It has been demonstrated that the chromosomal translocation t(7;11)(p15;p15) in patients with human acute myelogenous leukemia (AML) and chronic myelogenous leukemia (CML) invariably involves fusion of the nucleoporin gene, NUP98, on chromosome 11 and the class 1 HOX gene, HOXA9, on chromosome 7, and that the fusion gene NUP98-HOXA9 is an important gene in myeloid leukemogenesis. Here are reported 2 novel chromosome 7p15 targets of the t(7;11)(p15;p15) chromosomal translocation in 2 patients with CML and myelodysplastic syndrome (MDS). Southern blot and polymerase chain reaction (PCR) analyses of leukemia cell DNA failed to show rearrangement of HOXA9,whereas NUP98 was found to be rearranged in both cases. Reverse transcription-PCR analysis using a NUP98 primer and a degenerate primer corresponding to the third helix of the homeodomain of HOXA demonstrated that NUP98 was fused in-frame to HOXA11 in the patient with CML and toHOXA13 in the patient with MDS. The chromosomal breakpoints on 7p15 were located within introns of HOXA11 orHOXA13 genes. In both patients chimericNUP98-HOXA9 transcripts were also observed. These findings suggest that AbdB-type HOXA genes are common targets of t(7;11)(p15;p15) chromosomal translocations and that a single translocation can produce more than oneNUP98-HOXA fusion gene, presumably because of altered splicing.
Introduction
Chromosomal translocations are frequently associated with human leukemias and sarcomas.1 Various transcription factor genes are involved in these translocations. As a result of the gene fusion, chimeric transcription factors, which acquire novel function, are synthesized.2 Translocations involving chromosome 11p15 have been observed in patients with human acute myelogenous leukemia (AML), myelodysplastic syndrome (MDS), and chronic myelogenous leukemia (CML) and in one patient with T-cell acute lymphoblastic leukemia (T-ALL), with the breakpoint commonly occurring within the nucleoporin gene, NUP98.3-14 These translocations result in a fusion gene, consisting of NUP98and a target gene on a number of specific chromosomes. The genes commonly identified as fused to NUP98 are homeobox genes and include PMX1 on 1q23,3HOXD13 on 2q31,4 and HOXA9 on 7p15.5-8
Numerous HOX genes have been shown to have leukemogenic potential,15 and HOXA9 in particular appears to be important in myeloid leukemogenesis. Studies of gene expression in patients with AML suggest that the overexpression of HOXA9is associated with poor prognosis.16Hoxa7 andHoxa9 are the frequent targets of retroviral integration in the murine BXH2 myeloid leukemias.17 Murine bone marrow transplantation experiments involving the transfer of murine hematopoietic cells overexpressing Hoxa9 induce AML after a long latency period.18 In addition, constitutive expression of Hoxa9 immortalizes myeloid progenitors in vitro.19
We describe here 2 novel breakpoints within the HOXA cluster on 7p15 in 2 patients with CML or MDS and the chromosomal translocation t(7;11)(p15:p15). We demonstrate that HOXA11 andHOXA13 genes were involved in the translocation breakpoints and that NUP98-HOXA11 or NUP98-HOXA13 fusion gene mRNAs were synthesized. In addition, we present evidence to suggest that an NUP98-HOXA9 fusion gene was also transcribed, and we suggest this was attributed to alternative splicing.
Materials and methods
Patient material
Leukemia cells were obtained from a 58-year-old patient (Y) with CML associated with the chromosomal translocation t(7;11)(p15;p15). Details of the patient's clinical findings and karyotypic analysis will be reported.20 Cytogenetic and hematologic details of patients S have been described previously (patient 1 in7). Informed consent was obtained from both patients in accordance with the tenets of the Declaration of Helsinki. Approval was obtained from the Institutional Review Board for these studies.
DNA extraction and Southern blot analysis
High-molecular-weight genomic DNA was extracted from frozen leukemic cell suspensions. Samples consisting of 5 μg genomic DNA were subjected to restriction endonuclease digestion, agarose gel electrophoresis, Southern blot transfer, and hybridization as described previously.21 A 350-bp HaeIII fragment of the NUP98 gene was used as a probe to detect rearrangements of NUP98.5 Probes used to detect rearrangements of HOXA11 or HOXA13 were generated by genomic polymerase chain reaction (PCR) using primers specific for the introns and the second exon of each gene (Figure 2B).
Reverse transcription–polymerase chain reaction
Total RNA was extracted from leukemic cell suspensions using RNAzol (TelTest, Friendswood, TX). Reverse transcription (RT)–PCR was carried out as previously described.3 Hemi-degenerate RT-PCR was performed using a NUP98 forward 1 (5′-CTTGGTGCTGGACAGGCATC-3′) primer and a HOXA degenerate primer corresponding to the third helix of the HOXAhomeodomain (5′-(A/C)A(C/T)(C/G/T)C(G/T)(C/G)G(G/T)GTT(C/T)TG(A/G)AACCA-3′) in a Gene Amp 9600 thermal cycler (Perkin-Elmer, Norwalk, CT) with the following parameters: 94°C for 30 seconds, 55°C for 1 minute, and 72°C for 2 minutes, for a total of 35 cycles. PCR products were cloned into the pGEM-T easy vector (Promega, Madison, WI), and individual colonies were sequenced. PCR primers used for gene specific RT-PCR were as follows: NUP98 forward-2, 5′-CGGGATCCGCACAAATACCAGTGGGAATAG-3′; HOXA7 reverse, 5′-TGGGCGATTTCAATGCGG-3′; HOXA9 reverse, 5′-GGGCACCGCTTTTTCCGAGTG-3′; HOXA10 reverse, 5′-TGTCTGGTGCTTCGTGTAGG-3′; HOXA11 reverse, 5′-CAGCCGCTGGAGTCTTAGAGGAGTG-3′; and HOXA13 reverse, 5′-CGTATTCCCGTTCAAGTTC-3′.
Long-distance polymerase chain reaction
Samples consisting of 100 ng tumor DNA were used in the PCR in a 50-μL reaction volume containing dNTPs (25 nmol each), forward and reverse primers (30 pmol each), and 1× buffer 2 and enzyme mix (2.5 U) in the Expand Long Template PCR System (Roche, Mannheim, Germany). PCR was carried out in the same apparatus as above using the following parameters: 94°C for 2 minutes followed by 10 cycles of 94°C for 10 seconds, 65°C for 30 seconds, and 68°C for 10 minutes and then 25 cycles of 94°C for 10 seconds, 65°C for 30 seconds, and 68°C for 10 minutes with a 20-second auto extension per cycle. Primers used for long genomic PCR were: NUP98 3R2, 5′-CTCTTGGTGCTGGACAGGCATC-3′; HOXA9 LR3, 5′-GGCACCGCTTTTTCCGAGTGGAGCG-3′; HOXA11 LR1, 5′-CGCTGGAGTCTTAGAGGAGTGGATTTG-3′; and HOXA13 LR1, 5′-CGTGGCGTATTCCCGTTCAAGTTC-3′.
Results
Identification of the novel fusion genes, NUP98/HOXA11and NUP98/HOXA13, in myelogenous leukemias with t(7;11)(p15;p15)
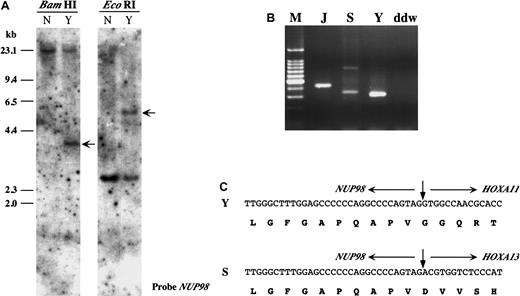
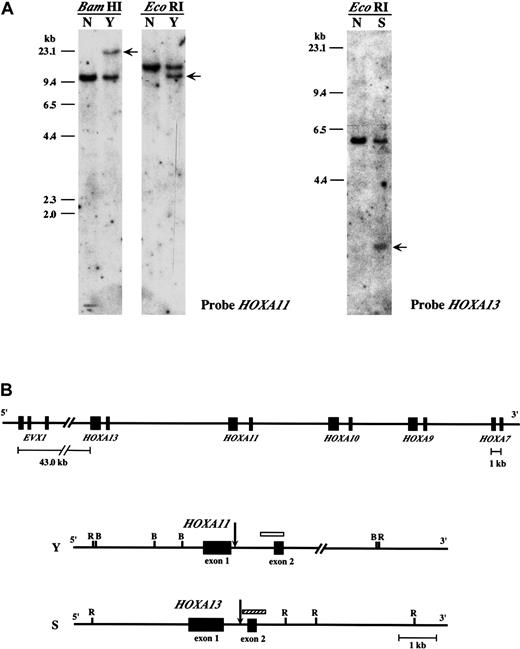
Karyotypic analysis of leukemic cells from a patient with CML (patient Y) revealed a t(7;11)(p15;p15) chromosomal translocation.20 Southern blot analysis using aNUP98 probe demonstrated rearrangement of theNUP98 gene (Figure 1A). However, no rearrangement of the HOXA9 gene was detected (data not shown). In a previous report we identified a patient (patient S) with MDS associated with a t(7;11)(p15;p15) translocation and a similar lack of rearrangement of HOXA9.7 These results suggested that other genes within the HOXA cluster on 7p15 might be involved in the chromosome 7 breakpoint in these patients. To identify candidate HOXA genes, hemi-degenerate RT-PCR was performed using a NUP98 specific primer and a degenerate primer corresponding to the third helix of theHOXA homeodomain. The size of the PCR products was not consistent with NUP98–HOXA9 gene fusion (Figure 1B). Sequence analysis of these PCR products showed that theNUP98 gene was fused in-frame to the HOXA11 gene in leukemic cells from the patient with CML (patient Y) and fused to HOXA13 in leukemic cells from the patient with MDS (patient S) (Figure 1C). Deduced amino acid sequences of the chimeric transcripts include the GLFG motif of the NUP98 protein and the DNA-binding homeodomains of HOXA11 or HOXA13, similar to previously described NUP98 homeobox fusion proteins.3-7 Southern blot analysis revealed rearrangements of HOXA11 orHOXA13 genes in these 2 patients (Figure2A) and indicated that the breakpoints on chromosome 7 were located within the introns of these genes (Figure 2B).
NUP98-HOXA11 and NUP98-HOXA13 fusions in AML and MDS.
(A) Southern blot analysis of the leukemic cell sample derived from t(7;11)(p15;p15) CML (patient Y). Normal human DNA (N) was used as a control. Rearranged bands in a patient with the t(7;11) translocation were detected by BamHI and EcoRI digestion (arrows). (B) Hemi-degenerate RT-PCR.NUP98-HOXA fusion transcripts were detected as products of different molecular sizes. M indicates 100-bp ladder molecular weight marker; J, patient J with known NUP98-HOXA9fusion5; S, patient S; Y, patient Y; ddw, H2O. (C) Nucleotide and deduced amino acid sequences ofNUP98-HOXA11 and NUP98-HOXA13 fusions in Y and S.
NUP98-HOXA11 and NUP98-HOXA13 fusions in AML and MDS.
(A) Southern blot analysis of the leukemic cell sample derived from t(7;11)(p15;p15) CML (patient Y). Normal human DNA (N) was used as a control. Rearranged bands in a patient with the t(7;11) translocation were detected by BamHI and EcoRI digestion (arrows). (B) Hemi-degenerate RT-PCR.NUP98-HOXA fusion transcripts were detected as products of different molecular sizes. M indicates 100-bp ladder molecular weight marker; J, patient J with known NUP98-HOXA9fusion5; S, patient S; Y, patient Y; ddw, H2O. (C) Nucleotide and deduced amino acid sequences ofNUP98-HOXA11 and NUP98-HOXA13 fusions in Y and S.
Breakpoints of the
HOXA11 and HOXA13 genes.(A) Southern blot analysis of leukemic cell samples derived from 2 patients with the t(7;11)(p15;p15) translocation (Y and S). (Left panel) Rearrangement of HOXA11 (arrows) was detected byBamHI and EcoRI digestion in patient Y. (right panel) Rearrangement of HOXA13 (arrow) was detected byEcoRI digestion in patient S. (B) Chromosome breaks on 7p15 in patient Y and S. (Top panel) Overview of the 5′ half of theHOXA cluster on chromosome 7. (bottom panel) Mapping of the breakpoints (arrows) within HOXA11 and HOX13 in patients Y and S, respectively. Locations of the probes used for Southern blot analysis are also shown as open (HOXA11) and hatched (HOXA13) boxes. R indicates EcoRI; B,BamHI.
Breakpoints of the
HOXA11 and HOXA13 genes.(A) Southern blot analysis of leukemic cell samples derived from 2 patients with the t(7;11)(p15;p15) translocation (Y and S). (Left panel) Rearrangement of HOXA11 (arrows) was detected byBamHI and EcoRI digestion in patient Y. (right panel) Rearrangement of HOXA13 (arrow) was detected byEcoRI digestion in patient S. (B) Chromosome breaks on 7p15 in patient Y and S. (Top panel) Overview of the 5′ half of theHOXA cluster on chromosome 7. (bottom panel) Mapping of the breakpoints (arrows) within HOXA11 and HOX13 in patients Y and S, respectively. Locations of the probes used for Southern blot analysis are also shown as open (HOXA11) and hatched (HOXA13) boxes. R indicates EcoRI; B,BamHI.
Simultaneous detection of NUP98-HOXA9 andNUP98-HOXA11 or NUP98-HOXA13 chimeric transcripts
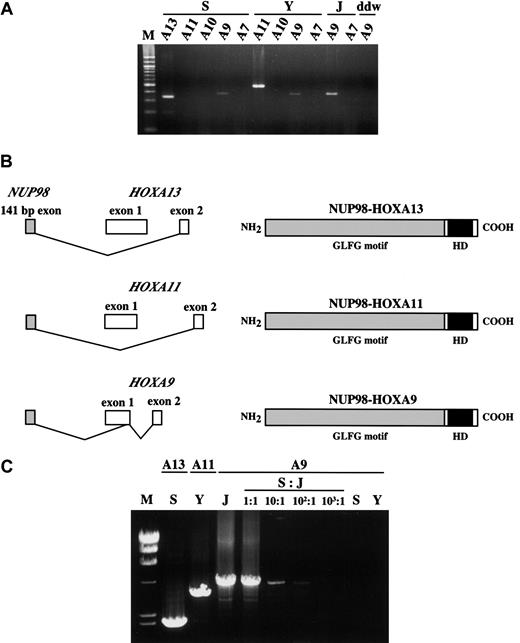
Despite the location of the 7p15 chromosomal breakpoint within theHOXA13 gene and the presence of the NUP98-HOXA13fusion transcript (patient S), evidence suggested aNUP98-HOXA9 chimeric transcript in a previous report.7 We hypothesized that 2 or more chimeras could potentially be produced because of alternative splicing induced by the chromosomal translocation and the densely clustered arrangement ofHOX genes in this chromosomal region. Subsequent RT-PCR analysis of leukemic cells from both patients showed evidence ofNUP98-HOXA9 chimeric transcripts (Figure 3A). In-frame fusions involving theNUP98 and HOXA9 genes and the identical structure of the predicted fusion protein were confirmed by sequence analysis of RT-PCR products generated using NUP98-specific and degenerate HOXA primers cloned into plasmids (Figure 3B). Sequence analysis of the multiple plasmid clones and semiquantitative RT-PCR suggested that these NUP98-HOXA9transcripts were rarely expressed comparing NUP98-HOXA11 orNUP98-HOXA13 in the patients S or Y, respectively (data not shown). We found no evidence of fusion between NUP98and any other HOXA gene located downstream ofHOXA13, nor were NUP98-HOXA7 fusion genes observed in patient J, who had a NUP98-HOXA9 break (Figure3A). To exclude the possibility that minor clones of leukemic cells bearing the NUP98-HOXA9 rearrangement developed in patients S and Y, LD-PCR was carried out. As expected, breakpoint-specific genetic fusion was amplified (Figure 3C). On the other hand, no NUP98-HOXA9 fusion was observed in S or Y samples. Series of dilutions of NUP98-HOXA9–positive DNA by the DNA samples originated from patients S and Y (data not shown) were also amplified, and it was confirmed that the method could detect at least 1% of the subpopulation with NUP98-HOXA9 (Figure 3C). Thus, it is unlikely that there existed a mixed population of leukemic cells with different translocations at HOXA9 and eitherHOXA11 or HOXA13.
Simultaneous detection of the
NUP98-HOXA9 and NUP98-HOXA13 orNUP98-HOXA11 transcripts in patients S and Y. (A) The 378-bp NUP98-HOXA9 products were detected in patients S, Y, and J, the patient with t(7;11) AML with a HOXA9break. However, NUP98-HOXA7 and NUP98-HOXA10chimeras were not detected. In addition, the NUP98-HOXA11chimera was not observed in patient S. ddw indicates water control. (B) Structures of fusion transcripts and predicted proteins ofNUP98-HOXA13, NUP98-HOXA11, andNUP98-HOXA9 of the current patients with leukemia. (C) LD-PCR showed NUP98-HOXA fusion genes. Reverse primers for each HOX gene were indicated at the top, and the sample identity was indicated at the middle. Serial dilutions of J sample by patient S were also subjected to long-distance-PCR to check the detection limit of the NUP98-HOXA9 fusion. M indicates λ/HindIII DNA size marker.
Simultaneous detection of the
NUP98-HOXA9 and NUP98-HOXA13 orNUP98-HOXA11 transcripts in patients S and Y. (A) The 378-bp NUP98-HOXA9 products were detected in patients S, Y, and J, the patient with t(7;11) AML with a HOXA9break. However, NUP98-HOXA7 and NUP98-HOXA10chimeras were not detected. In addition, the NUP98-HOXA11chimera was not observed in patient S. ddw indicates water control. (B) Structures of fusion transcripts and predicted proteins ofNUP98-HOXA13, NUP98-HOXA11, andNUP98-HOXA9 of the current patients with leukemia. (C) LD-PCR showed NUP98-HOXA fusion genes. Reverse primers for each HOX gene were indicated at the top, and the sample identity was indicated at the middle. Serial dilutions of J sample by patient S were also subjected to long-distance-PCR to check the detection limit of the NUP98-HOXA9 fusion. M indicates λ/HindIII DNA size marker.
Discussion
Chromosomal translocations involving 11p15 that result in fusion genes consisting of NUP98 and a target gene are observed in de novo5-9,12 and therapy-related leukemias.3,4,9-11,13 The fusion of NUP98 toHOXA9 is most frequently observed,5-8 and its transforming activity has been demonstrated in hematopoietic and nonhematopoietic cells.22,23 The current study suggests that several AbdB-like HOXA genes may play a role in leukemogenesis when fused to NUP98. Previous studies indicate that the N-terminal domain of NUP98 demonstrates potential transcriptional activity,3,22 suggesting that these chimeric proteins act as oncogenic transcription factors perhaps through abnormal interactions with the HOX cofactors PBX and MEIS.23 Unlike HOXA9, however, HOXA11 and HOXA13 lack the PBX-interaction motif deemed critical for cooperative DNA-binding with PBX,24-26 indicating that these chimeric proteins recognize target DNA sequences in a PBX-independent manner. In support of this, a HOXA9 mutant, incapable of interaction with PBX, nevertheless induced immortalization of myeloid progenitors.19 In addition, it has been shown that HOXA9 overexpression leads to the transformation of primary bone marrow cells through specific collaboration with MEIS1a but not PBX1b.18 Recently, expression of the fusion protein NUP98-HOXD13, which also lacks a PBX-interaction motif, has been shown to result in oncogenic transformation of murine bone marrow cells.27
Mammalian class I HOX genes are arranged in 4 clusters, and each cluster contains 9 to 12 genes.28 Expression of the individual HOX genes within the cluster is tightly regulated in time and space by the presence of regulatory elements within and outside the cluster.29,30 Coexpression ofNUP98-HOXA13 or NUP98-HOXA11 chimeras withNUP98-HOXA9 caused by aberrant splicing may be analogous to the colinear expression of individual Hox genes. Splicing of mRNA can be controlled by any mechanism that alters the relative rates of splice site selection.31 Thus, a splice acceptor site 5′ of HOXA9 exon 1B may be a stronger candidate for the splicing machinery, or the chromosomal translocation may itself alter the balance of competition among potential splice sites. The latter hypothesis is supported in part by the fact that gene fusion betweenHOXA9 exon 1B and any exons of the upstream HOXAgenes (EVX exon 1 through HOXA10 exon 2, including HOXA11 and HOXA13) has never been detected in the normal bone marrow cell, the current patient with t(7;11) leukemia, and any other leukemia cell lines examined, such as HL60, U937, and THP-1 (data not shown). A previous report suggested hybrid transcripts might exist between HOXA9 and otherHOXA genes in human adult and fetal tissues, though the possibility has not been proved.32 Alternatively, recognition of the requisite RNA sequence by splicing factors such as TRA2 may be affected by factors such as structural chromatin changes.33 In addition, we found no RT-PCR evidence of the presence of NUP98-HOXA10 or NUP98-HOXA7chimeras in patient Y, nor of NUP98-HOXA11,NUP98-HOXA10 or NUP98-HOXA7 chimeras in patient S. Moreover, no evidence of fusion of NUP98 with theHOXA7 gene was observed in 3 patients with AML with translocations that resulted in NUP98-HOXA9 fusion transcripts (data not shown). Thus, we postulate that the relative paucity of fusion transcripts involving NUP98 and otherHOXA genes suggests that there may be specific recognition mechanisms leading to a predilection for the HOXA9 splice site.
The amount and stability of each of the fusion mRNAs can also be functionally controlled by transcription termination and polyadenylation of the precursor mRNA.34 Many genes give rise to transcripts that use alternative polyadenylation sites, and it has been suggested that basal polyadenylation factors, splicing factors, and termination factors contribute cell type–specific mechanisms that lead to different 3′-end formation.35 EachHOXA gene has its own polyadenylation site, and this may result in a higher concentration of fusion precursor mRNA containingHOXA13 or HOXA11 sequences than precursor mRNA containing HOXA9 sequences.
The distance between the chromosomal breakpoint and HOXA9exon 1B in patient S was 33 kb. Such long-distance splicing has been described in a chromosomal translocation that resulted inNUP98-PMX1 gene fusion and 2 different chimeric transcripts.3 In that report the functioning chimera made use of the downstream exon, which was located 50 kb downstream of the breakpoint, whereas use of the exon within a few kilobases of the breakpoint resulted in a noncoding transcript. Nevertheless, the distances involved in our patients' translocations might have resulted in lower expression levels of the NUP98-HOXA9 chimeric transcript relative to those of the NUP98-HOXA11or NUP98-HOXA13 fusion genes. The functioning chimeric transcript was less expressed than the nonfunctioning transcript in NUP98-PMX1 fusion (3 and data not shown); therefore, the lower expression level of NUP98-HOXA9does not necessarily diminish its significance in leukemogenesis. Because high-quality antibodies to detect and to differentiate these chimeric proteins are unavailable, the relative amounts of these proteins remain unknown.
A recent report has found an association between a frameshift mutation of HOXA11 and megakaryocytic thrombocytopenia–radio-ulnar synostosis.36 It appears that the mutation abrogates DNA binding of HOXA11, suggesting that HOXA11 function is important in the proliferation and differentiation of early hematopoietic lineages. HOXA13 may also play an important role in hematopoiesis. In conclusion, we have identified the HOXA11 and HOXA13 genes as novel partners for NUP98 gene fusion in 2 patients with leukemia, suggesting the involvement of these genes in leukemogenesis. Our results also suggest that coexpression of different chimeric gene transcripts may be important in the neoplastic transformation of myeloid precursors.
We thank Yuriko Saiki and Miki Jishage for critical comments and Yukari Yamazaki and Mizuko Ohsaka for technical assistance.
Supported in part by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science; a Grant-in-Aid for Scientific Research on Priority Areas (C) from the Ministry of Education, Science, Sports and Culture; and grant 99-23107 from the Princess Takamatsu Cancer Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Takuro Nakamura, Department of Carcinogenesis, The Cancer Institute, Japanese Foundation for Cancer Research, 1-37-1 Kami-ikebukuro, Toshima-ku, Tokyo 170-8455, Japan; e-mail:takuro-ind@umin.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal