It was previously demonstrated that p53 status in human multiple myeloma (MM) cells regulates distinct cell cycle responses to CD40 activation. In this study, the production of vascular endothelial growth factor (VEGF) and migration in MM cells triggered by CD40 activation was examined, and the influence of p53 status in regulating this process was determined. Two human MM cell lines that express wild-type p53 at permissive (28°C) and mutant p53 at restrictive (37°C) temperatures were used as a model system. CD40 activation induces a 4-fold (RPMI 8226) and a 6-fold (SV) increase in VEGF transcripts, respectively, under restrictive, but not permissive, temperatures. VEGF expression is significantly induced after CD40 activation in patient MM cells expressing mutant p53. Increased VEGF transcripts result in increased protein and secretion levels, as evidenced by immunoblotting and enzyme-linked immunosorbent assay. In a double-chamber transmigration assay, CD40 activation of MM cells induced a 3-fold (RPMI 8226) and a 5-fold (SV) increase in migration under restrictive, but not permissive, conditions. A 2- to 8-fold induction in migration of patient MM cells expressing mutant p53 was similarly observed. Transduction of MM cells with a luciferase reporter under the control of a human VEGF promoter further indicated that CD40-induced VEGF expression was mediated through a transcriptional control mechanism. Finally, adenovirus-mediated wild-type p53 overexpression down-regulated CD40-induced VEGF expression and transmigration in MM cells expressing mutant p53. These studies demonstrate that CD40 induces VEGF secretion and MM cell migration, suggesting a role for CD40 in regulating MM homing and angiogenesis.
Introduction
CD40 plays an important role in the proliferation and differentiation of B lymphocytes. Ligation of cell surface CD40 on primary B cells induces homotypic adhesion, proliferation, immunoglobulin isotype switch–secretion, and production of cytokines that coordinate their activation and expansion. Multimerization of CD40 molecules by CD40L or anti-CD40 antibodies enhances the expression of cell surface molecules such as CD80 and CD86, LFA-1, VLA-4, and ICAM-1 on normal and leukemic B cells.1,2 We and others have demonstrated that CD40 is expressed on multiple myeloma (MM) patient cells and in MM cell lines3-6 and that the ligation of CD40 on MM cells up-regulates the expression of cell surface accessory molecules, transforming growth factor (TGF)–β1, and interleukin-6 (IL-6) secretion.6,7 We further demonstrated an induction of Ku86 autoantigen on the cell surface of CD40-activated MM cells that enhanced homotypic tumor cell adhesion and heterotypic binding of tumor cells to bone marrow stromal cells and fibronectin.8 Interestingly, CD40 activation induces the proliferation of some MM cells but triggers growth arrest and apoptosis in others.3,4,9,10 Specifically, we recently demonstrated that the cell cycle response to CD40 activation was p53 dependent because CD40 ligation of MM cells results in either growth arrest and apoptosis or proliferation, depending on the presence or absence of wild-type (wt) p53 function.10
Vascular endothelial growth factor (VEGF) is a key mediator of tumor angiogenesis, and overexpression of VEGF is associated with increased angiogenesis, growth, and metastasis in solid tumors. It may also play a role in the pathogenesis of hematologic malignancies, including MM.11,12 VEGF is expressed and secreted by MM cells and bone marrow stromal cells; moreover, it stimulates IL-6 secretion in bone marrow stromal cells and thereby augments paracrine IL-6–mediated MM cell growth.12 It may also account for the increased microvessel density observed in the BM of patients with MM, which correlates with disease progression and poor prognosis.13 VEGF binds to mononuclear cells, resulting in activation responses and chemotactic activity.14 We recently demonstrated that VEGF directly induces proliferation and triggers trans-filter migration of human MM cells expressing Flt-1 (VEGF receptor 1), suggesting an autocrine VEGF loop in MM.15 VEGF expression can be regulated by cytokines (eg, TGF-β, tumor necrosis factor-α, IL-1β, IL-6) in MM. IL-6 triggers tumor cell growth and survival, and it may promote angiogenesis through the induction of VEGF.16 Most recently, CD40L–CD40 interactions have been shown to induce VEGF expression by endothelial cells and monocytes17 and also by synovial fibroblasts.18 In addition, activation of CD40 induces migration, survival, growth, and neovascularization of Kaposi sarcoma cells in a SCID mouse model, promoting tumor cell growth and vascularization.19 Up-regulation of CD40 was observed in tumor vessels, further supporting a role for CD40 in tumor angiogenesis.
Oncogenes and tumor suppressor genes may control the expression and function of VEGF in human tumors. It has been found that transient transfection of the wt p53 tumor suppressor and of the v-srconcogene exert opposing effects on human VEGF gene promoter activity in human glioblastoma and transformed fetal kidney cells.20Mutant p53 potentiates the protein kinase C-mediated induction of VEGF expression,21 and p53 mutations occur frequently in human MM cell lines22 and are associated with advanced disease.23,24 Most p53 mutations found in human MM are single nucleotide substitutions that result in an amino acid change. For example, point mutations of p53 were detected in 7 of 52 (13%) patients and were specifically associated with more advanced and clinically aggressive MM.23 Gross rearrangements of the p53 gene are infrequent in this disease.25 The role of mutant p53 in promoting tumor angiogenesis in many human solid tumors is established26-28; conversely, the antiangiogenic effect of wt p53 in cancer gene therapy mediates transcriptional repression of VEGF expression.29-32 To date, however, p53-mediated regulation of bone marrow angiogenesis in MM is not well studied.
In this study, we examined whether CD40 activation triggers VEGF secretion and migration in human MM cells, suggesting a role for CD40 in regulating MM homing and angiogenesis. Our studies further demonstrate that p53 status regulates CD40-induced VEGF production and transmigration in human MM cells.
Materials and methods
Cell culture
RPMI 8226 (American Type Culture Collection [ATCC], Manassas, VA)10 and SV33 MM cell lines were cultured in culture medium (10% fetal calf serum [FCS]–RPMI 1640 medium supplemented with 2 mM L-glutamine, 25 μg/mL (μM) Pen/Strep). The p53 gene in the RPMI 8226 line is mutated Glu-285 (GAG) to Lys (AAG).10,22 p53 sequencing was performed on SV MM cell line and patient MM samples, as previously described.10 Both cell lines express wt p53 at permissive (28°C), but not at restrictive (37°C), temperatures. Freshly isolated MM cells (more than 90% CD38+CD45RA−) obtained after informed consent were prepared by antibody (Ab)-mediated negative selection, as previously described,33 using RosetteSep (StemCell Technologies, Vancouver, BC, Canada). Normal B cells were isolated from healthy donor bone marrow, after informed consent, as previously described.34 MM cells expressed CD40, assessed either by flow cytometric analysis or immunoblot analysis. An anti-CD40 monoclonal antibody (mAb; G28.5; ATCC) and a neutralizing antihuman CD40L mAb (5C8; ATCC) were prepared and purified using standard methods.
Northern blot analysis
Northern blot analysis was performed as previously described.34 Total RNA from 107 cells was prepared using RNeasy Mini Kit (Qiagen, Valencia, CA). Each RNA sample was resolved in 1.2% to 1.4% formaldehyde–agarose gels, transferred to Nitropure membrane (MSI, Westborough, MA), and probed with random primer-labeled VEGF cDNA. VEGF cDNA was amplified by reverse transcription–polymerase chain reaction (408 bp,VEGF121; 541bp, VEGF165) from total RNA of RPMI 8226 cells using primer pairs 5′-GAAGTGGTGAAGTTCATGGATGTC-3′ and 5′-CGATCGTTCTGTATCAGTCTTTCC-3′.11 These VEGF cDNAs were purified using agarose gel electrophoresis and were recovered using QIAEX II gel extraction kit (Qiagen, Chatsworth, CA), and they detected VEGF transcripts of approximately 4.4 and 3.7 kb. An oligo probe for housekeeping gene 18S rRNA was used for control loading. VEGF and 18S rRNA expression were quantitated by densitometry of autoradiograms using the Image Quant software program (Molecular Dynamics, Sunnyvale, CA).
Immunoblot analysis
Cell lysates were prepared using standard methods; immunoprecipitation and immunoblot analysis were performed as previously described.35 Reagents included anti-CD40 Ab C-20 (Santa Cruz Biotechnology, Santa Cruz, CA); Ab-5 anti-wt p53 (pAb 1620) mAb recognizing wt p53 and Ab-3 antimutant (mt) p53 (PAb 240) mAb recognizing mt p53 (Oncogene Science, Cambridge, MA); DO-1 horseradish peroxidase (HRP)–conjugated antipantropic p53 mAb (Santa Cruz Biotechnology); anti-hVEGF mAb (R&D Systems, Minneapolis, MN); and DM1A anti-α–tubulin mAb (Sigma, St Louis, MO).
Enzyme-linked immunosorbent assay
Cells (3 × 105/mL) were seeded onto 24-well plates in control medium (RPMI 1640 medium with 2% fetal bovine serum) or CD40-containing medium. Supernatants harvested from control and CD40-activated cell cultures were tested for soluble VEGF by enzyme-linked immunosorbent assay (ELISA) (R&D Systems), according to the manufacturer's instructions. VEGF was expressed as picograms VEGF protein per 5 × 106 cells. The minimum detectable level of VEGF was 10.0 pg/mL (pM).
Transwell migration assay
Cell migration was conducted in 24-well, 6.5-mm internal diameter Transwell cluster plates (Corning Costar, Cambridge, MA). Briefly, cells (105/75 μL) were loaded onto fibronectin (5 μg/mL [μM])–coated polycarbonate membranes (8-μm pore size) separating 2 chambers of a transwell. Medium–1% FCS (600 μL) containing agonist anti-CD40 mAb (G28.5) or sCD40L (Immunex, Seattle, WA), with or without blocking anti-CD40 mAb (M3; Genzyme, Cambridge, MA), neutralizing anti-CD40L mAb (5C8), or anti-VEGF mAb (R&D Systems), was added to the lower chamber of the Transwell cluster plates. Antagonist anti-CD40 mAb M3 blocks CD40-CD40L interaction. After 8 to 16 hours, cells migrating to the lower chamber were counted using a Coulter counter ZBII (Beckman Coulter) and by hemacytometer. Results were comparable using both methods.
Recombinant adenovirus
Adwtp53 expressing wt p53 (kindly provided by Dr Bert Volgestein) and control Adβ-gal recombinant adenoviruses35,36 were produced in 293 cells and were purified by 2 runs of ultracentrifugation through CsCl gradient, as published previously.35 Adenoviruses expressing luciferase cDNA controlled by full-length human VEGF promoter or control luciferase cDNA without any VEGF promoter sequences were generated to allow high transduction efficiency in MM cells. To obtain specific multiplicity of infection (MOI) achieving greater than 85% transduction efficiency for each MM cell line and patient MM sample, control Adβ-gal recombinant adenoviruses were first infected into MM cells at different MOIs (100-1000), and the β-galactosidase activity was quantified by an enhanced β-galactosidase assay kit (Gene Therapy System, San Diego, CA). To obtain transduction efficiencies of more than 85%, MOIs of 800, 200, and 400 for each adenovirus were used to infect RPMI 8226, SV, and patient MM5 cells, respectively.
Luciferase assay
A 2.4-kb KpnI-NheI genomic fragment (−2274 to +745 relative to transcription start site) containing the human VEGF promoter was cloned upstream of firefly luciferase cDNA in a pGL2-basic vector (Promega, Madison, WI), resulting in a pVEGF-Luc reporter construct.37 The DNA fragment containing sequences derived from the human VEGF promoter driving expression of luciferase was excised from pVEGF-Luc, purified, and subcloned into an adenoviral Ad5 left shuttle vector, as previously described.35 Briefly, the DF3-LacZ fragment in the adenoviral shuttle vector pDF3-LacZ was replaced with a DNA fragment containing a VEGF promoter upstream of luciferase cDNA. The resultant adenoviral shuttle vector pAdVEGF-Luc was cotransfected with the adenoviral packaging plasmid JM17 into 293 cells. Recombinant AdVEGF-Luc adenovirus was isolated from a single plaque, propagated in 293 cells, and purified.35 To allow more than 85% infection efficiency, MOIs of 800 and 200 were used for RPMI 8226 and SV MM cells, respectively. The Ad-Luc adenovirus containing luciferase cDNA without any VEGF promoter sequences was generated and used as a control. Twenty-four hours after adenovirus infection, transduced MM cells were left intact or were activated with sCD40L. Sixteen hours after CD40 activation, control and CD40-activated cells were harvested, cells were lysed in buffer, and the protein concentration was determined by Bradford assay. Total protein content was used for the normalization of luciferase activity. Luciferase activity using equal amounts of total cellular protein from each sample was measured using the Monolight 2010 Luminometer (Analytical Luminescence Laboratory, Frederick, MD) at room temperature and was expressed as relative light units (Luciferase Assay System; Promega). Experiments were performed in triplicate, and results were expressed as mean ± SE normalized luciferase activity.
Results
CD40 activation enhances VEGF production by MM cells expressing mutated p53
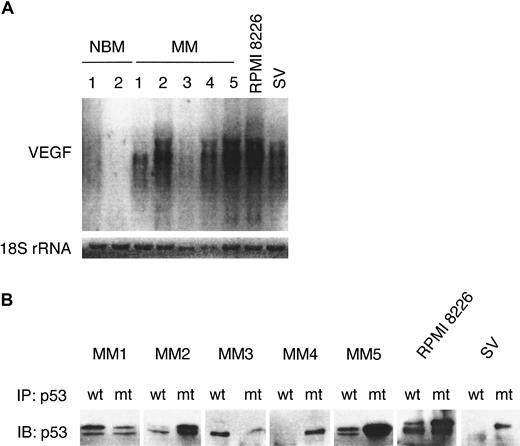
Because many tumors constitutively express VEGF, we first determined the expression of VEGF transcripts by Northern blot analysis in normal B cells from BM aspirates of healthy donors (NBM1-2), in purified MM cells from patients with MM (more than 90% CD38+CD45RA−) (MM1-5), and in MM cell lines RPMI 8226 and SV. Using immunoblot analysis (Figure1A) and direct immunofluorescence flow cytometry (Figure 1B), we confirmed the expression of CD40 in these patient MM cells, MM cell lines, and NBM samples. As shown in Figure2A, VEGF mRNA is expressed in MM cell lines RPMI 8226 and SV and in patient MM cells (MM1, MM2, MM4, MM5), but not in B cells from 2 healthy donors. Northern blotting for 18S rRNA was used as a loading control. The expression of VEGF transcripts in 3 additional NBM was undetectable (data not shown). These results are consistent with previous reports using reverse transcription–polymerase chain reaction to assay VEGF expression.12
CD40 expression in patient MM cells and cell lines.
CD40 expression on normal B cells (NBM1-2), patient MM cells (MM1-5), RPMI 8226, and SV MM cell lines was determined by (A) immunoblotting and (B) flow cytometry. Solid histogram, isotype control; open histogram, CD40.
CD40 expression in patient MM cells and cell lines.
CD40 expression on normal B cells (NBM1-2), patient MM cells (MM1-5), RPMI 8226, and SV MM cell lines was determined by (A) immunoblotting and (B) flow cytometry. Solid histogram, isotype control; open histogram, CD40.
MM cells express VEGF mRNA.
(A) Total RNA (5 μg) from control NBM1-2, patient MM1-5, RPMI 8226, and SV MM cell lines was size separated on a 1.4% agarose-formaldehyde gel, blotted, and probed with a 32P-labeled VEGF cDNA probe that recognizes VEGF transcripts of approximately 3.7 to 4.4 kb. A32P end-labeled oligonucleotide probe for 18S rRNA was used as the loading control. (B) Patient MM1-5 cells and 2 MM cell lines were lysed, immunoprecipitated with wt p53 and mutant (mt) p53 mAbs, and subjected to immunoblotting with an anti-p53-HRP mAb.
MM cells express VEGF mRNA.
(A) Total RNA (5 μg) from control NBM1-2, patient MM1-5, RPMI 8226, and SV MM cell lines was size separated on a 1.4% agarose-formaldehyde gel, blotted, and probed with a 32P-labeled VEGF cDNA probe that recognizes VEGF transcripts of approximately 3.7 to 4.4 kb. A32P end-labeled oligonucleotide probe for 18S rRNA was used as the loading control. (B) Patient MM1-5 cells and 2 MM cell lines were lysed, immunoprecipitated with wt p53 and mutant (mt) p53 mAbs, and subjected to immunoblotting with an anti-p53-HRP mAb.
Because loss or inactivation of the p53 tumor suppressor gene is associated with VEGF overexpression in solid tumors,28,38,39 we next characterized p53 status in these MM samples using conformation-specific mAbs directed against wtp53 (PAb 1620) and mtp53 (PAb 240) for immunoprecipitation, followed by immunoblotting with a pantropic p53 mAb (DO-1) (Figure 2B). Patients MM2, MM4, and MM5, as well as RPMI 8226 and SV MM cell lines, express predominantly mutant p53, whereas patients MM1 and MM3 express wt p53 at 37°C. Sequencing experiments on genomic DNA from these MM samples confirmed p53 mutations: Arg-248 (CGG) to Trp (TGG) in MM2; His-179 (CAT) to Arg (CGT) in MM4; Arg-273 (CGT) to His (CAT) in MM5; and Val-143 (GTG) to Ala (GCG) in SV MM cell line. MM1 and MM3 contain the wt p53 gene. The p53 mutation in RPMI 8226 has been previously reported.10 22 The expression of mutated p53 was easily detected in MM cells that have higher expression of VEGF (MM2, MM4, MM5, RPMI 8226, SV MM) (Figure 2A). In contrast, VEGF expression in patient MM1 and MM3 cells is weaker than that in patient MM samples with mutant p53 (MM2, MM4, MM5).
We have previously characterized the effect of CD40 activation on p53 transactivation in the RPMI 8226 MM line,10 which contains a temperature-sensitive p53 mutation. The SV MM line also contains a previously described temperature-sensitive missense mutation.40 These MM cell lines were, therefore, used as a model system to investigate the effect of CD40 on VEGF expression in human MM. As shown in Figure 3A, VEGF expression was induced 6 hours after CD40 activation with sCD40L (10 μg/mL [μM]) at restrictive (37°C), but not at permissive (28°C), temperatures in RPMI 8226 and SV cells. Because hypoxia easily induces VEGF transcripts in human umbilical vein endothelial cells and fibroblasts, we also exposed MM cells to hypoxia for 24 hours; a 2- to 4-fold increase in VEGF transcripts was observed in these samples (data not shown). In contrast, at permissive temperature (28°C), VEGF expression in both cell lines harboring temperature-sensitive mutant p53 was reduced (Figure 3A, 28°C), consistent with weaker VEGF expression in human lung and breast cancers that express wt p53.28 41 After CD40 activation, a decrease in VEGF mRNA expression was observed only at the permissive temperature when wt p53 was expressed (Figure 3A, 28°C). CD40 activation (24 hours at 37°C) also triggered a 2- to 8-fold induction in VEGF mRNA in patients MM2, MM4, and MM5 with mutant p53 (Figure 3B). When an agonist anti-CD40 G28.5 mAb (at 10 g/mL) was used to activate CD40, similar results were obtained (data not shown).
CD40 activation induces VEGF transcripts in human MM cells expressing mutant p53.
(A) CD40-activated RPMI 8226 (upper panel) and SV (lower panel) MM lines were cultured for indicated intervals (0-48 hours), and total RNA (5 μg) was subjected to Northern blotting using human VEGF cDNA, as described in Figure 1. After probing for VEGF mRNA, the blot was stripped and hybridized with an 18S rRNA probe. The fold induction relative to 18S rRNA as a control was calculated by densitometry. Both cell lines express mt p53 at 37°C (restrictive temperature) and wt p53 at 28°C (permissive temperature). (B) Total RNA (5 μg) from patient MM cells without (−) or with (+) CD40 activation (24 hours) at 37°C was subjected to Northern blotting for VEGF expression. Fold induction of VEGF relative to an 18S rRNA probe was calculated by densitometry.
CD40 activation induces VEGF transcripts in human MM cells expressing mutant p53.
(A) CD40-activated RPMI 8226 (upper panel) and SV (lower panel) MM lines were cultured for indicated intervals (0-48 hours), and total RNA (5 μg) was subjected to Northern blotting using human VEGF cDNA, as described in Figure 1. After probing for VEGF mRNA, the blot was stripped and hybridized with an 18S rRNA probe. The fold induction relative to 18S rRNA as a control was calculated by densitometry. Both cell lines express mt p53 at 37°C (restrictive temperature) and wt p53 at 28°C (permissive temperature). (B) Total RNA (5 μg) from patient MM cells without (−) or with (+) CD40 activation (24 hours) at 37°C was subjected to Northern blotting for VEGF expression. Fold induction of VEGF relative to an 18S rRNA probe was calculated by densitometry.
We next determined whether the induction of VEGF mRNA in CD40-activated MM cells was associated with increased VEGF protein production, confirmed by immunoblotting and ELISA. As can be seen in Figure4A, MM cells express the secreted 28- and 42-kd VEGF protein isoforms encoded by the predominantly expressed mRNA isoforms VEGF121 and VEGF165, and CD40 activation increases the expression of both isoforms. CD40 activation, using either sCD40L or anti-CD40 G28.5 mAb (0-10 μg/mL [μM]), triggered VEGF production in a dose-dependent fashion (Figure 4B). In these experiments, CD40-induced VEGF production in MM cells was not attributable to cellular proliferation because VEGF production was normalized for cell number. Incubation with sCD40L and anti-CD40 G28.5 induced equivalent amounts of VEGF. CD40 activation triggered VEGF secretion in a dose-dependent fashion. For example, the VEGF levels (n = 3) were 1203-1399/5 × 106 cells per pg/mL (pM) (RPMI 8226) and 226-238/5 × 106 cells per pg/mL (pM) (SV) in control cell supernatants versus 3018-3759/5 × 106 cells per pg/mL (pM) (RPMI 8226) and 1021-1205/5 × 106 cells per pg/mL (pM) (SV) in CD40-activated (10 μg/mL [μM]) cell culture supernatants (Figure4B, P < .05). VEGF levels were significantly increased by the addition of sCD40L or anti-CD40 G28.5 mAb in MM cells early (6 hours), and they persisted to 72 hours, compared with those of control cultures with media alone or with isotype control MOPC-21 immunoglobulin (Ig)G (Figure 4C). In contrast, the medium or isotype control MOPC-21 IgG-treated controls demonstrated no significant change in VEGF production.
CD40 induces VEGF secretion in a dose- and time-dependent fashion.
(A) RPMI 8226 and SV MM cell lysates (15 μg) at intervals (0-48 hours) after CD40 activation were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and were transferred to a polyvinylidene difluoride membrane. VEGF protein was detected with anti-VEGF mAb and was visualized by enhanced chemiluminescence. Anti-α–tubulin mAb was used as a loading control. Upper panel, RPMI 8226; lower panel, SV. Similar results were obtained in 2 independent experiments. (B) Cells were cultured at 37°C in triplicate with 0 to 10 μg/mL (μM) sCD40L (circle) or anti-CD40 mAb G28.5 (triangle). (C) Cells were cultured with sCD40L (10 μg/mL [μM]) (circle), anti-CD40 mAb G28.5 (10 μg/mL [μM]) (triangle), medium alone (diamond), or MOPC-21 isotype control IgG (square) at 37°C. VEGF levels in the supernatants at intervals were determined by ELISA. Data are expressed a means ± SE of culture triplicates. Open symbol, RPMI 8226; solid symbol, SV.
CD40 induces VEGF secretion in a dose- and time-dependent fashion.
(A) RPMI 8226 and SV MM cell lysates (15 μg) at intervals (0-48 hours) after CD40 activation were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and were transferred to a polyvinylidene difluoride membrane. VEGF protein was detected with anti-VEGF mAb and was visualized by enhanced chemiluminescence. Anti-α–tubulin mAb was used as a loading control. Upper panel, RPMI 8226; lower panel, SV. Similar results were obtained in 2 independent experiments. (B) Cells were cultured at 37°C in triplicate with 0 to 10 μg/mL (μM) sCD40L (circle) or anti-CD40 mAb G28.5 (triangle). (C) Cells were cultured with sCD40L (10 μg/mL [μM]) (circle), anti-CD40 mAb G28.5 (10 μg/mL [μM]) (triangle), medium alone (diamond), or MOPC-21 isotype control IgG (square) at 37°C. VEGF levels in the supernatants at intervals were determined by ELISA. Data are expressed a means ± SE of culture triplicates. Open symbol, RPMI 8226; solid symbol, SV.
Functional effect of CD40 activation on MM cell transmigration
We recently demonstrated that VEGF induces migration of MM and plasma cell leukemia (PCL) patient cells; moreover, the markedly enhanced migration in PCL cells compared with that in MM cells suggests an important role for VEGF in the transition of MM to PCL.15 Having shown that CD40 activation significantly induces VEGF expression at the mRNA and protein levels in MM cells with mutant p53, we next asked whether CD40 activation induced MM cell migration and, if so, whether CD40-induced transmigration of MM cells is p53 dependent. Cell migration was assayed by measuring the trans-filter migration activity of RPMI 8226 and SV MM cells and of patient MM cells, seeded on membranes precoated with fibronectin (5 μg/mL [μM]). As shown in Figure5A-C, the addition of sCD40L or agonist anti-CD40 G28.5 mAb to medium containing 1% FCS in the lower chamber induced the dose-dependent migration of MM cells at restrictive temperature (37°C), when mutated p53 was functional. For example, the addition of sCD40L or agonist anti-CD40 G28.5 mAb at 10 μg/mL (μM) in the lower chamber for 8 and 16 hours induced a 3-fold and a 4.2-fold activation of migration in RPMI 8226 and SV MM cells, respectively. In contrast, when either sCD40L or anti-CD40 mAb G28.5 was added at the lower chamber of the transwell, a dose-dependent decrease in the migration of MM cells was observed at permissive temperature (28°C), when wt p53 was activated. When patient MM cells were seeded on the filters precoated with fibronectin in the upper chamber of the transwell plate, the addition of sCD40L or anti-CD40G28.5 mAb to the lower chamber for 16 hours induced significant transmigration of MM cells—10-, 1.8-, and 12-fold for patients MM2, MM4, and MM5 with mutant p53, respectively. In contrast, similar treatment resulted in a decrease in patient MM1 and MM3 cells expressing wt p53 (Figure 5D). These results indicate that CD40 significant induced the motility of MM cells expressing mutant, but not wt, p53.
CD40 induces migration in human MM cells expressing mutant p53.
MM cells were plated on a polycarbonate membrane (8 μm pore size) precoated with fibronectin (5 μg/mL [μM]) in the transwell cluster plate and were activated using 0 to 10 μg/mL (μM) sCD40L (A) or anti-CD40 mAb (G28.5) (B) added to the lower chamber. Cells were cultured at restrictive temperature (37°C) with mt p53 or at permissive temperature (28°C) with wt p53. Migrated cells in the lower chamber were counted using a Coulter counter ZBII. (C) Isotype control antibody MOPC 21 (10 μg/mL [μM]) did not induce transmigration in MM cells at restrictive temperature. Results are representative of 2 independent experiments. (■) RPMI 8226 at 37°C; (▤) RPMI 8226 at 30°C; (▪) SV at 37°C; ( ) SV at 30°C. (D) Patient MM1-5 cells were subjected to transmigration assay, using sCD40L (10 μg/mL [μM]) in the lower chamber at 37°C. (▪) MM1; (
) SV at 30°C. (D) Patient MM1-5 cells were subjected to transmigration assay, using sCD40L (10 μg/mL [μM]) in the lower chamber at 37°C. (▪) MM1; ( ) MM2; (■) MM3; (░) MM4; (
) MM2; (■) MM3; (░) MM4; ( ) MM5. Increased transmigration is observed in MM cells expressing mutated p53 (MM2, MM4, MM5), but not in those with wt p53 (MM1, MM3), after CD40 activation.
) MM5. Increased transmigration is observed in MM cells expressing mutated p53 (MM2, MM4, MM5), but not in those with wt p53 (MM1, MM3), after CD40 activation.
CD40 induces migration in human MM cells expressing mutant p53.
MM cells were plated on a polycarbonate membrane (8 μm pore size) precoated with fibronectin (5 μg/mL [μM]) in the transwell cluster plate and were activated using 0 to 10 μg/mL (μM) sCD40L (A) or anti-CD40 mAb (G28.5) (B) added to the lower chamber. Cells were cultured at restrictive temperature (37°C) with mt p53 or at permissive temperature (28°C) with wt p53. Migrated cells in the lower chamber were counted using a Coulter counter ZBII. (C) Isotype control antibody MOPC 21 (10 μg/mL [μM]) did not induce transmigration in MM cells at restrictive temperature. Results are representative of 2 independent experiments. (■) RPMI 8226 at 37°C; (▤) RPMI 8226 at 30°C; (▪) SV at 37°C; ( ) SV at 30°C. (D) Patient MM1-5 cells were subjected to transmigration assay, using sCD40L (10 μg/mL [μM]) in the lower chamber at 37°C. (▪) MM1; (
) SV at 30°C. (D) Patient MM1-5 cells were subjected to transmigration assay, using sCD40L (10 μg/mL [μM]) in the lower chamber at 37°C. (▪) MM1; ( ) MM2; (■) MM3; (░) MM4; (
) MM2; (■) MM3; (░) MM4; ( ) MM5. Increased transmigration is observed in MM cells expressing mutated p53 (MM2, MM4, MM5), but not in those with wt p53 (MM1, MM3), after CD40 activation.
) MM5. Increased transmigration is observed in MM cells expressing mutated p53 (MM2, MM4, MM5), but not in those with wt p53 (MM1, MM3), after CD40 activation.
Induction of VEGF is associated with transmigration of MM cells after CD40 activation
We next tested whether the induction of VEGF is associated with the transmigration activity of MM cells after CD40 activation. RPMI 8226 or SV MM cells were plated on the fibronectin-coated filters separating 2 chambers in the transwell, and sCD40L (10 μg/mL [μM]), with or without a neutralizing anti-VEGF mAb (0-5 μg/mL [μM]), was added to the lower chamber. Eight to 16 hours later, cells in the lower chamber were collected and counted. As shown in Figure 6, anti-VEGF mAb alone (0.05-5 μg/mL [μM]) did not affect the mobility of unstimulated MM cells, but it did inhibit the transmigration of CD40-activated MM cells.
Neutralizing anti-VEGF mAb blocks CD40-induced MM cell migration.
MM cells were subjected to transmigration assay and exposed for 8 hours (RPMI 8226 cells) or 16 hours (SV cells) to CD40 activation by sCD40L (0 or 10 μg/mL [μM]), in the presence or absence of neutralizing anti-VEGF mAb (0-5 μg/mL [μM]) added to the lower chamber of the transwell. Migrated cells collected from the lower chamber were counted. Enhanced transmigration of cells induced by sCD40L was abrogated by neutralizing anti-VEGF mAb. (■) RPMI 8226; (▪) SV.
Neutralizing anti-VEGF mAb blocks CD40-induced MM cell migration.
MM cells were subjected to transmigration assay and exposed for 8 hours (RPMI 8226 cells) or 16 hours (SV cells) to CD40 activation by sCD40L (0 or 10 μg/mL [μM]), in the presence or absence of neutralizing anti-VEGF mAb (0-5 μg/mL [μM]) added to the lower chamber of the transwell. Migrated cells collected from the lower chamber were counted. Enhanced transmigration of cells induced by sCD40L was abrogated by neutralizing anti-VEGF mAb. (■) RPMI 8226; (▪) SV.
We next asked whether the inhibition of CD40-CD40L interaction could block VEGF expression and transmigration induced by the CD40 activation of MM cells. Multiple myeloma cells were treated with sCD40L (10μg/mL [μM]) for 24 hours, with or without a blocking anti-CD40L mAb 5C8, and total RNA (5 μg) from treated MM cells was subjected to Northern blotting for VEGF expression. As shown in Figure7A, 4- and 6-fold inductions of VEGF expression were observed in CD40-activated RPMI 8226 and SV MM cells, respectively. Conversely, anti-CD40L mAb (1 μg/mL [μM]) inhibited VEGF transcript induction by sCD40L (10 μg/mL [μM]) (1-fold reduction for RPMI 8226, 1.4-fold reduction for SV). Higher concentrations of anti-CD40L (10 μg/mL [μM]) completely blocked the induction of VEGF transcripts after CD40 activation. In the transmigration experiments in which sCD40L, with or without a blocking anti-CD40L mAb 5C8, was added to the lower chamber of the transwell, the transmigration activity triggered by sCD40L was inhibited in the presence of anti-CD40L mAb (Figure 7B). When compared with the medium control, the addition of anti-CD40L (5C8) or anti-CD40 (M3) mAbs alone at the lower chamber of the transwell did not induce migration. When an anti-CD40 mAb M3 that blocked CD40-CD40L interaction was used, a similar inhibition of CD40-induced VEGF expression and migration activity was observed (data not shown). VEGF was induced as early as 6 hours after CD40 activation, and the transmigration was observed later (8 hours to overnight), suggesting that the specific induction of VEGF by CD40 is associated with the transmigration of MM cells.
Effect of CD40 activation on VEGF production and migration is inhibited by 5C8 blocking anti-CD40L mAb.
MM cells were cultured in the presence or absence of sCD40L (10 μg/mL [μM]) and a 5C8 blocking anti-CD40L mAb (0-10 μg/mL [μM]) to confirm the specificity of sCD40L-triggered VEGF mRNA expression and cell migration. Blocking mAbs were added to the wells at the initiation of cultures at 37°C. (A) Twenty-four hours after indicated treatments, cells were collected and total RNA was prepared for Northern blotting (upper panel, RPMI 8226; lower panel, SV). Total RNA (5 μg) was used for analysis, and 18 rRNA served as an internal control. All autoradiographs are representative of 2 experiments with similar results. (B) MM cells were subjected to transmigration assay with the addition of sCD40L in the presence or absence of 5C8 blocking anti-CD40L mAb in the lower chamber of the transwell. (■) RPMI 8226 at 37°C; (▪) SV at 37°C. Identical effects of CD40 activation on the induction of VEGF were obtained with anti-CD40 mAb G28.5 (1 to 10 μg/mL [μM]) (data not shown).
Effect of CD40 activation on VEGF production and migration is inhibited by 5C8 blocking anti-CD40L mAb.
MM cells were cultured in the presence or absence of sCD40L (10 μg/mL [μM]) and a 5C8 blocking anti-CD40L mAb (0-10 μg/mL [μM]) to confirm the specificity of sCD40L-triggered VEGF mRNA expression and cell migration. Blocking mAbs were added to the wells at the initiation of cultures at 37°C. (A) Twenty-four hours after indicated treatments, cells were collected and total RNA was prepared for Northern blotting (upper panel, RPMI 8226; lower panel, SV). Total RNA (5 μg) was used for analysis, and 18 rRNA served as an internal control. All autoradiographs are representative of 2 experiments with similar results. (B) MM cells were subjected to transmigration assay with the addition of sCD40L in the presence or absence of 5C8 blocking anti-CD40L mAb in the lower chamber of the transwell. (■) RPMI 8226 at 37°C; (▪) SV at 37°C. Identical effects of CD40 activation on the induction of VEGF were obtained with anti-CD40 mAb G28.5 (1 to 10 μg/mL [μM]) (data not shown).
CD40 activation increases VEGF promoter activity in MM cells
Given that VEGF transcript expression was significantly enhanced by CD40 activation in MM cell lines and patient MM cells, we wanted to determine whether CD40 activation induces increased VEGF transcription. To test the transcriptional activity of VEGF induced by CD40 activation, a 2.4-kb genomic fragment containing human VEGF promoter upstream of luciferase reporter was transduced into RPMI 8226, SV, and patient MM5 cells. The transduction of VEGF-Luc reporter was performed using adenovirus infection at MOI 800, 200, and 400 for RPMI 8226, SV, and patient MM5 cells, respectively, to achieve greater than 80% transduction efficiency. Luciferase activity was assayed using equal amounts of cell lysates 6 hours after CD40 activation. As shown in Figure 8, the baseline luciferase activity by VEGF-Luc reporter was higher in RPMI 8226 cells than in SV cells. The higher baseline VEGF-Luc activity correlates with higher expression of constitutive VEGF transcript (Figure 1). Luciferase activity was significantly increased (approximately 4-fold for RPMI 8226, 6-fold for SV cells, and 8-fold for patient MM5) in the presence of sCD40L (10 μg/mL [μM]) (Figure 8). Similar induction of luciferase activity by VEGF-Luc reporter in the presence of sCD40L was obtained on cell lysates of MM cells 36 hours after CD40 activation (data not shown). These data, therefore, show that sCD40L induces VEGF promoter activity, indicating that increased transcription accounts, at least in part, for the increase of VEGF transcripts.
CD40 activation enhances VEGF promoter activity.
MM cells were first incubated overnight in 2% fetal bovine serum containing RPMI 1640 medium at 37°C. A 2.3-kb full-length human VEGF promoter-luciferase reporter (VEGF-Luc) was transduced into MM cells by adenoviruses. MM cells were cultured for 6 hours in the presence or absence of sCD40L, in duplicate wells for each condition. Luciferase activity was analyzed using equal amounts of total protein for each sample. Mock-transduced MM cells with Luc reporter without any VEGF promoter sequences (Luc) served as a negative control. Luciferase activity was consistently induced (4-fold for RPMI 8226, 5.5-fold for SV, and 6-fold for patient MM5 cells) in sCD40L-activated cells compared with unstimulated cells. Results are representative of 2 independent experiments performed in duplicate cultures. (■) RPMI 8226; (▪) SV; and ( ) patient MM5 cells.
) patient MM5 cells.
CD40 activation enhances VEGF promoter activity.
MM cells were first incubated overnight in 2% fetal bovine serum containing RPMI 1640 medium at 37°C. A 2.3-kb full-length human VEGF promoter-luciferase reporter (VEGF-Luc) was transduced into MM cells by adenoviruses. MM cells were cultured for 6 hours in the presence or absence of sCD40L, in duplicate wells for each condition. Luciferase activity was analyzed using equal amounts of total protein for each sample. Mock-transduced MM cells with Luc reporter without any VEGF promoter sequences (Luc) served as a negative control. Luciferase activity was consistently induced (4-fold for RPMI 8226, 5.5-fold for SV, and 6-fold for patient MM5 cells) in sCD40L-activated cells compared with unstimulated cells. Results are representative of 2 independent experiments performed in duplicate cultures. (■) RPMI 8226; (▪) SV; and ( ) patient MM5 cells.
) patient MM5 cells.
Overexpression of wt p53 in MM cells expressing mutated p53 abrogates CD40-induced VEGF production
Because the overexpression of wt p53 has been shown to down-regulate transcript and promoter activity of VEGF in a dose-dependent manner in human glioblastoma, colon cancer cell lines, leiomyosarcoma, and synovial sarcoma in vitro and in vivo,20,31,32 we sought to determine whether the overexpression of wt p53 in MM cells expressing mutated p53 down-regulates CD40-induced VEGF production. MM cells were first infected with control Adβ-gal or Adwtp53 adenoviruses; 6 hours later, cell lysates were subjected to immunoblotting for p53 expression. As shown in Figure 9A, the overexpression of p53 was induced only by Adwtp53 adenovirus transduction, whereas control Adβ-gal transduction did not change p53 expression. Next, MM cells were incubated with sCD40L (10 μg/mL [μM]), with or without Adwtp53 or control Adβ-gal adenoviruses. Twenty-four hours later, total RNA was prepared and subjected to Northern blotting for VEGF expression. As shown in Figure 9B, VEGF expression was decreased in the Adwtp53-transduced MM cells, consistent with previous studies in other human cancer cell lines.28-31 Although VEGF expression was induced by CD40 in the medium or Adβ-gal–transduced MM cells (4-fold for RPMI 8226; 5.5-fold for SV), no VEGF expression was induced in the presence of Adwtp53 adenoviruses. The level of VEGF transcript expression in CD40-activated MM cells transduced with Adwtp53 was approximately the same as that in control untreated MM cells, and the addition of control Adβ-gal adenoviruses did not result in any further changes in VEGF expression either in the absence or the presence of sCD40L. We performed similar experiments on patient MM cells. CD40-induced VEGF expression in patient MM5 cells was similarly inhibited by the overexpression of Adwtp53 (data not shown). These results provide direct evidence that CD40-induced VEGF expression is regulated by p53 in MM cells.
Adenovirus-mediated wt p53 overexpression down-regulates CD40-induced VEGF mRNA expression.
(A) Six hours after adenovirus infection into MM cells, whole cell lysates (15 μg) were subjected to immunoblotting using HRP-conjugated antipantropic p53 (anti-p53-HRP) mAb. Enhanced expression of p53 was observed in RPMI 8226 and SV MM cell lines. Control adenoviruses expressing the β-gal reporter gene (Adβ-gal) were used at the same MOIs as Adwtp53 (MOIs of 800 and 200 for RPMI 8226 and SV cells, respectively). As an additional control, nontransduced MM cells were used as an internal control. (B) MM cells were cultured with control (Adβ-gal) or wt p53 (Adwtp53) adenoviruses in the presence or absence of sCD40L for 48 hours. At the end of treatment, total RNA (5 μg) was subjected to Northern blotting, and an 18S rRNA transcript was used as a loading control. Steady state mRNA expression was quantitated by densitometry of autoradiograms. (■) RPMI 8226; (▪), SV. (C) Adwtp53-transduced MM cells were subjected to transmigration assay in the presence or absence of sCD40L (10 μg/mL [μM]) in the lower chamber of the transwell.
Adenovirus-mediated wt p53 overexpression down-regulates CD40-induced VEGF mRNA expression.
(A) Six hours after adenovirus infection into MM cells, whole cell lysates (15 μg) were subjected to immunoblotting using HRP-conjugated antipantropic p53 (anti-p53-HRP) mAb. Enhanced expression of p53 was observed in RPMI 8226 and SV MM cell lines. Control adenoviruses expressing the β-gal reporter gene (Adβ-gal) were used at the same MOIs as Adwtp53 (MOIs of 800 and 200 for RPMI 8226 and SV cells, respectively). As an additional control, nontransduced MM cells were used as an internal control. (B) MM cells were cultured with control (Adβ-gal) or wt p53 (Adwtp53) adenoviruses in the presence or absence of sCD40L for 48 hours. At the end of treatment, total RNA (5 μg) was subjected to Northern blotting, and an 18S rRNA transcript was used as a loading control. Steady state mRNA expression was quantitated by densitometry of autoradiograms. (■) RPMI 8226; (▪), SV. (C) Adwtp53-transduced MM cells were subjected to transmigration assay in the presence or absence of sCD40L (10 μg/mL [μM]) in the lower chamber of the transwell.
We also performed transmigration assays on adenovirus-transduced MM cells. When MM cells transduced with Adwtp53 (for 6 hours) were directly seeded on the fibronectin-precoated filter in the upper chamber of the transwell, decreased transmigration of transduced MM cells was observed (RPMI 8226, from 3.4-fold to 1.8-fold; SV, from 4.7-fold to 2.2-fold) in response to sCD40L (10 mg/mL) in the lower chamber when compared with the transmigration of untransduced or control Adβ-gal–transduced MM cells (Figure 9C). Similarly, the overexpression of wt p53 in patient MM5 cells expressing mutated p53 reduced (by 2-fold) the transmigration induced by sCD40L (data not shown). These data indicate that induced VEGF expression and transmigration of MM cells after CD40 activation is p53 dependent.
Discussion
In this study, we defined the role of CD40-CD40L interactions in the regulation of VEGF expression in human MM cell lines and freshly isolated patient MM cells and the influence of p53 status on this process. Our results show that CD40 activation significantly increases VEGF production and transmigration in MM cells expressing mutant p53, but not in MM cells expressing wt p53. MM cell lines RPMI 8226 and SV, with temperature-sensitive p53 mutations, were used to demonstrate VEGF induction after CD40 activation at restrictive (37°C) mutant p53 but not at permissive (28°C) wt p53 temperatures. Significantly, VEGF transcript expression was also induced after CD40 activation in patient MM cells (MM2, MM4, MM5) expressing mutant p53, but not in patient MM cells (MM1, MM3) expressing wt p53. We used luciferase reporter assays with full-length human VEGF promoter to demonstrate that the induction of VEGF after CD40 activation is controlled, at least in part, by a transcriptional mechanism. Using an adenovirus-mediated transfer of wt p53, we further showed that the overexpression of wt p53 down-regulates CD40-induced VEGF production and transmigration in MM cells. These studies help to identify a VEGF role in MM pathophysiology. Because p53 mutations occur more frequently in patients with MM during advanced stages of the disease, our demonstration of enhanced VEGF expression and migration after CD40 activation in MM cells expressing mutant p53 supports the role of p53 in MM tumor progression. Furthermore, the association of mutant p53 and CD40 activation on VEGF production also suggests a role for VEGF in MM progression.
Although CD40 activation generates multiple biologic sequelae in human MM cells, the induction of VEGF has not previously been explored. Most reports thus far are in favor of a paracrine action by VEGF in MM, whereby VEGF secreted by MM cells augments IL-6 production in stromal cells.12 However, we recently showed that MM cells express Flt-1 receptor (VEGFR-1) and Flt-1 activation by VEGF on MM cell lines,15 suggesting that VEGF may have important autocrine effects on tumor cell transmigration and survival. Our current study demonstrating CD40 induction of VEGF in MM cells also suggests autocrine effects of VEGF on MM cells. In addition, it was shown recently that CD40 induces the cell motility of Kaposi sarcoma and human umbilical vein endothelial cells in vitro, which may enhance tumor cell invasion of tissues and endothelial cell organization.19 Because cell migration involves a dynamic regulation of cell adhesion and our prior studies show an up-regulation of cell surface adhesion molecules on MM cells after CD40 activation,6,8 we investigated here whether CD40 activation modulated MM cell motility using the transmigration assay. Interestingly, we observed increased MM cell transmigration induced by CD40, correlating with tumor cell p53 status. These data, coupled with the clinical observation that patients with advanced stages of MM express high CD40 levels,5 suggest a role for CD40 in MM homing or invasion.
It has been postulated that p53 dysfunction may play an important role in MM tumor progression. Specifically, wt p53 regulates MM cell growth and apoptosis and attenuates IL-6 production, whereas loss of p53 function deregulates IL-6 production. In addition, the presence of a mutated p53 allele may potentiate autonomous MM cell growth and secretion of angiogenic factors (ie, VEGF), which enhance the emergence of MM cell clones bearing only the mutated p53 allele. Our findings that MM cells expressing mutated p53 produced more VEGF after CD40 stimulation supports this view.
It has been shown that wt p53 protein down-regulates VEGF promoter activity in cell lines in a dose-dependent manner.20 Recently, it was reported that the introduction of wt p53 into sarcoma cells repressed the transcription and decreased the expression of VEGF.32 Endothelial cell growth and migration were also decreased when cells were treated with conditioned medium from sarcoma cells expressing wt p53 compared with media from sarcoma cells expressing mutant p53.32 In the current study, the overexpression of wt p53 in MM cell lines and freshly isolated patient MM cells expressing mutant p53 down-regulated CD40-induced VEGF production, indicating that VEGF secretion by MM cells can be regulated by wt p53. This result suggests the potential usefulness of adenovirus-mediated delivery of wt p53 in gene therapy for MM.42 Our ongoing studies are examining the growth-inhibiting and potentially therapeutic benefits of CD40 activation of MM cells heterozygous for p53. In addition, because wt p53 suppresses angiogenesis by the regulation of thrombospondin (TSP-1) expression in human fibroblasts43 and because preclinical studies have shown that wt p53 protein enhances the expression of TSP-1, the down-regulation of TSP-1 may be observed when alterations of the p53 protein occur. Studies are ongoing to determine whether TSP-1 expression is increased in MM cells expressing wt p53 after CD40 activation to further define the role of CD40 in angiogenesis and progression of MM.
CD40 ligation is known to regulate multiple genes through transcriptional mechanisms, and our study demonstrates that VEGF expression in MM cells after CD40 activation is also primarily controlled by a transcriptional mechanism. Specifically, we showed that sCD40L increased luciferase activity in MM cells transduced with the VEGF-Luc reporter. Thus far, the CD40 responsive element in the promoter region of CD40-induced genes has not been identified. However, in a previous report, the induction of VEGF expression by IL-6 was found to be mediated by specific DNA motifs located on the putative promoter region of VEGF and by specific elements located in the 5′-UTR.16 One DNA control element mediating IL-6 response has been identified between −796 and −804 in human VEGF promoter.16 Given that the VEGF-Luc reporter used in the current study contains these regions, the observed induction of luciferase activity by CD40 activation could have been indirectly regulated by IL-6. We have shown that CD40 activation also triggers IL-6 secretion in MM cell lines and patient MM cells.6Therefore, we cannot rule out the possibility that the effects of sCD40L on the transmigration of MM cells may, at least in part, be a result of indirect (non-VEGF) mechanisms. Because CD40 activation results in the expression of multiple cytokines, chemokines, and adhesion molecules, which themselves may have effects on VEGF expression and cell motility, studies are under way to delineate the direct role of CD40 in regulating these processes.
In conclusion, we have demonstrated that CD40 activation induces p53-dependent VEGF expression in human MM cells. Specifically, CD40 activation enhanced VEGF expression and transmigration in MM cells with mutant p53. Our current study validates the important role of VEGF and p53 status in MM disease progression.
We thank Dr Bert Vogelstein and Dr Robert Taylor for reagents.
Supported by a Multiple Myeloma Research Foundation Fellowship (Y.-T.T.) and a Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Dept of Adult Oncology, Dana-Farber Cancer Institute, M557, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.



![Fig. 4. CD40 induces VEGF secretion in a dose- and time-dependent fashion. / (A) RPMI 8226 and SV MM cell lysates (15 μg) at intervals (0-48 hours) after CD40 activation were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and were transferred to a polyvinylidene difluoride membrane. VEGF protein was detected with anti-VEGF mAb and was visualized by enhanced chemiluminescence. Anti-α–tubulin mAb was used as a loading control. Upper panel, RPMI 8226; lower panel, SV. Similar results were obtained in 2 independent experiments. (B) Cells were cultured at 37°C in triplicate with 0 to 10 μg/mL (μM) sCD40L (circle) or anti-CD40 mAb G28.5 (triangle). (C) Cells were cultured with sCD40L (10 μg/mL [μM]) (circle), anti-CD40 mAb G28.5 (10 μg/mL [μM]) (triangle), medium alone (diamond), or MOPC-21 isotype control IgG (square) at 37°C. VEGF levels in the supernatants at intervals were determined by ELISA. Data are expressed a means ± SE of culture triplicates. Open symbol, RPMI 8226; solid symbol, SV.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1419/6/m_h80422116004.jpeg?Expires=1765956786&Signature=fCa8~SEZeg0m0AgbAoqLrySzojQh8SX05G2wUXJknWWexXLoD~7HLAx~LLJ5lQ2eGdeNVOxN5EQ3sieyVIpFVcSPbKWuGXTLmyziZGIqTrlZpj0VfnQHnqXcpvUPaM2VJCQfwdZU~1078vYqU7LYlEDKmUw8G7QxwdgbwUHeomaGRQPyJqg8mEm8QZOLvVi-DJDOUsXRpNwi9VBVTWx1xFbO049M9hnXjqrEe6x-JOqgnCM9EkmpEZvXp6FYa4~xdGxQSYS2jF5c-QELVSd7pl60e7xJTIgz~BC1ud8p3zwtF9nPFSzJ4I8oCsuEKYg8u126BaOM1VpniBeB7L5d8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. CD40 induces migration in human MM cells expressing mutant p53. / MM cells were plated on a polycarbonate membrane (8 μm pore size) precoated with fibronectin (5 μg/mL [μM]) in the transwell cluster plate and were activated using 0 to 10 μg/mL (μM) sCD40L (A) or anti-CD40 mAb (G28.5) (B) added to the lower chamber. Cells were cultured at restrictive temperature (37°C) with mt p53 or at permissive temperature (28°C) with wt p53. Migrated cells in the lower chamber were counted using a Coulter counter ZBII. (C) Isotype control antibody MOPC 21 (10 μg/mL [μM]) did not induce transmigration in MM cells at restrictive temperature. Results are representative of 2 independent experiments. (■) RPMI 8226 at 37°C; (▤) RPMI 8226 at 30°C; (▪) SV at 37°C; () SV at 30°C. (D) Patient MM1-5 cells were subjected to transmigration assay, using sCD40L (10 μg/mL [μM]) in the lower chamber at 37°C. (▪) MM1; () MM2; (■) MM3; (░) MM4; () MM5. Increased transmigration is observed in MM cells expressing mutated p53 (MM2, MM4, MM5), but not in those with wt p53 (MM1, MM3), after CD40 activation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1419/6/m_h80422116005.jpeg?Expires=1765956786&Signature=mbI2XVy25gEoAAkObPndpgLm9VTZEbqmateMSaISoYHPGJo4LY9IHoZ24fESwzhn-Gi~4LRa9XaloyyKZSnIZcXk~rS~ESOH4wxKM6U81KPLbWxbp4~-~Y2ddyudqoRuqTRgr~8sHw7kyhFBG00czupLe5FoXM9nbf2IwO2rMHnquo~OWZ3fnCYKorg5K-G-IwlRpK5ZuWPxIweWRoiTp~x9Laf4lR9D2SgR4faNZM09bIHl3AO-BBeMLiUQRcH2VorGxsriAgmthyp2rsYVExFqxJb~dVXiqZqjIhKBkvh3SSFAwsGtGpUv-faa9pq5pkBi9KYRIORzd93UVNd4kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Neutralizing anti-VEGF mAb blocks CD40-induced MM cell migration. / MM cells were subjected to transmigration assay and exposed for 8 hours (RPMI 8226 cells) or 16 hours (SV cells) to CD40 activation by sCD40L (0 or 10 μg/mL [μM]), in the presence or absence of neutralizing anti-VEGF mAb (0-5 μg/mL [μM]) added to the lower chamber of the transwell. Migrated cells collected from the lower chamber were counted. Enhanced transmigration of cells induced by sCD40L was abrogated by neutralizing anti-VEGF mAb. (■) RPMI 8226; (▪) SV.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1419/6/m_h80422116006.jpeg?Expires=1765956786&Signature=CNlqx55gsSjKRvVDNHEKY~pl4jCIWVJBwaQC5baJm-dlZKEAvrzuAnGMSEeuxn8dgKYwpSIxd8K9PuzLJ3Nw3SqOoxnr7AD~4DYAtgmvj552Yy8nrSMenrtNHtpZy5BeCc7sKvOuaPbE694lkbDjTWkh5MVJC2C1qg0UNxsU-8PiMNSvS6nzLgTKjDspsV4BB6NzsYdJbE3lXGhXN3eCyJUy6n9OV58cNRWrLV2fuE2lScEW3LyWOtnvdOWC9hpugUluTiPvRGy1aDnOCq-zPXFe6gLp5QoT8izcHJK8CeF9dY3bAoEO06GVTi48GVTsOoQIHx1AHSH~9VhKO7drVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Effect of CD40 activation on VEGF production and migration is inhibited by 5C8 blocking anti-CD40L mAb. / MM cells were cultured in the presence or absence of sCD40L (10 μg/mL [μM]) and a 5C8 blocking anti-CD40L mAb (0-10 μg/mL [μM]) to confirm the specificity of sCD40L-triggered VEGF mRNA expression and cell migration. Blocking mAbs were added to the wells at the initiation of cultures at 37°C. (A) Twenty-four hours after indicated treatments, cells were collected and total RNA was prepared for Northern blotting (upper panel, RPMI 8226; lower panel, SV). Total RNA (5 μg) was used for analysis, and 18 rRNA served as an internal control. All autoradiographs are representative of 2 experiments with similar results. (B) MM cells were subjected to transmigration assay with the addition of sCD40L in the presence or absence of 5C8 blocking anti-CD40L mAb in the lower chamber of the transwell. (■) RPMI 8226 at 37°C; (▪) SV at 37°C. Identical effects of CD40 activation on the induction of VEGF were obtained with anti-CD40 mAb G28.5 (1 to 10 μg/mL [μM]) (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1419/6/m_h80422116007.jpeg?Expires=1765956786&Signature=mpCcRRq9KjdjSdiKd~YbY3O9Z4dcgQo0LWIFWMSsqTYGsv1ZxvHXq82QDtdrlfO0camXKinhiM2~jNQshnzCwO6-2~0YDPyBtGLFICttKpPjlCRoLrlOsqtIjj~0U9Uro-WCV5cYqVk0u-uN9oVoS7fo3LMzUZY1bcPXHcOWu-iXwFcGD~UcZiuCaWmy6y~wqsk-zcaAq19RqKDD5GoPxRzO-mQdkGCxi-U4mUVYWskR7041GmQWTm1DJBaN6nyeSXeAuaFdPYTTQfSYYP9rYuFOtNPvo8pvUsq3~3rXlFagu5nzsbFHfAktGl1g6nZu-uZlHXIFXkuXr2nnuCAUfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 9. Adenovirus-mediated wt p53 overexpression down-regulates CD40-induced VEGF mRNA expression. / (A) Six hours after adenovirus infection into MM cells, whole cell lysates (15 μg) were subjected to immunoblotting using HRP-conjugated antipantropic p53 (anti-p53-HRP) mAb. Enhanced expression of p53 was observed in RPMI 8226 and SV MM cell lines. Control adenoviruses expressing the β-gal reporter gene (Adβ-gal) were used at the same MOIs as Adwtp53 (MOIs of 800 and 200 for RPMI 8226 and SV cells, respectively). As an additional control, nontransduced MM cells were used as an internal control. (B) MM cells were cultured with control (Adβ-gal) or wt p53 (Adwtp53) adenoviruses in the presence or absence of sCD40L for 48 hours. At the end of treatment, total RNA (5 μg) was subjected to Northern blotting, and an 18S rRNA transcript was used as a loading control. Steady state mRNA expression was quantitated by densitometry of autoradiograms. (■) RPMI 8226; (▪), SV. (C) Adwtp53-transduced MM cells were subjected to transmigration assay in the presence or absence of sCD40L (10 μg/mL [μM]) in the lower chamber of the transwell.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1419/6/m_h80422116009.jpeg?Expires=1765956786&Signature=BUFRMpEXeBwnDqCgvGNNiW-iYuKa53wD51vgbr108pu9s2V9Smp3MoCzk1p8JaDK34P34tBGyvbViiiKAKQnbit8-sqGa3jAWoUL2lsEMp~pi9PPOTbHJGehSYgf~z7kJQGIwanJpbR48gv6srVGygq~h2dzHVQqaXCZVOlANSIFnzvoH66e~Qsxw7wNLjrZi4w46-g2k6vcszj~Goz9adu53oLOd~xSCj9DGSclVnJ1E~FGV6IVMK1Yc1jxvi70ldjoJCf33twvTA~qz1ainlnWrKcvqbm7h3Qo8gdDkGzJj8b3sWpYllH0BK~StC181CVLsSX~jt7hNWuyKzFEHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal