stripped and reprobed with anti-p38 MAP kinase that recognizes phosphorylated and nonphosphorylated forms to show equal loading.
Failure of C2-ceramide to inhibit p38 MAP kinase phosphorylation.
(A) PMNs were treated with 10 μM C2-ceramide or buffer for 30 minutes at 22°C. Cells were then stimulated with 50 ng/mL G-CSF for 10 minutes at 37°C, alone or followed by 1 μM FMLP for 1 or 3 minutes. Cells were lysed with Triton X-100 and samples run on 10% SDS-PAGE. Western blots were probed with antibody against phosphorylated p38 MAP kinase. One representative experiment of 3. (B) The Western blot from panel A was stripped and reprobed with anti-p38 MAP kinase that recognizes phosphorylated and nonphosphorylated forms to show equal loading.
Discussion
We have demonstrated in this study that C2-ceramide inhibited both PMN degranulation and NADPH oxidase activity. In turn, the DAG analog DiC10 restored function when used at concentrations found incapable of activating PMNs themselves, indicating that the ceramide effect on PLD was bypassed by replacement of its downstream product DAG. DAG probably then contributed to granule-membrane fusion. PLD activity, stimulated either by G-CSF and FMLP or by cytochalasin B and FMLP, was inhibited by the same concentrations of C2-ceramide shown to inhibit oxidase function and degranulation. Similarly, C2-ceramide inhibits PLD activity stimulated by PMN phagocytosis of IgG-opsonized erythrocytes or in adherent PMNs stimulated by FMLP.7,24C2-ceramide inhibits FMLP-stimulated PLD activity in PMN-like differentiated HL-60 cells as well as in a cell-free system.29 Others have linked PLD activation with oxidant production and degranulation.6,8,16 44 Therefore, we concluded that C2-ceramide inhibition of these functions is through inhibition of PLD.
PLD is activated by small GTPases of the ARF and Rho families, perhaps synergistically with other activators such as PKC-α or phosphatidylinositol 4,5-bisphosphate.41 In PMN-like differentiated HL-60 cells, the mechanism for C2-ceramide inhibition of PLD has been found to be inhibition of translocation of ARF1 and RhoA from the cytosol to the membrane fraction.29Similarly, ARF1 and RhoA translocate to the plasma membrane during stimulation of PMNs.42 However, we were unable to demonstrate ceramide inhibition of this translocation, possibly because Abousalham et al29 used 50 μM C2-ceramide, while we used 10 μM, the concentration that inhibited superoxide release and degranulation. Our data are consistent with those of Venable et al,28 who found no ceramide inhibition of PKC-α translocation but observed that C2-ceramide inhibited the PKC-α–mediated increase in PLD activity. Their data indicated that ceramide might inhibit the interaction of PKC-α with the ARF1-PLD complex in the plasma membrane. In our studies, translocation of proteins was intact in the presence of C2-ceramide, also suggesting that the target for ceramide is in the plasma membrane.
C2-ceramide inhibition of function is consistent with a negative modulatory role of endogenous ceramide. Previously, we determined that ceramide formation correlated with the termination of the respiratory burst in adherent PMNs24 and of phagocytosis of opsonized red cells by PMNs.7When PMNs in suspension were either activated with FMLP or primed with FMLP followed by challenge with antibody-coated red cells, plasma membrane–associated neutral sphingomyelinase activity rose significantly at a time when both the respiratory burst23and phagocytosis7 were terminated, respectively. In this model, we postulate that sphingomyelinase is activated by agonists along with, or soon after, effectors of phagocytosis, oxidant production, and degranulation. In suspended PMNs, phosphorylation, kinase and lipase activation, and molecular translocation begin within 1 minute after stimulation. Maximum rates of phagocytosis, oxidase activity, and degranulation are achieved within 5 minutes, after which these processes slow or cease.1,4,7,26,36,38,45 Ceramide, the product of sphingomyelinase, begins to accumulate in the cell with a time lag as compared with the initiators of function7 24and, reaching significant levels several minutes later, may result in PLD inhibition and termination of function.
The results in the present study demonstrate differential C2-ceramide inhibition of organelle release: lactoferrin release is most potently inhibited by ceramide, gelatinase less so, and secretory vesicles not at all. This is the reverse order of controlled exocytosis shown by Sengelov et al,38 where PMNs were stimulated by FMLP or manipulation of cytosolic calcium, and secretory vesicles were most readily released, followed by tertiary (gelatinase) and secondary (lactoferrin) granules. These observations suggest that easily mobilizable granules may be more difficult to inhibit, perhaps relating to different signaling pathways, levels of PLD involvement, or targets of ceramide inhibition involved in granule mobilization.
Various studies have shown that ERK1 and ERK2 are involved in PMN oxidase activation, possibly by phosphorylating p47phox.3,45,46 C2-ceramide inhibits ERK activation in phagocytosing PMNs.7 Additionally, C2-ceramide treatment of PMNs inhibits activation of p21-activated kinases, which may phosphorylate p47phox.21However, we found that C2-ceramide did not substantially inhibit p47phox phosphorylation, suggesting that ERK or p21-activated kinase is responsible for little or no phosphorylation of p47phox. Indeed, mutation of ERK target serines 345 and 348 to alanines has no significant effect on oxidase activity,18 and MEK inhibitor PD098059 does not consistently block superoxide production (unpublished observations, April 1999, and Kuroki and O'Flaherty47). Our data do not rule out the possibility that p47phox is inactivated by inhibiting ERK phosphorylation of a particular residue while other residues are normally phosphorylated, but even so, this would be consistent with a downstream effect of PLD inhibition. Translocation of PKC-β, which phosphorylates p47phox, was not affected by C2-ceramide, consistent with the lack of effect of C2-ceramide on p47phox phosphorylation.
C2-ceramide did not inhibit phosphorylation and activation of p38 MAP kinase. The evidence for p38 involvement in NADPH oxidase activation includes inhibition of superoxide production by SB203580 (unpublished observations, April 1999, and Rane et al22) and in vitro phosphorylation of p47phox by p38 MAP kinase.2 Thus, p38 regulation of the oxidase appears to be separate from the PLD pathway inhibited by ceramide.
Although C2-ceramide did not noticeably affect phosphorylation or translocation of p47phox, PKC-β, or activation of p38 MAP kinase, we cannot exclude additional possible effects of ceramide. C2-ceramide inhibits translocation of Rab4, implicated in exocytosis, and Rho, which may regulate the cytoskeleton in addition to (or through) its effect on PLD.29,48-50However, we previously found that C2-ceramide did not inhibit chemotaxis,7 suggesting that at least part of the cytoskeleton is functional.
The data presented suggest that accumulation of endogenous ceramide may participate in the termination of oxidant release and degranulation as well as phagocytosis. Exogenous C2-ceramide may inhibit release of reactive oxygen intermediates to the extracellular environment, while not necessarily inhibiting assembly of oxidase in the phagolysosome or on granule membranes. However, C2-ceramide also prevents formation of the phagolysosome by inhibiting ingestion and possibly also granule membrane fusion with the phagolysosome, believed to be mediated by DAG produced by PA phosphohydrolase from the PLD product PA.30
Supported by National Institutes of Health grants AI20065 (L.A.B.) and DK41487 and DK39255 (J.A.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laurence A. Boxer, University of Michigan, Dept of Pediatrics, Hematology/Oncology, L2110 Women's Hospital, Box 0238, 1500 E Medical Center Dr, Ann Arbor, MI 48109; e-mail:laboxer@med.umich.edu.



![Fig. 3. C2-ceramide inhibitition of PLD activity in PMNs stimulated for superoxide production and degranulation. / PMNs were labeled with 1-O-[3H]-octadecyl-sn-glycero-3 phosphocholine. PMNs were treated with lipids as in Figure 1, then incubated with 1% ethanol for 5 minutes at 37°C. Cells were then stimulated at 37°C with G-CSF (50 ng/mL, 10 minutes) (panel A) or cytochalasin B (5 μg/mL, 3 minutes) (panel B) followed by FMLP (1 μM, 5 minutes). Cell pellets were extracted with chloroform and methanol. Lipid products were separated by means of TLC, and [3H]-labeled PEt was removed and counted in a scintillation counter. Data represent the mean ± SEM of 4 experiments. *Significantly different from control;P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1434/6/m_h80422119003.jpeg?Expires=1767707919&Signature=DqnTT~lnDle4fCHKiGEQ8wY6-eNiOYFLmRI6jIZ~BmNS3biANHXk75hSE~e8UCm2wav7eXsqqBf6X1~CG69f3fHgw3YYcyo5mOPFO~rsLtoxqdFLCRcZqIe-KV1cxYBrupalgbjTs6Aws34gWgJ9oEInbyoguBPvih70JOvevUl5HK1365A5W55q34N1rg3ZFRRhp8YqM7ouTJmZwX3-NfJl3FiWlWoAxgZspnnKQS2ZPX6YWcczNZNWf8kcj3oTAMEebNUKtb~36Z5iDws3e75-9--yB6NVtGteAaCOsOAlaxK2qHFrK6Ejmfzmo4vtfmuh-ULGEFL~M7UM3N786A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Failure of C2-ceramide to inhibit p47phox phosphorylation or translocation. / (A) PMNs were labeled with H3[32P]O4, washed, and exposed to 10 μM C2-ceramide or buffer for 30 minutes at 22°C. Cells were then stimulated at 37°C with 50 ng/mL G-CSF (10 minutes) and then 1 μM FMLP (5 minutes) or 100 ng/mL PMA (5 minutes) as indicated. PMNs were lysed with 1% Igepal CA-630, and p47phox was immunoprecipitated. Samples (equal volumes) were run on 10% SDS-PAGE and exposed to x-ray film. One representative experiment of 3. (B) Samples (equal volumes) from panel A were loaded on a 25-cm-tall 10% SDS-PAGE gel to separate p47phox from the 55-kd IgG band. Proteins were transferred to PVDF, and the membrane was probed with anti-p47phox to show equivalent loading. (C) PMNs were treated with C2-ceramide and stimulated as indicated for panel A. Cells were probe sonicated and fractionated on 15% to 35% discontinuous sucrose gradients as described in “Materials and methods” to isolate plasma membrane. Equal amounts of protein were run on 10% SDS-PAGE, and Western blots were probed with antibody against p47phox. One representative experiment of 3. U indicates unstimulated; G, G-CSF; F, FMLP; C2, C2-ceramide; and P, PMA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1434/6/m_h80422119006.jpeg?Expires=1767707919&Signature=GvkgQbX2STEkQYrqn6Iot~QqfICPhadvVhiFBU5RqQTFdtYEQrbL3jl28hQ~yBVTIQMR2jOw0KryiUnwg2XOIHuETzCM8X435ADHSNrCUEjJRGfer9Ys4J-d8VLtmiPXA6R-YBNPQwauk6tdh-e5cMJXXVDJidpt5ngdzOOLo7zM4uOpH~VGRWhofy~xn~evSBpYSfuLmo4I1pIz0zMCHH6IhMCf-RbEu-6VDnU4ageS5k172uTmzSPrJr1Z53Mla57IReiYWx9n1Iw5RJZCXE6IruHmpXcV4UBX6iFUAz5ftWXdjswaZMsd5qLFtc8XfdRvlI4fOgGTYxyHc6dpsg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal