Interleukin 18 (IL-18) was discovered as an interferon-γ (IFN-γ)–inducing factor and plays important roles in natural killer (NK) cell activation. IL-18 also induces proinflammatory cytokines; chemokines; helper T-cell 2 (TH2) cytokines (eg, IL-4, IL-13); and immunoglobulin E (Ig-E) and IgG1 production. The combination of IL-18 plus IL-2 or IL-12 up-regulates IFN-γ gene expression and NK cytotoxicity and has synergistic antitumor activity in vivo and in vitro. Here it is reported that daily administration of IL-18 with IL-2, but not of IL-18 or IL-2 alone, induces lethal lung injury in normal mice, but not in IL-18 receptor α (IL-1 receptor–related protein)–deficient (IL-18 receptor α−/−) mice. Marked interstitial infiltration of lymphocytes, composed mainly of NK cells, was found in the lungs of IL-18/IL-2–treated mice. Increased cytokine and chemokine levels were observed in the sera and lungs of IL-18/IL-2–treated mice. Administration of IL-18/IL-2 was also lethal to mice treated with a metalloproteinase inhibitor, which inhibited tumor necrosis factor–α and Fas-ligand release. While IFN-γ−/− mice were partially resistant to the treatment, IL-4−/−, IL-13−/−, IL-4/IL-13−/−, and Stat6−/− mice were sensitive to IL-18/IL-2, indicating that these genes were not involved in the host response. The lethal effect by IL-18/IL-2 was completely eliminated in severe combined immunodeficient mice pretreated with antiasialo-GM1 antibody and normal mice pretreated with anti-NK1.1 but not with anti-CD4 or anti-CD8, monoclonal antibody. These results suggest that specific cytokines, chemokines, and NK cells are involved in the pathogenesis of interstitial pneumonia. These results suggest that the clinical use of this interleukin may result in unexpected physiological consequences.
Introduction
Interstitial pneumonia represents a heterogeneous group of idiopathic interstitial lung diseases that have a grave prognosis. It can be clinically classified as an acute and a chronic form (Bouros et al1; Michaelson et al2). Acute interstitial pneumonia (AIP) is clinically characterized by a rapid onset of respiratory failure and has a grave prognosis, with greater than 70% mortality in 3 months, despite mechanical ventilation. AIP is thought to be synonymous with Hamman-Rich syndrome, occurring in patients without pre-existing lung diseases. It also physiologically resembles acute respiratory distress syndrome (ARDS) and occurs in a subset of patients with idiopathic ARDS. Chronic interstitial pneumonia is also known as usual interstitial pneumonia (UIP). It is characterized clinically by the insidious onset of a cough and shortness of breath that slowly progresses to respiratory failure and is characterized histopathologically by the random, nonuniform foci of inflammation and fibrosis of the lung. UIP is often observed in aged adults and in patients with a variety of conditions, including viral infections, rheumatoid diseases, and radiation treatment. Additionally, lethal UIP is often found in patients treated with chemotherapy. Bleomycin- and busulphan-induced3 4 lung fibrosis has been reported in patients undergoing treatment for malignancies, such as squamous cell carcinoma and myeloid leukemia. Prevention of interstitial pneumonia/lung fibrosis is one of the most important issues in patients treated with chemotherapy.
Histologically, diffuse infiltration of mononuclear and polymorphonuclear leukocytes into the lung is observed in the early stage of human interstitial pneumonia. Following the interstitial inflammation, florid fibroblast proliferation within both the interstitium and the alveolar space is found (proliferative stage). The same pathology is observed in the lung fibrosis animal model.1-8 Thus, the interstitial inflammation is thought to be essential for the fibroblast proliferation of lung fibrosis. It has become clear that multiple mediators may be involved in establishing interstitial pneumonia/lung fibrosis, including cytokines, chemokines, oxygen radicals, eicosanoids, prostaglandin, and apoptosis-related genes.1-8 However, the pathogenesis of interstitial pneumonia is not well understood.
Interleukin 18 (IL-18) was originally discovered as an interferon-γ (IFN-γ)–inducing factor in studies of IFN-γ production in a Proprionobacterium acnes–induced model of toxic shock.9 IL-18 synergistically induces IFN-γ production in helper T-cell 1 (TH1) clones and cell lines and in natural killer (NK) cells when IL-12, IL-2, antigens, and possibly IFN-α are added as costimulating signals.10-12 IL-18, like IL-12, augments NK activity through the induction of constitutively expressed IL-18 receptor (IL-18R) on NK cells.13 A previous study reported that IL-18Rα (IL-1R–related protein) is selectively expressed on the surface of TH1 but not TH2 cells.14 On the basis of these reports, IL-18 was thought to be a strong cofactor for TH1 cell development. However, we have demonstrated that IL-18, in combination with IL-2 but not with IL-12, can be a strong cofactor for the expression of a TH2 cytokine, IL-13, in T cells and in a unique NK population.15,16 More recently, we and other groups reported that IL-18 potentially induced TH2 cytokine (IL-4, IL-10, IL-13) and immunoglobulin E (IgE) production, suggesting that IL-18 can act as a cofactor for both TH1 and TH2 cell development.17-23When these results are considered together, IL-18 may play an important role in the development of inflammatory and immune responses. However, a role for IL-18 in interstitial lung diseases has not been established.
We have reported that administration of IL-18 induced serum IgE production and that IL-18 plus IL-2 treatment induced higher serum IgE and IgG1 levels than seen with either IL-18 alone or IL-2 alone in normal mice.17 Here we report that daily administration of IL-18 with IL-2, but not IL-18 or IL-2 alone, induced a synergistic lethal effect caused by lung injury in normal but not in IL-18Rα–deficient (IL-18Rα−/−) mice. When a high dose of IL-18 (more than 1 μg) with IL-2 (50 000 IU) was administered daily to normal juvenile C57BL/6 (B6) mice, the mice died within 4 to 7 days. Histologically, severe infiltration of mononuclear cells (mainly NK cells) and polymorphonuclear leukocytes was observed. Administration with a lower dose of IL-18 plus IL-2 (50 000 IU) resulted in a prolonged survival time of these mice, and lung histology showed chronic (persistent) lymphocyte infiltration with collapsed alveolar spaces. The lethal effect induced by IL-18/IL-2 was completely eliminated by the depletion of NK cells. Thus, our results suggest that these cytokines and NK cells can play an important role in the pathophysiology of lung injury and interstitial pneumonia.
Materials and methods
Mice
C57BL/6 (B6), Balb/c, 129, Balb/c athymic nude, and C.B-17 severe combined immunodeficient (SCID) mice used in this study were obtained from Charles River Japan (Yokohama, Japan). B6 × 129 background IL-18Rα (IL-1R related protein)–deficient– (IL-18Rα−/−),24 B6 IL-4−/−,17 B6 × 129 IL-13−/−,25 B6 × 129 IL-4/IL-13−/−,26 B6 Stat6−/−,27 and control B6 × 129 mice were generated and maintained. B6 background IFN-γ–deficient (IFN-γ−/−) (IFN-γ knockout [GKO]) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice in this study were maintained under specific pathogen-free conditions and used for experiments at 5 to 9 weeks of age. All procedures were approved by the Committee on the Ethics of Animal Experiments, Kurume University (approval No. 570). Animal care was provided in accordance with the procedures outlined by NIH.48
Reagents
Recombinant human IL-2 (rhIL-2) was obtained from Hoffmann-La Roche (Nutley, NJ). Recombinant mouse (rm) IL-18 (rmIL-18) was obtained from Pepro Tech (Rocky Hill, NJ). Endotoxin levels are undetectable (less than 0.1 ng/μg) in these recombinant cytokines. Metalloproteinase inhibitor [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsuccinyl]-L-phenylglycineN-methylamide (KB-R7785)28 was kindly provided by Dr Kohichiro Yoshino (Japan Organon, Osaka). Purified sterile antimouse CD4 (GK1.5) and CD8 (2.43) were provided by Dr Craig Reynolds (Biological Resources Branch, National Cancer Institute–Frederick, MD), and NK1.1 monoclonal antibody (mAb) (PK136) was kindly provided by Dr Goro Matsuzaki (Kyusyu University, Fukuoka, Japan). Endotoxin levels in these monoclonal antibodies were below detectable limits (less than 0.1 ng/μg).
In vivo treatment of mice with IL-18 and IL-2
Mice were treated once a day with an intraperitoneal injection of 200 μL rhIL-2 and/or rmIL-18, as indicated in “Results.” These cytokines were suspended in sterile 200 μL phosphate-buffered saline (PBS), and mice treated with 200 μL PBS were used as controls. Following the treatment, mice were bled and killed, and wet lung, heart, and body weight were measured. The lungs were dried in an oven at 50°C for more than 48 hours, and dry lung weight was measured at 3 time points.
In vivo administration of the metalloproteinase inhibitor
Metalloproteinase inhibitor (KB-R7785) was suspended in sterile 0.5% carboxymethyl cellulose (CMC) at 10 mg/mL. Mice were treated once a day by an intraperitoneal injection of 0.2 mL KB-R7785, and 0.2 mL 0.5% CMC was used as a vehicle control. Administration of KB-R7785, used to prevent tumor necrosis factor α (TNF-α) and Fas ligand (FasL) release in this study, was not toxic as previously reported.28
Pretreatment with antiasialo-GM1 Ab and anti-NK1.1 mAb
Female SCID mice were pretreated by an intraperitoneal injection of 1 mg (100 μL) antiasialo-GM1 Ab (Wako, Tokyo, Japan) on days 0 and 7 to eliminate the NK cell population. SCID mice were then treated with control PBS or with IL-2 (100 000 IU) plus IL-18 (1 μg) once a day for 10 days. Normal rabbit serum (Dako Japan, Kyoto) was used as control.
Antimouse CD4 (GK1.5, rat IgG2b), CD8 (2.43, rat IgG2b), and NK1.1 (PK136, mouse IgG2a) mAbs were used for in vivo depletion of T and NK cells as previously reported.17 Normal B6 mice were pretreated with 500 μg of each mAb at days 0 and 7. Mice were then daily treated with IL-2 (100 000 IU) plus IL-18 (1 μg) for 10 days. Purified rat IgG (Sigma Chemical, St Louis, MO) and mouse serum immunoglobulin were used as controls.
Histological examinations
For the histological analysis, mice were euthanized by an intraperitoneal injection of pentobarbital sodium (5 to 10 mg per mouse). After opening of the thorax, the lungs were immediately fixed by intratracheal instillation of 10% buffered formalin for 15 minutes, as previously reported.29 After the gross examination, the extracted tissues were placed in 10% formalin. Sections (4 μm thickness) were cut from paraffin-embedded tissues and placed on poly-l-lysine–coated slides and then incubated overnight at 55°C to 60°C. Deparaffinized sections were stained with hematoxylin and eosin (HE), and sequential sections were alternatively stained with the Elastica van Gieson (EVG) or Azan methods.
Lymphocyte preparation and surface antigen analysis by flow cytometry
B6 mice were treated with control PBS and with IL-2 (50 000 IU) plus IL-18 (1 μg) once a day as described above. At 6 hours after the fifth injection (4 days after treatment), mice were killed, and the lung tissues and pleural effusion were immediately harvested into PBS. Lung tissues were finely minced with surgical blades and then suspended with cold PBS. The cell suspension was passed through a cell strainer (Becton Dickinson, Franklin Lakes, NJ) and then washed with PBS. Spleen lymphocytes were also isolated as previously described.15 Then, 3-color analysis was performed by means of a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, Mountain View, CA). Antimouse CD16/CD32 mAb was used to block the nonspecific binding. More than 30 000 cells were analyzed in each fluorescence-activated cell sorter (FACS) analysis.
Cytokine assays in the lung and serum
Total RNA was isolated from the lungs by the RNeasy Midi Kit (Qiagen, Valencia, CA). Cytokine and chemokine messenger RNA (mRNA) expression was analyzed by RNase protection assay by means of the RiboQuant kit (PharMingen, Franklin Lakes, NJ) and33P-uridine 5′-triphosphate–labeled riboprobe, set as previously described.15 For enzyme-linked immunosorbent assay (ELISA) measurement, the lung tissue was suspended and homogenized 1:4 (wt:vol) in sterile PBS containing 0.1% Tween 20 and centrifuged at 20 000g for 15 minutes; the supernatants were collected and stored at −80°C until assay, as previously reported.30 The lung tissue supernatants and sera were assayed by sandwich ELISA (R&D Systems, Minneapolis, MN).
Hydroxyproline assay
Hydroxyproline assay was performed as described elsewhere.31
Statistical analysis
Results are expressed as the mean ± SD for the number per group. The difference between groups were analyzed by Wilcoxon signed rank test and, if appropriate, by paired t test. Survival curves were analyzed by the Kaplan-Meier log rank test.P < .05 was considered to be statistically significant.
Results
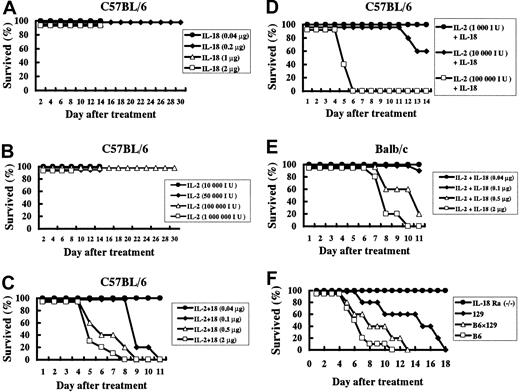
IL-18 plus IL-2, but not IL-18 or IL-2 alone, induces death in normal mice
Once-a-day administration of IL-18 (0.04 to 2 μg/d) or IL-2 alone (1000 to 1 million [Mil] IU/d) for 8 to 14 days did not induce lethality in normal B6 mice. Moreover, all B6 mice treated daily with 50 000 IU IL-2 or 0.2 μg IL-18 for 30 days survived (Figure 1A-B). However daily administration of IL-18 plus IL-2 was lethal in B6 mice in an IL-18 and IL-2 dose–dependent manner (IL-18 2 μg versus 0.5 μg, 0.1μg, or 0.04 μg, P < .05; IL-2 100 000 IU versus 10 000 IU or 1000 IU, P < .05) (Figure 1C-D). IL-18 plus IL-2, but neither cytokine alone, was able to induce death in normal B6, Balb/c, 129, and B6 × 129 mice. Although Balb/c, 129, and B6 × 129 mice were more resistant to the lethal effects of this cytokine combination than B6 mice (Figure 1E-F), overall the lethal effects induced by IL-18 plus IL-2 were not strain specific. Next, B6 × 129 IL-18Rα−/− and wildtype B6 × 129 mice were treated daily with IL-18 (1 μg) plus IL-2 (50 000 IU) for 28 days. None of the IL-18/IL-2–treated IL-18Rα−/− mice died, whereas all wildtype mice died within 18 days (Figure 1F). Moreover, histological analysis revealed that no tissue damage was found in the IL-18/IL-2–treated IL-18Rα−/− mice (data not shown), suggesting that the IL-18 plus IL-2 toxicity is IL-18 dependent.
Lethal effect of IL-18 with IL-2 on normal but not IL-18Rα–deficient mice.
(A) Juvenile C57BL/6 (B6) mice (n = 5 to 10 per group) were treated daily with various doses of IL-18 (0.04 to 2 μg). (B) B6 mice (n = 5 to 10 per group) were treated daily with various doses of IL-2 (10 000 to 1 Mil IU). (C) B6 mice (n = 10 per group) were treated daily with various doses of IL-18 (0.04 to 2 μg) plus IL-2 (50 000 IU). (D) B6 mice (n = 10 per group) were treated daily with various doses of IL-2 (1000 to 100 000 IU) plus IL-18 (0.5 μg). (E) Balb/c mice (n = 10 per group) were treated daily with various doses of IL-18 (0.04 to 2 μg) plus IL-2 (50 000 IU). (F) IL-18Rα−/−, 129, B6 × 129, and B6 (n = 5 to 10 per group) mice were treated daily with IL-18 (1 μg) plus IL-2 (50 000 IU) for 28 days.
Lethal effect of IL-18 with IL-2 on normal but not IL-18Rα–deficient mice.
(A) Juvenile C57BL/6 (B6) mice (n = 5 to 10 per group) were treated daily with various doses of IL-18 (0.04 to 2 μg). (B) B6 mice (n = 5 to 10 per group) were treated daily with various doses of IL-2 (10 000 to 1 Mil IU). (C) B6 mice (n = 10 per group) were treated daily with various doses of IL-18 (0.04 to 2 μg) plus IL-2 (50 000 IU). (D) B6 mice (n = 10 per group) were treated daily with various doses of IL-2 (1000 to 100 000 IU) plus IL-18 (0.5 μg). (E) Balb/c mice (n = 10 per group) were treated daily with various doses of IL-18 (0.04 to 2 μg) plus IL-2 (50 000 IU). (F) IL-18Rα−/−, 129, B6 × 129, and B6 (n = 5 to 10 per group) mice were treated daily with IL-18 (1 μg) plus IL-2 (50 000 IU) for 28 days.
IL-18 plus IL-2 induces interstitial NK cell infiltration in the lung but not other tissue damage
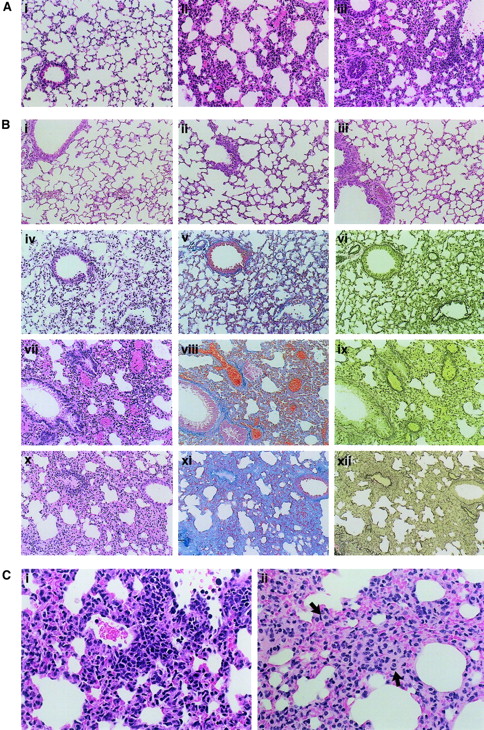
Initially, we found that daily treatment with IL-18/IL-2 induced a lethal effect accompanied by pleural effusion in juvenile (younger than 10-week-old) female mice. Male or female mice older than 10 weeks also succumbed to IL-18/IL-2 treatment, but were more resistant than the juvenile female mice (data not shown). These results suggested that the body weight and sex could influence the lethality induced by IL-18/IL-2 treatment. In a general histological evaluation, moderate thymus atrophy and splenomegaly were found in IL-18/IL-2–treated mice, similar to that observed in IL-2–treated mice.16,32 Striking pathological changes in IL-18/IL-2–treated mice were limited to the lung while other organs, such as heart, liver, kidney, and intestine, showed only congestion. No brain damage was found in IL-18/IL-2–treated mice (data not shown). Daily administration of a high dose of IL-18 (1 μg IL-18 plus 50 000 IU IL-2) resulted in an acute lethal effect in juvenile B6 mice, usually within 4 days. Necropsy of these mice revealed that the alveolar wall and general interstitium had many nuclei with an interstitial infiltrate of mononuclear cells. Infiltrations of polymorphonuclear cells were also observed in the lesion, which was randomly distributed in the entire lung tissues. We did not observe that the lesions were dominated by hyaline membrane and proteinaceous debris, which is characteristic of the diffuse alveolar damage in humans (Figure 2A,C). The mice treated with a relatively low dose of IL-18 (0.1 to 0.2 μg) plus IL-2 (50 000 IU) demonstrated prolonged survival (longer than 1 week). In these mice, thickening of alveolar walls and interstitium was increased in a time-dependent manner and was accompanied by an infiltration of mononuclear cells, polymorphonuclear cells, and foam cells, which subsequently resulted in an architectural destruction and collapsed alveolar spaces (Figure 2B-C). In contrast, no significant histological change was found in the lungs of B6 mice treated daily with IL-18 (0.1 μg) alone or IL-2 (50 000 IU) alone for 30 days (Figure 2B). FACS analysis revealed that more than 40% of lymphocytes in the lung and pleural effusion of IL-18/IL-2–treated B6 mice were CD3−NK1.1+ NK cells, while CD3−NK1.1+ NK cells were infrequently observed in the lungs of control PBS-treated mice (Figure3). Moreover, CD8+ T cells, but not CD3−NK1.1+ NK cells, were increased in the spleen of IL-18/IL-2–treated B6 mice. In contrast, CD3−NK1.1+ NK cells, CD8+ T cells, and CD3+NK1.1+ T cells were increased in the spleen of B6 mice treated with IL-2 alone as previously reported16 32 (data not shown). Thus, our histopathological and FACS analyses suggest that NK cells accumulate predominantly in the lung. This increase in NK cells may result in a specific toxic effect on the lung but not other organs following the IL-18 plus IL-2 treatment.
Pulmonary histology of IL-18/IL-2–treated mice.
(A) Acute lethal effect. B6 mice were daily administered PBS alone (Ai) or a high dose of IL-18 (1 μg) plus 50 000 IU IL-2 (Aii-Aiii). Necropsy was performed after 2 (Aii) and 4 (Ai,Aiii) days of the treatment, and the lung tissue was microscopically observed with HE staining. Original magnification at the observation at × 200. (B) Chronic effect. B6 mice were daily administered a low dose of IL-18 (0.1 μg) plus 50 000 IU IL-2 (Biv-Bxii), PBS alone (Bi), IL-2 (50 000 IU) alone (Bii), or IL-18 (0.1 μg) alone (Biii), and were killed at the time point indicated (day 8, Biv-Bvi; day 18, Bvii-Bix; day 30, Bi-Biii; day 36, Bx-Bxii). The lung tissue was microscopically observed with HE (Bi-Biv,Bviii,Bx), Azan (Bv,Bviii,Bxi), or EVG (Bvi,Bix,Bxii) staining. Original magnification at the observation at × 200. (C) Morphology of the infiltrating cells. B6 mice were daily administered a high dose of IL-18 (1 μg) (Ci) or a low dose of IL-18 (0.1 μg) (Cii) plus 50 000 IU IL-2 for 4 and 36 days, respectively. The lung tissue was observed with HE staining. Foam cells (arrows) are present in the thick alveolar walls and general interstitium. Original magnification at the observation at × 400.
Pulmonary histology of IL-18/IL-2–treated mice.
(A) Acute lethal effect. B6 mice were daily administered PBS alone (Ai) or a high dose of IL-18 (1 μg) plus 50 000 IU IL-2 (Aii-Aiii). Necropsy was performed after 2 (Aii) and 4 (Ai,Aiii) days of the treatment, and the lung tissue was microscopically observed with HE staining. Original magnification at the observation at × 200. (B) Chronic effect. B6 mice were daily administered a low dose of IL-18 (0.1 μg) plus 50 000 IU IL-2 (Biv-Bxii), PBS alone (Bi), IL-2 (50 000 IU) alone (Bii), or IL-18 (0.1 μg) alone (Biii), and were killed at the time point indicated (day 8, Biv-Bvi; day 18, Bvii-Bix; day 30, Bi-Biii; day 36, Bx-Bxii). The lung tissue was microscopically observed with HE (Bi-Biv,Bviii,Bx), Azan (Bv,Bviii,Bxi), or EVG (Bvi,Bix,Bxii) staining. Original magnification at the observation at × 200. (C) Morphology of the infiltrating cells. B6 mice were daily administered a high dose of IL-18 (1 μg) (Ci) or a low dose of IL-18 (0.1 μg) (Cii) plus 50 000 IU IL-2 for 4 and 36 days, respectively. The lung tissue was observed with HE staining. Foam cells (arrows) are present in the thick alveolar walls and general interstitium. Original magnification at the observation at × 400.
Infiltration of CD3−NK1.1+ NK cells into the lungs of IL-18/IL-2–treated mice.
B6 mice were treated with control PBS and IL-18 (1 μg) plus IL-2 (50 000 IU) for 4 days; lung lymphocytes were prepared; and FACS analysis was performed as described in “Materials and methods.” Fluorescein isothiocyanate (FITC)–antimouse CD8a mAb, phycoerythrin (PE)–antimouse CD4 mAb, Cy-Chrome–antimouse CD3ε mAb, PE–antimouse NK1.1 mAb, and FITC-, PE-, Cy-–conjugated isotype-matched immunoglobulin were used for FACS analysis.
Infiltration of CD3−NK1.1+ NK cells into the lungs of IL-18/IL-2–treated mice.
B6 mice were treated with control PBS and IL-18 (1 μg) plus IL-2 (50 000 IU) for 4 days; lung lymphocytes were prepared; and FACS analysis was performed as described in “Materials and methods.” Fluorescein isothiocyanate (FITC)–antimouse CD8a mAb, phycoerythrin (PE)–antimouse CD4 mAb, Cy-Chrome–antimouse CD3ε mAb, PE–antimouse NK1.1 mAb, and FITC-, PE-, Cy-–conjugated isotype-matched immunoglobulin were used for FACS analysis.
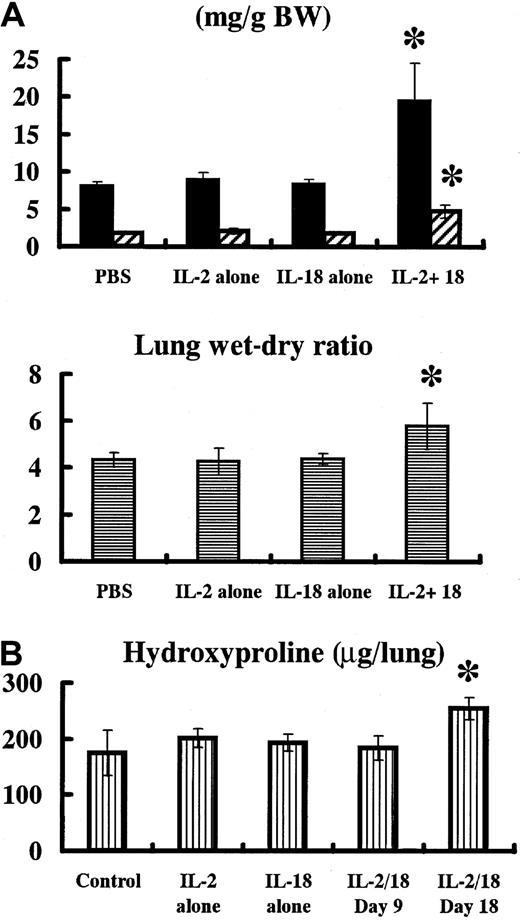
To verify whether IL-18/IL-2 can induce lung failure, we treated B6 mice daily with a control 200 μL PBS, 50 000 IU IL-2, 1 μg IL-18, or 50 000 IU IL-2 plus 1 μg IL-18 for 8 days (n = 7 per group). Then, mice were killed, and wet lung, dry lung, heart weight, and body weight were measured. All of the IL-18/IL-2–treated B6 mice died between 4 and 7 days after treatment, while all of the B6 mice treated with PBS, IL-2 alone, or IL-18 alone survived. Both wet and dry lung weights in IL-18/IL-2–treated B6 mice were significantly (P < .05) higher than in control PBS-, IL-2–, and IL-18–treated B6 mice, although no significant difference in heart weight due to treatment was observed in these mice (data not shown). The lung wet-dry ratio in IL-18/IL-2–treated B6 mice was also significantly (P < .05) higher than that in control PBS-, IL-2–, and IL-18–treated B6 mice (Figure4A). The same results were observed in B6 mice treated daily with a low dose of IL-18 (0.1 μg) plus IL-2 (50 000 IU) for 30 days (data not shown).
Wet and dry lung weight of IL-18/IL-2–treated mice.
(A) B6 mice (n = 7 per group) received control PBS, IL-2 (50 000 IU) alone, IL-18 alone (1 μg), or IL-18 (1 μg) plus IL-2 (50 000 IU) daily for 8 days. Mice were bled and killed, and wet lung, dry lung, and body weight (BW) were measured. Wet (▪) and dry (▨) lung weight and lung wet-dry ratio in IL-18/IL-2–treated mice were significantly (P < .05) higher than that in PBS, IL-2, and IL-18–treated mice. (B) Hydroxyproline content of lungs of IL-18/IL-2–treated mice. B6 mice (n = 5 per group) were daily given control PBS, IL-2 (50 000 IU) alone, IL-18 alone (0.1 μg), or IL-18 (0.1 μg) plus IL-2 (50 000 IU) for 18 days. Then, mice were killed, and hydroxyproline content of lungs was measured. *Significantly (P < .05) higher than those in B6 mice treated with control PBS, IL-2 alone, IL-18 alone, and IL-18/IL-2 (day-9 treatment).
Wet and dry lung weight of IL-18/IL-2–treated mice.
(A) B6 mice (n = 7 per group) received control PBS, IL-2 (50 000 IU) alone, IL-18 alone (1 μg), or IL-18 (1 μg) plus IL-2 (50 000 IU) daily for 8 days. Mice were bled and killed, and wet lung, dry lung, and body weight (BW) were measured. Wet (▪) and dry (▨) lung weight and lung wet-dry ratio in IL-18/IL-2–treated mice were significantly (P < .05) higher than that in PBS, IL-2, and IL-18–treated mice. (B) Hydroxyproline content of lungs of IL-18/IL-2–treated mice. B6 mice (n = 5 per group) were daily given control PBS, IL-2 (50 000 IU) alone, IL-18 alone (0.1 μg), or IL-18 (0.1 μg) plus IL-2 (50 000 IU) for 18 days. Then, mice were killed, and hydroxyproline content of lungs was measured. *Significantly (P < .05) higher than those in B6 mice treated with control PBS, IL-2 alone, IL-18 alone, and IL-18/IL-2 (day-9 treatment).
Hydroxyproline content of lungs of IL-18/IL-2–treated mice
We treated B6 mice daily with 200 μL PBS (control), 50 000 IU IL-2, 0.1 μg IL-18, or 50 000 IU IL-2 plus 0.1 μg IL-18 for 18 days (n = 5 each group). Then, mice were killed, and hydroxyproline content of lungs was measured. Our repeated experiments revealed a slight but significant increase of hydroxyproline content in the lungs of mice treated with IL-2 (50 000 IU) plus 0.1 μg IL-18 for 18 days (P < .05 versus PBS, IL-2, IL-18, and IL-18/IL-2 [treated on day 9]) (Figure 4B).
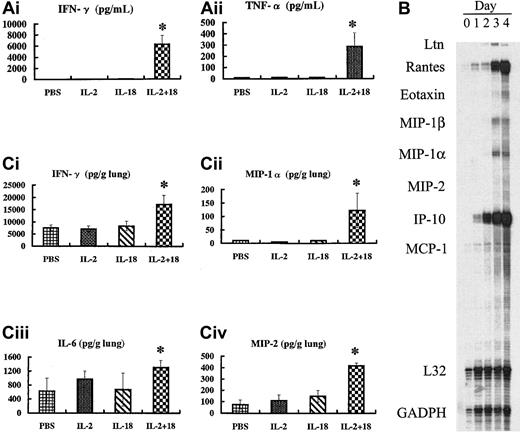
Cytokine and chemokine expression in the serum and lungs of IL-18/IL-2–treated mice
Next, we analyzed serum IFN-γ and TNF-α protein levels in IL-18/IL-2–treated mice, as previous studies have reported that TNF-α and IFN-γ are involved in human interstitial pneumonia as well as the animal lung fibrosis model.1,2,7 8 Serum IFN-γ and TNF-α protein levels in IL-18/IL-2–treated B6 mice were significantly (P < .05) higher than those in B6 mice treated with control PBS, IL-2 alone, or IL-18 alone (Figure5A). We then analyzed lung cytokine and chemokine levels in IL-18/IL-2–treated mice. B6 mice were treated with control PBS, IL-2 (50 000 IU) alone, IL-18 (1 μg) alone, or IL-2 (50 000 IU) plus IL-18 (1 μg) once a day. At 2 hours after every injection, mice were killed; the lung tissue was immediately harvested; and total RNA was isolated. An RNase protection assay revealed that various cytokine (IFN-γ IL-6, TNF-α/β) and chemokine (lymphotactin [Ltn], RANTES, eotaxin, macrophage inflammatory protein (MIP)–1α, MIP-1β, MIP-2, IP-10) mRNA expression was induced in the lungs of IL-18/IL-2– but not IL-18– or IL-2–treated B6 mice (data not shown). Moreover, the chemokine and cytokine mRNA expression was up-regulated 1 day after treatment with IL-18/IL-2, while little cytokine or chemokine mRNA or protein was observed without stimulation (Figure 5B). We confirmed that IFN-γ, IL-6, MIP-2, and MIP-1α protein levels in the lungs of IL-18/IL-2–treated B6 mice were significantly (P < .05) higher than those in control PBS-, IL-2–, and IL-18–treated B6 mice (Figure 5C).
Increased cytokine and chemokine levels in IL-18/IL-2–treated mice.
(A) B6 (n = 8 per group) mice were treated with control PBS, IL-2 (50 000 IU) alone, IL-18 (1 μg) alone, or IL-2 (50 000 IU) plus IL-18 (1 μg) once a day. At 6 hours after the third injection, mice were killed; the sera were immediately harvested; and serum cytokine levels were measure by ELISA. (B) B6 mice were treated with IL-2 (50 000 IU) plus IL-18 (1 μg) once a day for 4 days. At 2 hours after every injection, mice were killed; the lung tissue was immediately harvested; and total RNA was isolated. Total cytoplasmic RNA (1 μg) was used for chemokine mRNA analysis by means of the multiprobe RNase protection assay. (C) B6 mice (n = 10 per group) were treated with control PBS, IL-2 (50 000 IU) alone, IL-18 (1 μg) alone, or IL-2 (50 000 IU) plus IL-18 (1 μg) once a day, and 6 hours after the third injection, mice were killed. ELISA assayed the lung tissue supernatants as described in “Materials and methods.” *Significantly (P < .05) higher than in B6 mice treated with control PBS, IL-2 alone, and IL-18 alone.
Increased cytokine and chemokine levels in IL-18/IL-2–treated mice.
(A) B6 (n = 8 per group) mice were treated with control PBS, IL-2 (50 000 IU) alone, IL-18 (1 μg) alone, or IL-2 (50 000 IU) plus IL-18 (1 μg) once a day. At 6 hours after the third injection, mice were killed; the sera were immediately harvested; and serum cytokine levels were measure by ELISA. (B) B6 mice were treated with IL-2 (50 000 IU) plus IL-18 (1 μg) once a day for 4 days. At 2 hours after every injection, mice were killed; the lung tissue was immediately harvested; and total RNA was isolated. Total cytoplasmic RNA (1 μg) was used for chemokine mRNA analysis by means of the multiprobe RNase protection assay. (C) B6 mice (n = 10 per group) were treated with control PBS, IL-2 (50 000 IU) alone, IL-18 (1 μg) alone, or IL-2 (50 000 IU) plus IL-18 (1 μg) once a day, and 6 hours after the third injection, mice were killed. ELISA assayed the lung tissue supernatants as described in “Materials and methods.” *Significantly (P < .05) higher than in B6 mice treated with control PBS, IL-2 alone, and IL-18 alone.
Metalloproteinase inhibitor cannot prevent IL-18/IL-2–induced lethality
Our present study showed that TNF-α production was induced in the sera and lungs of IL-18/IL-2–treated mice. Thus, we wished to determine whether a metalloproteinase inhibitor (KB-R7785) that inhibits TNF-α and FasL release28 can prevent this lethal lung fibrosis model. All of the B6 mice treated with KB-R7785/IL-18 plus IL-2 or with vehicle/IL-18 plus IL-2 succumbed within 8 days, although KB-R7785 did not demonstrate any toxicity in control PBS-treated B6 mice (Figure 6A). Our results showed that KB-R7785 did not inhibit the lethal effects of IL-18/IL-2 treatment.
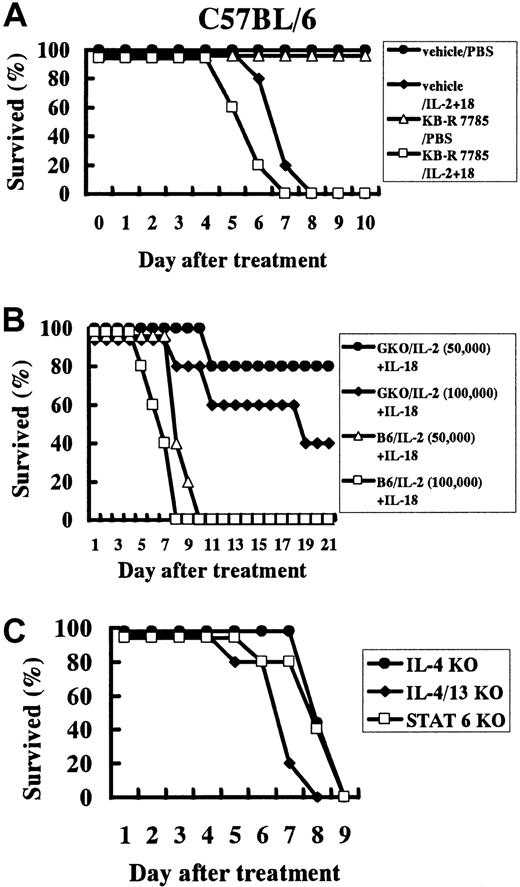
Effect of a metalloproteinase inhibitor on IL-18/IL-2 lethality and effect of IL-18/IL-2 in gene knockout mice.
(A) Effect of a metalloproteinase inhibitor on IL-18/IL-2 lethality. Metalloproteinase inhibitor (KB-R7785) was suspended in sterile 0.5% CMC at 10 mg/mL. B6 mice (n = 10 per group) were treated daily with an intraperitoneal injection of 0.2 mL KB-R7785 or 0.5% CMC (vehicle). Then mice were daily treated with IL-18 (1 μg) plus IL-2 (50 000 IU) or control PBS for 10 days. (B) (C) Effect of IL-18/IL-2 in IFN-γ−/− (GKO) (panel B), IL-4−/−, IL-13−/−, IL-4/IL-13−/−, and Stat6−/− mice (panel C). Mice (n = 5 to 20 per group) were treated daily with IL-18 (1 μg) plus IL-2 (50 000 IU) for 21 days.
Effect of a metalloproteinase inhibitor on IL-18/IL-2 lethality and effect of IL-18/IL-2 in gene knockout mice.
(A) Effect of a metalloproteinase inhibitor on IL-18/IL-2 lethality. Metalloproteinase inhibitor (KB-R7785) was suspended in sterile 0.5% CMC at 10 mg/mL. B6 mice (n = 10 per group) were treated daily with an intraperitoneal injection of 0.2 mL KB-R7785 or 0.5% CMC (vehicle). Then mice were daily treated with IL-18 (1 μg) plus IL-2 (50 000 IU) or control PBS for 10 days. (B) (C) Effect of IL-18/IL-2 in IFN-γ−/− (GKO) (panel B), IL-4−/−, IL-13−/−, IL-4/IL-13−/−, and Stat6−/− mice (panel C). Mice (n = 5 to 20 per group) were treated daily with IL-18 (1 μg) plus IL-2 (50 000 IU) for 21 days.
IFN-γ–, IL-4–, IL-13–, IL-4/IL-13–, and Stat6-deficient mice are susceptible to IL-18/IL-2–induced lethality
Initially, B6 IFN-γ−/− (GKO) and wildtype B6 mice were treated daily with PBS, IL-2 (50 000 IU), IL-18 (1 μg), or IL-18 (1 μg) plus IL-2 (50 000 IU) for 21 days. Three repeated experiments revealed that 80% (16 of 20) of IL-18/IL-2–treated GKO mice survived, while all of control B6 mice (n = 20) died. Next, we treated GKO and B6 mice with a high dose of IL-18 (1 μg) plus a high dose of IL-2 (100 000 IU) for 21 days. These experiments revealed that 40% (4 of 10) of GKO mice treated with IL-18 (1 μg) plus IL-2 (100 000 IU) survived, while all of the control wildtype B6 mice (n = 10) died within 8 days (Figure 6B). Our previous study showed that IL-18 in combination with IL-2 induced TH2 cytokine (IL-4, IL-13), IgE, and IgG1 production in vivo17 and in vitro.16 More recently, Yoshimoto et al20 reported that IgE induction by IL-18 was Stat6 dependent. Therefore, we wished to analyze whether the lethal effect induced by IL-18/IL-2 is dependent on IL-4, IL-13, or Stat6. We treated B6 IL-4−/−, B6 × 129 IL-13−/−, B6 × 129 IL-4/IL-13−/−, B6 Stat6−/−, and control wildtype mice once a day with IL-18 (1 μg) plus IL-2 (50 000 IU) for 10 days. All of these knockout and control wildtype B6 and B6 × 129 mice died within 10 days after the treatment (Figure6C).
NK-cell depletion prevents the lethal effect caused by IL-18/IL-2 administration
We have analyzed juvenile SCID (T-cell– and B-cell–deficient) and Balb/c athymic nude (T-cell–deficient) mice to determine whether the IL-18 plus IL-2 toxicity is NK cell dependent. SCID, nude, and control Balb/c mice (n = 10) were treated daily with control PBS, IL-2 (50 000 IU) alone, IL-18 (1 μg) alone, or IL-18 (1 μg) plus IL-2 (50 000 IU). All of the IL-18/IL-2–treated SCID, nude, and Balb/c mice died within 14 days after treatment, while all mice treated with PBS, IL-2 alone, or IL-18 alone survived (data not shown). Next, we pretreated SCID mice with antiasialo-GM1 Ab at days 0 and 7 to deplete NK cells, and then treated the mice daily with IL-18 (1 μg) and IL-2 (100 000 IU). Surprisingly, lethality was completely eliminated by the pretreatment of SCID mice with antiasialo-GM1 Ab but not with control rabbit serum (Figure7A). Next, we pretreated B6 mice with anti-NK1.1 mAb, anti-CD4 mAb, anti-CD8 mAb, rat IgG, or mouse immunoglobulin at days 0 and 7 and then treated the mice daily with IL-18 (1 μg) plus IL-2 (100 000 IU). IL-18/IL-2 lethality was completely eliminated by the pretreatment with anti-NK1.1 mAb. However, all of the IL-18/IL-2–treated B6 mice pretreated with anti-CD4 mAb, anti-CD8 Ab, or control antibody succumbed within 9 days (Figure7B).
Effect of NK cell depletion on IL-18/IL-2 lethality.
(A) Effect of antiasialo-GM1 Ab. SCID mice (n = 5) were pretreated with 1 mg (100 μL) antiasialo-GM1 [asialo] Ab or control normal rabbit serum (NRS) on days 0 and 7 to deplete the NK-cell population. The NK-cell–depleted SCID mice were then treated daily with control PBS or IL-18 (1 μg) plus IL-2 (100 000 IU) once a day for 10 days. (B) Effect of anti-NK1.1 mAb. B6 mice (n = 5) were pretreated with 0.5 mg anti-NK1.1 mAb, CD4 mAb, CD8 mAb, or control antibody on days 0 and 7. Mice were then treated daily with IL-18 (1 μg) plus IL-2 (100 000 IU) once a day for 10 days.
Effect of NK cell depletion on IL-18/IL-2 lethality.
(A) Effect of antiasialo-GM1 Ab. SCID mice (n = 5) were pretreated with 1 mg (100 μL) antiasialo-GM1 [asialo] Ab or control normal rabbit serum (NRS) on days 0 and 7 to deplete the NK-cell population. The NK-cell–depleted SCID mice were then treated daily with control PBS or IL-18 (1 μg) plus IL-2 (100 000 IU) once a day for 10 days. (B) Effect of anti-NK1.1 mAb. B6 mice (n = 5) were pretreated with 0.5 mg anti-NK1.1 mAb, CD4 mAb, CD8 mAb, or control antibody on days 0 and 7. Mice were then treated daily with IL-18 (1 μg) plus IL-2 (100 000 IU) once a day for 10 days.
Discussion
In this study, we have demonstrated that daily administration of a high dose of IL-18 (2 μg) alone or IL-2 (1 Mil IU) alone did not induce lethality in normal mice. However, in our experimental model, coadministration of IL-18 plus IL-2 induced a lethal effect in normal mice in an IL-18 or IL-2 dose–dependent manner. A high dose of IL-18 (more than 1 μg with 50 000 IU IL-2) resulted in acute death to juvenile B6 mice, usually within 4 days. In the mice treated with a high dose of IL-18 plus IL-2, severe lymphocyte infiltration, mainly by NK and polymorphonuclear cells, was found in the alveolar walls and general interstitium within 2 days, suggesting acute lung injury. In the mice treated with a relatively low dose of IL-18 (plus 50 000 IU IL-2) for 3 to 4 weeks, marked cellular infiltration and an architectural destruction associated with collapsed alveolar spaces were found in surviving B6 mice. In contrast, no tissue damage was observed in brain, heart, liver, kidney, and intestine in IL-18/IL-2–treated mice. Therefore, our present study revealed that coadministration of IL-18 plus IL-2 induced lethality by both acute and chronic lung injury in normal mice in an IL-18 dose–dependent manner.
In the late 1980s, lymphokine-activated killer cells and IL-2 were administered to patients with metastatic cancer.33 The therapy produced significant side effects, mainly owing to the administration of large amounts of IL-2. Patients developed a capillary leak syndrome resulting in anasarca and multiple organ failure in an IL-2 dose–dependent manner. In some cases, the increase in vascular permeability led to varying degrees of interstitial pulmonary edema.33,34 Moreover, similar observations were reported in murine experimental models; the toxicity of large amounts of IL-2 administration was shown to be systemic, mediated by NK cells35 and TNF production.36 For example, Rosenstein and his colleagues37 reported that female 8- to 16-week-old B6 mice treated 3 times a day by an intraperitoneal injection of more than 200 000 IU IL-2 showed systemic vascular leak. Anderson et al38 also reported that administration of 600 000 IU IL-2 twice a day for 7 days induced lethality caused by vascular leak syndrome and hepatocyte necrosis in normal B6D2F1 mice. Here we report that administration of IL-2 alone (10 000 to 1 000 000 IU) once a day was not toxic, although IL-2 (more than 10 000 IU) induced lethal lung injury when combined with IL-18 (0.5 μg) in B6 mice (Figure 1). Thus, our present results and previous studies indicate that IL-2 alone cannot induce toxicity when B6 mice are treated with a relatively low dose of IL-2 once a day, although systemic (greater than twice a day) administration of a high dose of IL-2 alone is toxic. In our present study, a pharmacological blockade (matrix metalloproteinase inhibitor28) of TNF production did not prevent the toxicity of IL-18 plus IL-2, which is in contrast to the report of Fraker et al,36 where the toxicity induced by high-dose IL-2 was prevented by anti-TNF antibody. These results suggest that TNF-independent mechanisms are involved in the pathogenesis of lung injury induced by a relatively low dose of IL-2 plus IL-18, while toxicity (systemic vascular leak) by a high dose of IL-2 is TNF dependent.
Bleomycin, a member of a glycopeptide group of antibiotics, is well known to induce lung fibrosis in humans as well as in rodents.3,5 Our present study showed that administration of IL-18 plus IL-2 induced acute lung injury, usually within 4 days, when a high dose of IL-18 (more than 1 μg) along with 50 000 IU IL-2 was administered to juvenile B6 mice. However, acute lung injury in these mice was not accompanied by hyaline membrane formation, which is considered to be the histological sign of human acute lung injury, such as AIP and ARDS.1,39 Similar observations were reported in the mouse model of bleomycin-induced lung fibrosis; formation of hyaline membrane was often found in the lungs of bleomycin-treated patients but not in a mouse bleomycin-fibrosis model.1,7,39 These results suggest that species difference accounts for the hyaline membrane formation in the process of acute lung injury. Administration of low-dose IL-18 (less than 0.2 μg) plus IL-2 (50 000 IU) resulted in a prolonged survival time. In these mice, interstitial infiltration of mononuclear cells and polymorphonuclear cells was observed followed by collapsed alveolar spaces. We also observed foam cells in the collapsed alveolar lesions. Moreover, the mean wet lung weight, lung wet-dry ratio, and, especially, dry lung weight in IL-18/IL-2–treated mice were significantly higher than in control PBS-, IL-2–, and IL-18–treated mice. Furthermore, the hydroxyproline content of the lung in IL-2 (50 000 IU)/IL-18 (0.1 μg)–treated mice (day 18), representing a quantitative analysis of collagen deposition,31 was significantly higher than in control mice (Figure 4). It has been reported that thickening of the alveolar walls with an accumulation of lymphocytes and foam cells was characteristic of the early stage of fibrous alveolitis, which subsequently augments the gradual increase of collagen synthesis.1,39 Thus, our histological and biochemical findings in the mice treated with low-dose IL-18 (less than 0.2 μg) plus IL-2 (50 000 IU) appear to represent an early stage of lung fibrosis. However, in these mice, we did not observe the distinct honeycombing structure that is usually observed in patients with end-stage pulmonary fibrosis1,39 (Figure 2). Thus, our lung injury model may be characteristic of persistent lymphocyte infiltration rather than distinct lung fibrosis. Lymphocytic interstitial pneumonia (LIP) is characterized by heavy lymphoid infiltrates but a lesser degree of fibrosis.1,2 The multiple nodular lymphocytic lesions resembling lymphoid hyperplasia or lymphoma that is prominent in patients with LIP were not observed in our present model. Thus, our mouse model of IL-18/IL-2–induced lung injury represents a novel animal model for elucidating lymphocytic infiltration (especially of NK cells) into the lung. We have found that IL-18/IL-2 lethal lung injury was not strain specific, but mortality differences were found between the highly susceptible B6 and the more resistant 129 strain. Similar strain differences are seen in the bleomycin-induced lung fibrosis model. For example, C57BL/6J and C3H/HeN mice are considered to be susceptible to bleomycin fibrosis, and Balb/c and C3H/fKam mice are relatively bleomycin resistant.40,41 However, our present study showed that all of the Balb/c mice succumbed to the IL-18/IL-2 treatment (Figure 1), suggesting that different mechanisms are involved in the pulmonary toxicity induced by bleomycin and IL-18/IL-2 treatment. In fact, it has been reported that administration of bleomycin induced distinct lung fibrosis associated with myofibloblastic proliferation,40 41 but less fibroblastic proliferation was found in our IL-18/IL-2–induced lung injury model. Here we propose that our mouse model of IL-18/IL-2–induced lethal lung injury represents a new animal model for human interstitial pneumonia.
Previous studies have suggested that cytokines, such as IFN-γ and TNF-α, and apoptosis-related genes could be involved in the pathogenesis of interstitial pneumonia/lung fibrosis.1,2Moreover, IL-18 can synergistically induce IFN-γ TNF-α, and FasL gene expression when IL-12 or costimulating signals are added,10-12 Therefore, we wished to address the role of these genes in our lung injury model. First, we examined the effect of a metalloproteinase inhibitor (KB-R7785) that inhibits TNF-α and FasL release28 in our experimental model. Our results showed that KB-R7785 did not inhibit the lethal effects of IL-18/IL-2 treatment. Moreover, our repeated experiments showed that IFN-γ–deficient mice were partially resistant to the lethal effects of IL-18/IL-2 treatment, suggesting that there may be a threshold level of specific cytokine/chemokine or receptor gene expression that must be reached before toxicity is seen and that these levels were not achieved in all of the GKO mice. Furthermore, IL-18/IL-2 induced death in IL-4−/−, IL-13−/−, IL-4/IL-13−/−, Stat6−/−, and IL-6−/− mice (data not shown). These results suggest that the lethal effects of IL-18 plus IL-2 treatment were not due simply to the expression of cytokines or FasL. Although IL-18 exerts some of its proinflammatory effects through IFN-γ induction, recent studies have reported that IL-18 activates nuclear factor–κB and induces proinflammatory cytokines, such as TNF-α and IL-1β, and chemokines, such as IL-8 and MIP-1α.10-12In this study, we demonstrated that administration of IL-18 with IL-2 induced expression of both proinflammatory cytokines (IFN-γ, TNF-α, IL-6) and chemokines (eg, MIP-2, MIP-1α) in the lungs and sera. Thus, these results suggest that IL-18 in synergy with IL-2 could induce pulmonary interstitial lymphocyte infiltration, resulting in lung injury through up-regulating expression of multiple, specific proinflammatory cytokines, chemokines, and their receptors. We are currently investigating the expression of other immunomodulators, including additional proinflammatory cytokines/chemokines, to determine the validity of this hypothesis.
Our present study shows that in vivo administration of IL-18/IL-2 induces death in both athymic nude (T-cell–deficient) and SCID (T- and B-cell–deficient) mice. FACS and histological analysis found a diffuse lymphocyte infiltration, composed mainly of CD3−NK1.1+ NK cells, in the interstitial connective tissue of the lung of IL-18/IL-2–treated mice. Furthermore, the lethal effect by IL-18/IL-2 was completely blocked by the NK-cell depletion with antiasialo-GM1 Ab and anti-NK1.1 mAb in SCID and normal B6 mice, respectively. As described above, previous studies have shown that treatment with higher doses of IL-2 leads to elevated NK activity and toxicity and results in tissue damage, including lung and liver injury in several strains of mice, whereas treatment with antiasialo-GM1 Ab was protective.35 It is known that IL-18 can activate NK activity in synergy with IL-12, but independently of IL-12.13 Thus, our results raise the possibility that IL-18 synergistically up-regulates IL-2 toxicity and induces lethal interstitial pneumonia by elevating NK activity.
Many lines of evidence support the model that administration of IL-2, IL-12, and IL-18 can be a particularly effective antitumor treatment. Moreover, combination therapy with IL-12, IL-2, and IL-18 has demonstrated synergistic antitumor activity.32,42-44However, it has been reported that in vivo administration of IL-12 can induce sepsis, shock, and death accompanied with intestinal damage in synergy with IL-2 or IL-15.45 Recent studies have reported that daily administration of IL-18 and IL-12 can synergistically induce liver injury, diarrhea, hemorrhagic colitis, and fatty liver and can result in a fatal inflammatory response in mice but not in IFN-γ−/− mice.46,47 These results suggest that IL-18 may up-regulate IFN-γ–dependent cytotoxicity induced by IL-12 and have an important role in causing liver injury and bleeding from the intestine, especially when administered along with IL-12. However, in our experimental model, we have found that daily administration of IL-18 plus IL-2 induced interstitial pneumonia but not tissue damage in liver, intestine, heart, brain, and kidney in normal mice. Moreover, IFN-γ plays a significant role in the lung injury related to IL-18/IL-2 treatment, since up-regulation of IFN-γ was found in response to this therapy, and lethal effects of IL-18/IL-2 were attenuated in IFN-γ−/− mice. IFN-γ also provides a plausible mechanism by which NK cells may be involved in pulmonary toxicity caused by IL-18/IL-2. Recent studies have reported the same observation; an attenuated response of in IFN-γ−/− mice in bleomycin-induced pulmonary inflammation and fibrosis.41 Thus, our results suggest that IL-18 may have different pathological effects when IL-18 is administered with IL-2 as compared with IL-12, and IFN-γ may play an important role in the pathogenesis of lung injury resulting from IL-18/IL-2 therapy.
Interstitial lung diseases, including lung fibrosis, AIP, ARDS, and UIP, have a grave prognosis. Current therapeutic strategies for interstitial pneumonia/lung fibrosis are hardly effective, although glucocorticoids are often used in therapy.1 2 Our results raise the possibility that blocking of IL-18, IL-2, and/or IFN-γ expression in vivo (eg, anti–IL-18, anti–IL-18R mAb, IL-18 binding protein [BP]) may have clinical benefit in the treatment of interstitial pneumonia/lung fibrosis.
In conclusion, we have shown that administration of IL-18 in combination with IL-2, but not IL-18 alone or IL-2 alone, induced lethal lung injury accompanied by marked interstitial lymphocyte infiltration in normal mice. This lethal effect was completely eliminated by the depletion of NK cells. These results suggest that IL-18, IL-2, and NK cells may play an important role in the pathogenesis of lung injury and interstitial pneumonia. Our study offers a potential model for the pathogenesis of interstitial pneumonia, which currently has an unknown etiology.
We thank Ms Chitose Harada, Ms Maki Tsuruta, Ms Emiko Kuma, and Ms Yumi Furushiro for technical assistance; Dr Kohichiro Yoshino (Japan Organon, Osaka) for providing metalloproteinase inhibitor (KB-R7785); and Dr Goro Matsuzaki (Kyusyu University, Fukuoka, Japan) for providing anti-NK1.1 mAb.
Supported by Kurume University School of Medicine Alumni Foundation (Japan), the Ishibashi Foundation for the Promotion of Science (Tokyo, Japan), Kaibara Morikazu Medical Science Promotion Foundation (Fukuoka, Japan), Mitsui Medical Science Promotion Foundation (Tokyo, Japan), The Promotion and Mutual Aid Corporation for Private Schools of Japan, and Grant-in-Aid for Scientific Research on Priority Areas (C) “Medical Genome Science” from the Ministry of Education, Science, Sports and Culture of Japan (T.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tomoaki Hoshino, Department of Internal Medicine 1, Kurume University School of Medicine, 67 Asahi-machi, Kurume 830-0011, Japan; email: hoshino@med.kurume-u.ac.jp.


![Fig. 7. Effect of NK cell depletion on IL-18/IL-2 lethality. / (A) Effect of antiasialo-GM1 Ab. SCID mice (n = 5) were pretreated with 1 mg (100 μL) antiasialo-GM1 [asialo] Ab or control normal rabbit serum (NRS) on days 0 and 7 to deplete the NK-cell population. The NK-cell–depleted SCID mice were then treated daily with control PBS or IL-18 (1 μg) plus IL-2 (100 000 IU) once a day for 10 days. (B) Effect of anti-NK1.1 mAb. B6 mice (n = 5) were pretreated with 0.5 mg anti-NK1.1 mAb, CD4 mAb, CD8 mAb, or control antibody on days 0 and 7. Mice were then treated daily with IL-18 (1 μg) plus IL-2 (100 000 IU) once a day for 10 days.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/4/10.1182_blood.v99.4.1289/6/m_h80422124007.jpeg?Expires=1769415955&Signature=Ippz1Wtlfll-q396-RfBvEhYKIGtZaM0YnER4e0jNSfjUU1LwPvTRvkM3sDyiaHzKCgG8FalrHbO3ghhjSCj8jT-dYvJmNlyNG0NVfus9h5C56S-duwqw5hrfmwWTcZTm4ZT6pffJm1JGebMlf8A2XBzSh20MY-jott0HGJqo0jRGbQ1Av2Fxtya5BhLjt6ztJgrx7SccTcPqGHUq1wggXMwLdYZEH78PCvmEJ6TT9GJ0gDG7lL-kz7kh7NHYXAcrl6mLaM9cI1qFwKzG3l3GdE4971xwTSlvZWjHZ6qifUKU5XZnIjGtXd4XnIgUlsjzXaBu9rcA3uyW2czq8wo2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal