During mammalian development, definitive hematopoietic stem cells (HSCs) arise in the aorta-gonad-mesonephros (AGM) region and colonize the fetal liver (FL) before hematopoiesis occurs in the bone marrow. The FL is a unique hematopoietic organ where both HSCs and mature blood cells are actively generated along with functional maturation of hepatic cells as a metabolic organ. To characterize HSCs and FL microenvironments during development, this study establishes a coculture system composed of AGM-originated HSCs and FL nonhematopoietic cells. The results demonstrate that FL cells support significant expansion of lineage-committed hematopoietic cells as well as immature progenitors. More important, long-term repopulating activity was amplified from AGM-originated HSCs in this coculture system. Engraftment of HSCs to the bone marrow was strongly enhanced by coculture. In addition, AGM HSCs produced significantly more hematopoietic cells than E14.5 and E18.5 FL HSCs in vitro. These results suggest that the FL microenvironment not only stimulates expansion of the hematopoietic system, but also possibly modifies the characteristics of AGM HSCs. Thus, this coculture system recapitulates the developmental process of HSCs and the FL microenvironment and provides a novel means to study the development of hematopoiesis.
Introduction
Development of the hematopoietic system is a complex process that takes place in several distinct hematopoietic microenvironments.1-3 The initial hematopoietic activity, known as primitive hematopoiesis, appears in the blood island of the yolk sac (YS) at embryonic day 7.5 (E7.5) in mice. Adult-type definitive hematopoiesis begins in the aorta-gonad-mesonephros (AGM) region at E10.54,5 and thereafter shifts to the fetal liver (FL) at E12.5, where massive production of various hematopoietic cells occurs. Then, near birth, hematopoiesis shifts to the bone marrow and spleen. Therefore, the FL is a main hematopoietic organ during the embryonic period. The transition of hematopoietic sites accompanies changes in the characteristics of hematopoietic activity.3Primitive hematopoiesis in the YS is characterized by production of nucleated erythrocytes with fetal hemoglobin and by the absence of lymphoid and myeloid cells except for macrophages.6-8However, YS cells are unable to reconstitute all types of hematopoietic cells in lethally irradiated adult mice for more than several months (long-term repopulating hematopoietic stem cells [LTR HSCs]), which is the most important characteristic of definitive HSCs.9HSCs with such activity first appear in the AGM region at E10.5 and thereafter colonize the FL and bone marrow. Therefore, the AGM region has been considered the origin of definitive HSCs. Interestingly, however, YS-derived hematopoietic cells were shown to reconstitute the entire hematopoietic system when grafted into the liver of busulfan-treated newborn recipients.10-12 These findings suggest that YS-derived hematopoietic cells acquire the HSC activity by the interaction with a specific hematopoietic microenvironment in the liver of newborn mice in which hematopoiesis is sustained. In addition, the CD34+/c-Kit+ rather than the CD34−/ c-Kit+ population in YS was able to reconstitute hematopoiesis in this transplantation system,11 distinguishing YS HSCs from bone marrow HSCs.13 Thus, the characteristics of HSCs are different among the hematopoietic organs, and the microenvironment created by each tissue appears to affect the stem cell activity and expression of cell-surface antigens in HSCs.
Although HSCs in bone marrow replicate at a constant rate to maintain hematopoiesis throughout life,14,15 HSCs in embryonic organs are believed to proliferate actively during development.3 Accumulating evidence suggests that definitive HSCs differentiate from their precursors, hemangioblasts, in the AGM at about E10.5 to E11.516 and colonize to the FL, where the most dramatic expansion of HSCs occurs from E11.5 through E14.5.17,18 Thereafter, the expansion of HSCs declines along with development18 and is essentially terminated around birth. Thus, the proliferative potential of HSCs can be different from stage to stage during development. The FL provides a distinct hematopoietic microenvironment that enables significant proliferation of HSCs, especially in the early phase of its development. On the other hand, fetal hepatic cells undergo their own maturation process to become a center of metabolism, while they function as a major hematopoietic tissue.19 20 The maturation process that leads to expression of various liver-specific genes is also regulated by extracellular signals such as hormones, cytokines, and extracellular matrices. Thus, in the FL, the 2 different cellular systems coexist and undergo their own developmental processes.
To clarify the mechanism of hematopoietic development in the AGM region, we previously developed a primary culture system of AGM-derived cells and demonstrated that oncostatin M (OSM), an interleukin-6 (IL-6) family cytokine, stimulates development of multilineage progenitors from the AGM cells in vitro.21 We also established a primary culture system of nonhematopoietic cells from E14.5 liver and found that OSM in the presence of dexamethasone (Dex), a synthetic glucocorticoid, induces maturation of fetal hepatocytes, as evidenced by morphologic changes that closely resemble differentiated hepatocytes, expression of various liver enzymes, accumulation of intracellular glycogen, lipid synthesis, and clearance of ammonia.22,23 Moreover, those FL cells also supported hematopoiesis in vitro and interestingly, such activity declined along with liver development.24 OSM is expressed in CD45+ hematopoietic cells in FL, whereas the OSM receptor is expressed predominantly in hepatocytes, suggesting that OSM is a paracrine factor in FL.22
Because HSCs generated in AGM are expected to proliferate in FL, we describe here a coculture system in which HSCs derived from E11.5 AGM (AGM HSCs) are cultivated in the microenvironment created by FL nonhematopoietic cells. AGM HSCs proliferated more efficiently than FL HSCs in the in vitro FL microenvironment, and, most important, LTR-HSC activity was significantly increased in this system. These culture systems enabled us to analyze the characteristics of HSCs and microenvironments in AGM and FL separately and provided novel in vitro models to study the development of hematopoiesis and hepatocytes.
Materials and methods
Mice
C57BL/6 mice were purchased from Nihon SLC (Hamamatsu, Japan). To distinguish the donor cells from host cells after transplantation and coculture, we isolated donor cells from transgenic mice expressing the green fluorescent protein25 (GFP mice were from Dr M. Okabe, Osaka University). GFP+ male mice were mated with GFP− female mice; therefore, the donor GFP+ cells were derived exclusively from the GFP+ embryos but not from contamination of maternal blood cells. The GFP mice were maintained and mated in the institutional animal facility according to our institutional guideline. The time at midday was considered as E0.5 for plugged mice.
Antibodies and cytokines
Monoclonal antibodies used for cell-surface analysis, phycoerythrin-conjugated anti–Mac-1 (M1/70), anti–Gr-1 (RB6-8C5), anti–Thy-1.2 (30-H12), anti-B220 (RA3-6B2), anti–Ter-119 (Ter-119), anti–c-Kit (3C1), biotinylated anti-CD34 (RAM34), and Sca1 (E13-161.7) were obtained from Pharmingen (San Diego, CA). The biotinylated antibody was visualized by allophycocyanin (APC)-conjugated streptavidin (Molecular Probe, Eugene, OR). Murine IL-3 was produced in silkworms and purified as described previously.26 IL-6 and erythropoietin (EPO) were provided by Ajinomoto (Tokyo, Japan) and Kirin Brewery (Tokyo, Japan), respectively.
AGM culture
Primary culture of the AGM cells was performed as described previously.21 In brief, the AGM region was excised from E11.5 GFP+ C57BL/6 embryos and dissociated into a single-cell suspension by trypsin digestion. The cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 15% fetal calf serum (FCS) in the presence of various cytokines: 100 ng/mL stem cell factor (SCF), 1 ng/mL basic fibroblast growth factor (bFGF; Gibco-BRL, Carlsbad, CA), 10 ng/mL murine leukemia inhibitory factor (mLIF), and 10 ng/mL mOSM. After 10 days of incubation, nonadherent hematopoietic cells spontaneously generated in cultures were harvested and analyzed for expression of cell-surface markers and progenitor activities.
AGM/FL coculture
FL cells were isolated from the E14.5 embryos as described previously22 and used as hematopoietic stromal cells.24 The cells were suspended in DMEM supplemented with 10% FCS, Insulin-Ferritin-X solution (Gibco-BRL), and 1 × 10−7 M Dex, and inoculated onto 0.1% gelatin-coated plastic dishes. A few hours later, the cells were washed extensively with phosphate-buffered saline to eliminate hematopoietic cells. Two days later, sources of hematopoietic cells were overlaid onto FL cells and cultured in DMEM supplemented with 15% FCS in the presence of various cytokines, 100 ng/mL SCF, 10 ng/mL mOSM, Insulin-Ferritin-X solution, and 1 × 10−7 M Dex. After incubation for 10 days, the cells were harvested for further analysis.
Flow cytometry
Cells generated in vitro or from the recipient mice were filtered through a nylon mesh to remove cell aggregates and debris. After staining with a monoclonal antibody according to the manufacturer's protocol, cells were analyzed by fluorescence-activated cell sorting (FACS) with the FACS-Calibur system or sorted by the FACS-Vantage system (Becton Dickinson, San Jose, CA) for further analysis
Culture colony-forming unit assay
For analysis of the progenitor activity of cells generated during cultures, donor-derived GFP+ hematopoietic cells were sorted by FACS Vantage (Becton Dickinson) and applied for assays. Two thousand hematopoietic cells were suspended in 1 mL α-minimal essential medium containing 0.8% methylcellulose, 30% FCS, 1% deionized bovine serum albumin, 100 μM 2-mercaptoethanol, 10 ng/mL IL-3, 100 ng/mL IL-6, 2 U/mL EPO, and 100 ng/mL SCF and plated onto plastic dishes (35 mm in diameter). Colony types were judged from the morphology under microscopic observation on day 14 after plating.27
In vivo repopulating assay
Adult mice (C57BL/6 males, 9-12 weeks old) were exposed to a single dose of 10 Gy from a 137Cs source. At this dose, all irradiated mice died within 2 weeks. Test cells were filtered through a nylon mesh of 70 μm (Cell Strainer; Becton Dickinson, San Jose, CA) and injected intravenously into the tail vein. Normal bone marrow cells (2 × 105) were coinjected with the test cells for radioprotection. Under this experimental condition, 10% to 20% of mice undergoing transplantation died within 2 weeks. Several months later, peripheral blood cells were collected from the tail vein and analyzed by flow cytometry. To examine the tissue distribution of donor cells, we killed the recipient mice; collected blood cells from the bone marrow, thymus, spleen, and peripheral blood; and analyzed them by flow cytometry.
Results
Establishment of a coculture system composed of AGM HSCs and FL nonhematopoietic cells
Definitive HSCs generated in AGM immediately colonize to the FL, in which they replicate themselves and generate numerous mature blood cells. To clarify the interaction between HSCs and the FL microenvironment, we developed the coculture system (Figure1). AGM cells isolated from transgenic mice expressing GFP were inoculated in subconfluent cultures of nonhematopoietic FL cells from nontransgenic mice and incubated in the presence of cytokines and hormones. After 10 days of incubation, numerous cells floating above the hepatic stromal layer were generated. Flow cytometric analysis of hematopoietic cell-surface markers as well as microscopic observation indicated that these floating cells were hematopoietic cells (data not shown). To compare the differences in the hematopoietic microenvironments between AGM and FL, we counted the number of GFP+ floating cells by FACS and found that the AGM/FL coculture produced more hematopoietic cells than the AGM culture (Figure 2). It is therefore likely that the FL microenvironment is more suitable than that of AGM for AGM HSCs to produce hematopoietic cells.
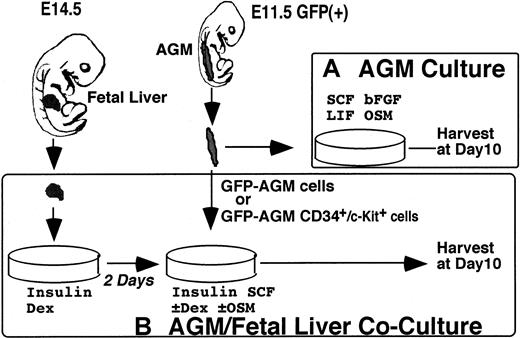
Primary culture systems used in this study.
(A) AGM culture. AGM culture was developed to analyze hematopoiesis of the AGM region in vitro. Tissue of the AGM region isolated from E11.5 embryos was dissociated into a single-cell suspension and cultured in the presence of SCF, bFGF, LIF, and OSM for 10 days. Then, floating hematopoietic cells spontaneously generated in the culture were harvested and analyzed for expression of cell-surface markers and progenitor activities. (B) AGM/FL coculture. The AGM/FL coculture system was designed to analyze the interaction between AGM HSCs and the FL hematopoietic microenvironment. Nonhematopoietic FL cells from E14.5 embryos were first cultured for 2 days to create a hematopoietic microenvironment, and then AGM-derived cells (either whole or CD34+/c-Kit+ cells) were overlaid. Ten days later, floating hematopoietic cells generated from input cells over the stromal layer were harvested and analyzed as in (A). To distinguish donor cells from recipients, GFP+ embryos were used as input cells in both systems.
Primary culture systems used in this study.
(A) AGM culture. AGM culture was developed to analyze hematopoiesis of the AGM region in vitro. Tissue of the AGM region isolated from E11.5 embryos was dissociated into a single-cell suspension and cultured in the presence of SCF, bFGF, LIF, and OSM for 10 days. Then, floating hematopoietic cells spontaneously generated in the culture were harvested and analyzed for expression of cell-surface markers and progenitor activities. (B) AGM/FL coculture. The AGM/FL coculture system was designed to analyze the interaction between AGM HSCs and the FL hematopoietic microenvironment. Nonhematopoietic FL cells from E14.5 embryos were first cultured for 2 days to create a hematopoietic microenvironment, and then AGM-derived cells (either whole or CD34+/c-Kit+ cells) were overlaid. Ten days later, floating hematopoietic cells generated from input cells over the stromal layer were harvested and analyzed as in (A). To distinguish donor cells from recipients, GFP+ embryos were used as input cells in both systems.
Expansion of AGM-derived cells by coculture with FL cells.
Floating hematopoietic cells generated under various culture conditions were harvested after 10 days of incubation. GFP+ cells were counted by FACS. Under these culture conditions, 40% to 80% of floating cells were GFP+. Results are shown as fold expansion relative to the number of input cells. Note that FL cells provided a better microenvironment for blood cell expansion than did AGM cells. Data shown are the mean ± SD of at least triplicate experiments.
Expansion of AGM-derived cells by coculture with FL cells.
Floating hematopoietic cells generated under various culture conditions were harvested after 10 days of incubation. GFP+ cells were counted by FACS. Under these culture conditions, 40% to 80% of floating cells were GFP+. Results are shown as fold expansion relative to the number of input cells. Note that FL cells provided a better microenvironment for blood cell expansion than did AGM cells. Data shown are the mean ± SD of at least triplicate experiments.
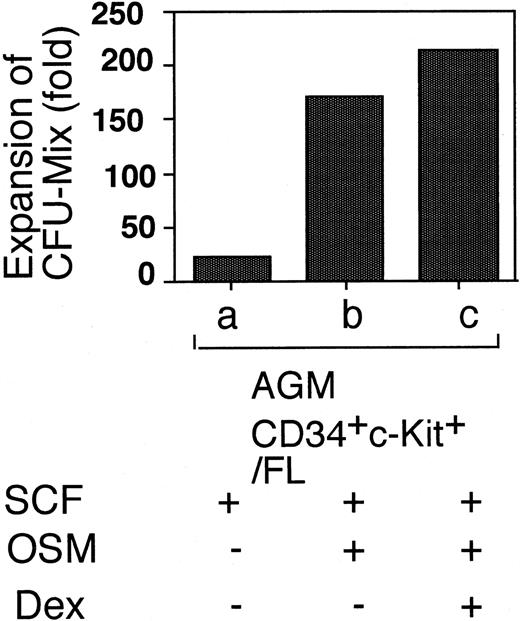
We next examined the factors required for hematopoiesis in the 2 culture systems. SCF was essential for hematopoiesis in both AGM culture and AGM/FL coculture (data not shown). In contrast to AGM culture, which requires OSM for hematopoiesis, OSM was not absolutely required for the production of hematopoietic cells in AGM/FL coculture (Figure 2, lane b). In agreement with our previous observation24 that hematopoiesis from FL-derived HSCs in the FL microenvironment in vitro was inhibited by OSM and Dex, the number of cells produced in AGM/FL coculture was significantly reduced in the presence of OSM and Dex (Figure 2, lanes b-d).
Characteristics of AGM HSCs and FL HSCs
Because LTR HSCs in AGM were reported to express CD34 and c-Kit,28 we fractionated AGM-derived cells into 2 populations: the CD34+/c-Kit+ cells and the remainder (ie, CD34+/c-Kit−, CD34−/c-Kit+, and CD34−/c-Kit− cells), and examined their potential to generate hematopoietic cells in the AGM/FL coculture system (Figure 3). We found that hematopoietic cells were produced only from the CD34+/c-Kit+ population in AGM, but not from the remaining cell populations (data not shown). Furthermore, the efficiency of expansion was higher in the culture with CD34+/ c-Kit+ cells than in the culture with the whole AGM cells. These findings indicate that the CD34+/c-Kit+ cells in AGM produce hematopoietic cells, and the remaining cells neither give rise to hematopoietic cells nor enhance hematopoiesis in the coculture system.
Expansion of AGM- or FL-derived CD34+/c-Kit+ cells in the FL microenvironment.
CD34+/c-Kit+ cells were sorted from E11.5 AGM, E14.5 FL, or E18.5 FL and cultured in the E14.5 FL microenvironment. The number of GFP+ floating cells generated over the stromal layer was counted by FACS on day 10. Under these culture conditions, 40% to 80% of floating cells were GFP+. Results are shown as fold expansion relative to the number of input CD34+/c-Kit+ cells in each culture condition. (Lane a) Whole AGM cells from 3 embryos containing 1.8 × 104 CD34+/c-Kit+ cells were cultured in the microenvironment created by the AGM cells. (Lanes b-d) Whole AGM cells (1 × 105) containing 2 × 103 CD34+/c-Kit+ cells were cocultured in the FL microenvironment in the presence of various factors, as indicated. (Lanes e-m) CD34+/c-Kit+cells (2 × 103) from different sources were cultured in the FL microenvironment under various culture conditions. Data shown are the mean ± SD of at least triplicate experiments.
Expansion of AGM- or FL-derived CD34+/c-Kit+ cells in the FL microenvironment.
CD34+/c-Kit+ cells were sorted from E11.5 AGM, E14.5 FL, or E18.5 FL and cultured in the E14.5 FL microenvironment. The number of GFP+ floating cells generated over the stromal layer was counted by FACS on day 10. Under these culture conditions, 40% to 80% of floating cells were GFP+. Results are shown as fold expansion relative to the number of input CD34+/c-Kit+ cells in each culture condition. (Lane a) Whole AGM cells from 3 embryos containing 1.8 × 104 CD34+/c-Kit+ cells were cultured in the microenvironment created by the AGM cells. (Lanes b-d) Whole AGM cells (1 × 105) containing 2 × 103 CD34+/c-Kit+ cells were cocultured in the FL microenvironment in the presence of various factors, as indicated. (Lanes e-m) CD34+/c-Kit+cells (2 × 103) from different sources were cultured in the FL microenvironment under various culture conditions. Data shown are the mean ± SD of at least triplicate experiments.
Because LTR HSCs in FL are present in the lineage−/CD34+/ c-Kit+population,28 production of hematopoietic cells from the CD34+/c-Kit+ cells derived from E14.5 and E18.5 FL in the FL microenvironment was compared with that from E11.5 AGM. The AGM-derived CD34+/c-Kit+ cells produced the highest number of hematopoietic cells among the 3 sources of HSCs (Figure 3). Because the hematopoietic activity of the E18.5 FL CD34+/c-Kit+ cells was considerably less than that of E14.5 FL, it is likely that the potential of HSCs to generate hematopoietic cells declines along with the progression of embryonic development. The effect of OSM was also different in the 2 sources of HSCs; OSM enhanced the generation of hematopoietic cells from the AGM-derived CD34+/c-Kit+ cells, whereas it suppressed the production of hematopoietic cells from the FL-derived CD34+/c-Kit+ cells at E14.5 and E18.5 in coculture with E14.5 FL cells. Dex in the presence of OSM further reduced the production of hematopoietic cells. These findings indicate that OSM has different effects on hematopoiesis between AGM HSCs and FL HSCs in the same FL microenvironment.
Because the floating hematopoietic cells were likely to be a mixture of lineage-committed cells and immature cells, we examined hematopoietic progenitor activity of these cells by in vitro colony-formation assays (Table 1). The colony-forming activity was found only in CD34+/c-Kit+ cells in AGM. Whereas the frequency of colony-forming unit-culture (CFU-C) was not significantly different among the culture conditions, CFU-GEMM was markedly expanded in the presence of OSM (Figure4). In conclusion, OSM enhanced the production of total hematopoietic cells and immature hematopoietic progenitors from AGM HSCs. Although the addition of Dex suppressed the production of total hematopoietic cells, it enhanced the production of immature progenitors from AGM HSCs in the FL microenvironment. Because OSM and Dex induce maturation of hepatic cells,22maturation of nonhematopoietic FL cells may provide a more suitable microenvironment for expansion of immature progenitors, but not for production of mature blood cells from AGM HSCs.
In vitro colony-formation assay
| Cells . | CFU-C (/104 cells) . | CFU-GEMM (/104 cells) . | CFU-C (/dish) . | CFU-GEMM (/dish) . |
|---|---|---|---|---|
| AGM CD34+/c-Kit+ | 54 ± 15 | 7 ± 7 | 12.6 | 1.4 |
| AGM (CD34+/c-Kit+)− | 0.15 ± 0.14 | 0 | — | — |
| AGM CD34+/c-Kit+/FL coculture | ||||
| SCF | 50 ± 12 | 0.4 ± 0.5 | 1.1 × 104 | 92 |
| SCF + OSM | 75 ± 30 | 1 ± 2 | 2.6 × 104 | 330 |
| SCF + OSM + Dex | 40 ± 5 | 2 ± 0.8 | 0.7 × 104 | 360 |
| Cells . | CFU-C (/104 cells) . | CFU-GEMM (/104 cells) . | CFU-C (/dish) . | CFU-GEMM (/dish) . |
|---|---|---|---|---|
| AGM CD34+/c-Kit+ | 54 ± 15 | 7 ± 7 | 12.6 | 1.4 |
| AGM (CD34+/c-Kit+)− | 0.15 ± 0.14 | 0 | — | — |
| AGM CD34+/c-Kit+/FL coculture | ||||
| SCF | 50 ± 12 | 0.4 ± 0.5 | 1.1 × 104 | 92 |
| SCF + OSM | 75 ± 30 | 1 ± 2 | 2.6 × 104 | 330 |
| SCF + OSM + Dex | 40 ± 5 | 2 ± 0.8 | 0.7 × 104 | 360 |
GFP+ cells were sorted from coculture of GFP+ AGM-derived CD34+/c-Kit+ with FL cells in the presence of cytokines, as indicated, at day 10. For comparison, CD34+/c-Kit+ cells and the remainder (CD34+/c-Kit−, CD34−/c-Kit+, and CD34−/ c-Kit− cells; AGM [CD34+/c-Kit+]−) of primary E11.5 AGM cells were also applied to colony-formation assays. The number and type of colonies were determined at day 14. The number of each type of colony was normalized to 1 × 104 cells applied to the assay. In AGM/FL coculture, 2 × 103CD34+/c-Kit+ cells were cocultured with FL cells for 10 days, and resulted in 2.3 × 106, 3.3 × 106, and 1.8 × 106 cells with SCF, SCF + OSM, and SCF + OSM + Dex, respectively.
AGM indicates aorta-gonad-mesonephros; FL, fetal liver; SCF, stem cell factor; OSM, oncostatin M; Dex, dexamethasone; CFU-GEMM, colony-forming unit-granulocyte, erythrocyte, macrophage, and megakaryocyte.
Expansion of CFU-GEMM in the AGM/FL coculture.
Immature hematopoietic progenitors in floating cells generated in vitro were examined by colony assays. GFP+ cells were sorted from total hematopoietic cells generated and used for an in vitro colony-formation assay. As a control, CD34+/c-Kit+ cells in freshly isolated E11.5 AGM were similarly tested. Fold expansion relative to the control level is shown in the figure. The number and type of colonies were determined at day 10. Data shown are mean values of at least triplicate experiments.
Expansion of CFU-GEMM in the AGM/FL coculture.
Immature hematopoietic progenitors in floating cells generated in vitro were examined by colony assays. GFP+ cells were sorted from total hematopoietic cells generated and used for an in vitro colony-formation assay. As a control, CD34+/c-Kit+ cells in freshly isolated E11.5 AGM were similarly tested. Fold expansion relative to the control level is shown in the figure. The number and type of colonies were determined at day 10. Data shown are mean values of at least triplicate experiments.
Repopulating activity of HSCs generated in vitro
Because the most important characteristic of HSCs is the ability to reconstitute the entire hematopoietic system in vivo, we transplanted the GFP+ hematopoietic cells generated in vitro into lethally irradiated adult mice through the tail vein and analyzed the donor contribution in the peripheral blood of recipient mice. We compared the repopulating activity in the following 3 cell populations: total hematopoietic cells generated in the AGM culture originating from 6 embryos, and the cells generated in the AGM/FL coculture originating from either whole AGM cells (1 × 105) or the CD34+/c-Kit+fraction of AGM cells (2 × 103), both of which were equivalent to one third of the AGM cells from a single embryo. Two months after transplantation, GFP+ cells in the peripheral blood of recipients were analyzed by FACS (Figure5A). GFP+ cells were found in the myeloid (Mac-1+, Gr-1+ cells), erythroid (Ter119+ cells), and lymphoid (B220+, Thy-1+ cells) compartments, as described previously.16 When the cells derived from the AGM culture were transplanted, approximately 12% of peripheral blood cells were found to be GFP+ (Figure 5A, lane a). However, the cells from the AGM/FL coculture originating from only one third of a single embryo were sufficient to achieve a comparable level of donor contribution (Figure 5A, lanes c, f), suggesting that the number of in vivo repopulating cells was increased during the AGM/FL coculture. As in immature progenitors, the repopulation activity was dependent on the presence of OSM during coculture, and the addition of Dex further elevated the efficiency of repopulation (Figure 5A, lanes d, g). These findings indicate that fetal hepatic cells provide a better hematopoietic microenvironment than AGM for the maintenance and expansion of the HSC activity of AGM HSCs.
In vivo repopulating activity of cells generated in vitro.
(A) To analyze HSC activity in vivo, hematopoietic cells generated in vitro under various culture conditions were transplanted into lethally irradiated adult mice. Donor contribution in peripheral blood of recipient mice was analyzed by FACS at 2 months after transplantation. Donor cells used are as follows: (lane a) cells derived from AGM culture originating from 6 embryo equivalents (see Figure 1); (lanes b-d) cells from AGM/FL coculture originating from one-third embryo equivalent of whole AGM cells (approximately 1 × 105 cells); and (lanes e-g) CD34+/c-Kit+ cells (2 × 103) derived from AGM (one-third embryo equivalent). Data shown are the mean ± SD of 3 to 5 experiments. (B) LTR activity of cells either freshly isolated from the E11.5 AGM region or generated in vitro. Mice were killed at various times, and peripheral blood cells were analyzed for donor contribution by FACS. Data are the mean ± SD values of 3 to 5 experiments.
In vivo repopulating activity of cells generated in vitro.
(A) To analyze HSC activity in vivo, hematopoietic cells generated in vitro under various culture conditions were transplanted into lethally irradiated adult mice. Donor contribution in peripheral blood of recipient mice was analyzed by FACS at 2 months after transplantation. Donor cells used are as follows: (lane a) cells derived from AGM culture originating from 6 embryo equivalents (see Figure 1); (lanes b-d) cells from AGM/FL coculture originating from one-third embryo equivalent of whole AGM cells (approximately 1 × 105 cells); and (lanes e-g) CD34+/c-Kit+ cells (2 × 103) derived from AGM (one-third embryo equivalent). Data shown are the mean ± SD of 3 to 5 experiments. (B) LTR activity of cells either freshly isolated from the E11.5 AGM region or generated in vitro. Mice were killed at various times, and peripheral blood cells were analyzed for donor contribution by FACS. Data are the mean ± SD values of 3 to 5 experiments.
To analyze LTR-HSC activity in the cultured cells, we examined the donor contribution in peripheral blood for a longer period after transplantation (Figure 5B). The donor contribution by freshly isolated AGM cells in recipient mice was 15% at 1 month after transplantation and reached 53% by 3 months after transplantation. This level was maintained for at least 5 months. Flow cytometric analysis of hematopoietic lineage markers in the recipient indicated that donor cells were present in myeloid, lymphoid, and erythroid compartments (data not shown). Consistent with previous studies,4,5these results indicate that E11.5 AGM contained LTR HSCs. On the other hand, donor contribution of cultured AGM cells was approximately 14% during the first 4 months after transplantation and thereafter declined to 2%, indicating that the majority of hematopoietic progenitor cells generated in AGM culture were short-term repopulating cells (STRCs)/committed progenitors and that AGM culture was unable to maintain LTR HSCs effectively. In sharp contrast, when AGM cells were cultivated in the FL microenvironment in the presence of SCF and OSM, a high level of donor contribution (up to 70%) was readily achieved within 1 month and was maintained for at least 5 months. These results indicate that AGM/FL cocultured cells contained a significant number of LTR HSCs and STRCs. It is generally accepted that LTR HSCs contribute to hematopoiesis, but STRCs disappear at 5 months after transplantation.29 30 The level of donor contribution by AGM/FL cocultured cells was higher (82%) than that by freshly isolated AGM cells (52%), even though the number of cells added to the AGM/FL coculture was 9-fold less than that to the AGM culture. It is therefore likely that the FL microenvironment increases LTR HSCs derived from AGM in the presence of SCF and OSM.
Engraftment of HSCs to the bone marrow
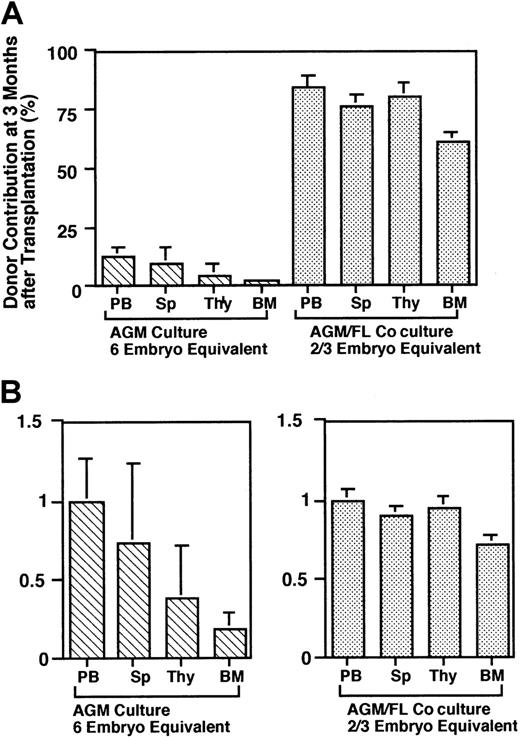
The donor contribution in the peripheral blood as shown above strongly suggested that LTR HSCs are generated in AGM/FL coculture from AGM HSCs, whereas AGM culture produces mainly STRCs. Therefore, there is a clear difference in characteristics of hematopoietic progenitor cells generated from AGM HSCs between the AGM and FL microenvironments. Because hematopoiesis from STRCs is not sustained longer than 4 months after transplantation,29 30we analyzed the donor contribution in various hematopoietic organs at 3 months after transplantation (Figure 6A). The cells generated in AGM culture were poorly engrafted in every organ tested. The efficiency of engraftment of progenitor cells to hematopoietic organs relative to peripheral blood cells revealed that engraftment to the bone marrow was particularly low in comparison with other organs (Figure 6B, left panel). The level of donor contribution in peripheral blood was similar to that in spleen (Figure 6B, left panel), suggesting that spleen is the dominant hematopoietic site for cultured AGM cells. In contrast, the AGM/FL cocultured cells contributed at high levels to all organs including the bone marrow (Figure 6A), and there was no substantial difference in the efficiency of engraftment to each organ (Figure 6B, right panel). Taken together with the results of the long-term repopulation analysis, the data suggest that cultivation of AGM cells in the AGM microenvironment generates mainly STRCs, which reside in the spleen even 3 months after transplantation. In contrast, AGM/FL coculture in the presence of OSM generates both STRCs/committed progenitor cells and LTR HSCs from AGM HSCs, which effectively colonize in spleen and bone marrow.
Donor contribution in hematopoietic tissues.
(A) Recipient mice were killed at 3 months after transplantation, and the levels of the donor contribution in hematopoietic tissues were determined by FACS. (B) Relative contribution of donor cells in various hematopoietic tissues. To compare the contribution of donor cells among various hematopoietic tissues in recipients, the levels of the donor contribution in various tissues relative to that in peripheral blood cells were calculated. The cells from AGM cultures were poorly colonized, particularly in the bone marrow.
Donor contribution in hematopoietic tissues.
(A) Recipient mice were killed at 3 months after transplantation, and the levels of the donor contribution in hematopoietic tissues were determined by FACS. (B) Relative contribution of donor cells in various hematopoietic tissues. To compare the contribution of donor cells among various hematopoietic tissues in recipients, the levels of the donor contribution in various tissues relative to that in peripheral blood cells were calculated. The cells from AGM cultures were poorly colonized, particularly in the bone marrow.
Discussion
HSCs are the best characterized stem cell system and have been used to study various concepts in stem cell biology.31 To clarify the characteristics of HSCs and the microenvironment during embryonic development, we have developed a primary coculture system composed of AGM-derived hematopoietic cells and nonhematopoietic FL cells. This system is capable of supporting AGM HSCs to generate a number of lineage-committed progenitors and LTR HSCs. The cell population in AGM contributing to the production of hematopoietic cells in the FL microenvironment was found exclusively in CD34+/c-Kit+ cells, consistent with the previous findings that HSCs in both AGM and FL were detected only in the CD34+/c-Kit+ fraction.28 HSCs change their proliferation potential during development, and AGM HSCs produced significantly more hematopoietic cells than FL HSCs in the FL microenvironment. Furthermore, E18.5 FL HSCs produced fewer hematopoietic cells than E14.5 FL HSCs in the coculture system (Figure3, lanes h-m). These findings strongly suggest that the proliferation potential of HSCs to produce hematopoietic cells decreases along with development. Alteration of HSC characteristics is also suggested by their response to OSM in the FL microenvironment: OSM enhanced the generation of hematopoietic cells from the AGM-derived CD34+/ c-Kit+ cells, whereas it suppressed hematopoiesis from the CD34+/ c-Kit+ cells derived from FL at E14.5 and E18.5 in coculture with E14.5 FL cells.
The most striking difference between the AGM culture and the AGM/FL coculture was their ability to support expansion of hematopoietic progenitors and LTR HSCs. Consistent with previous observations,5,28 we also found LTR-HSC activity in freshly isolated E11.5 AGM cells (Figure 5B). The donor contribution in peripheral blood in our system was relatively lower than those in previous reports.5 28 In previous studies, donor cells with a genetic marker were detected by polymerase chain reaction (PCR)–based methods, whereas we used the GFP transgenic mouse as a source of donor cells and detected donor cells in the recipients by flow cytometry. Although PCR is very sensitive to detect donor cells, flow cytometry provides a more quantitative means to estimate donor contribution. Thus, the difference between our results and those of previous studies may be due to a difference in the detection systems. Although the AGM culture increased the number of total hematopoietic cells, cultured AGM cells exhibited mainly STRC/committed progenitor activity. In sharp contrast, the AGM cells grown in the FL microenvironment showed remarkable LTR-HSC activity (Figure 5B). Whereas 6 embryos were required for LTR using freshly isolated AGM cells, AGM/FL cocultured cells originated from only two thirds of a single embryo were sufficient to reconstitute hematopoiesis with higher levels of donor contribution as early as 1 month after engraftment, and it lasted at least 5 months. These findings indicate that LTR HSCs and STRC/committed progenitor cells are generated from AGM HSCs in coculture and contribute to high levels of hematopoietic reconstitution immediately after transplantation.
The efficiency of the in vivo repopulating activity was highest when coculture was performed in the presence of both OSM and Dex, consistent with the generation of immature progenitors (Figure 4). These findings apparently contradict the results shown in Figures 2 and 3, as well as previous observations24 showing that the number of total hematopoietic cells generated in the FL microenvironment was reduced in the presence of both OSM and Dex regardless of the source of hematopoietic cells. However, as described previously,24lineage-committed cells were more susceptible to OSM and Dex in the FL microenvironment. Therefore, it is conceivable that OSM and Dex affect the multilineage progenitor (CFU GEMM) and/or lineage-committed cells. Our previous findings showed that the combination of OSM and Dex causes functional maturation of hepatic cells in primary FL culture to express metabolic enzymes.22 By this combination, LTR-HSC activity was strikingly expanded while the production of mature blood cells was suppressed. In conclusion, these findings suggest that our culture system is an excellent in vitro model of FL development, in which expansion of LTR HSCs from AGM HSCs and maturation of hepatic cells progress coordinately.
The cells from the AGM/FL coculture were efficiently repopulated not only in the peripheral blood, but also in various hematopoietic organs including the bone marrow at 3 months after transplantation. In contrast, the cells from the AGM culture poorly colonized in recipients, particularly in the bone marrow (Figure 6). These findings are well correlated with the previous observation that colonization to the bone marrow is a necessary step for transplanted HSCs to maintain their LTR activity immediately after transplantation.32Consistent with this, the donor contribution in the peripheral blood by the cells from AGM culture gradually decreased to a level similar to that in the bone marrow. It was shown previously that the bone marrow homing activity of HSCs derived from adult bone marrow declines by in vitro culture, despite a significant increase in the number of hematopoietic cells.33 Because the spleen contains abundant committed progenitors, our results with transplantation of AGM cultured cells indicate that transplanted STRCs/committed progenitor cells also reside in spleen. The AGM/FL coculture system allowed dramatic expansion of multilineage progenitor cells as well as LTR HSCs with high efficiency of engraftment to the bone marrow. These findings also support that engraftment in bone marrow is necessary for maintenance of LTR-HSC activity in the recipient. Because LTR-HSC activity was amplified in vitro, this system will be useful for gene transfer to LTR HSCs by retroviral vectors. Although the molecular basis of how HSCs repopulate to hematopoietic organs is yet to be investigated, it is possible that engraftment is mediated by soluble as well as membrane-bound proteins such as chemokines and integrins. Therefore, the FL microenvironment stimulated with OSM and Dex may modulate expression of the receptors for such molecules in HSCs. Alternatively, it may selectively expand HSCs expressing these molecules. The coculture system also recapitulates these alterations of HSCs in vitro.
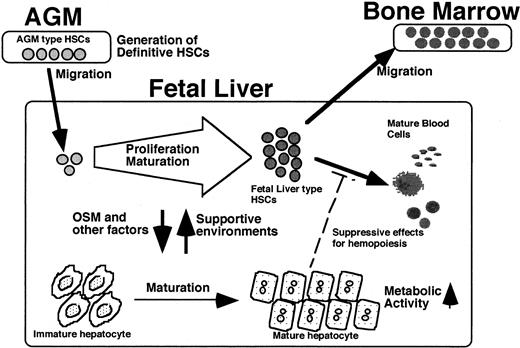
Taking these findings together with our previous observations,22-24 a model for development of the hematopoietic system during embryonic life can be suggested (Figure7). Definitive HSCs first arise in the AGM region from hemangioblasts. Because the AGM microenvironment poorly supports hematopoiesis, HSCs immediately colonize to the FL, where they proliferate as authentic LTR HSCs and produce mature hematopoietic cells with the help of the FL microenvironment. During this process, highly proliferative AGM HSCs change their characteristics to FL HSCs, with less proliferative potential. However, as reported previously,22 fetal hepatic cells are also stimulated with factors such as OSM, which are produced by hematopoietic cells, and undergo their own developmental program to become a metabolic organ rather than a hematopoietic microenvironment. Thus, the 2 different cellular systems existing in the same organ coordinately regulate their developmental processes. The present in vitro system provides a novel means to dissect such complex processes at the cellular and molecular levels.
A possible model for coordinated development of HSCs and liver.
LTR HSCs with CD34+/c-Kit+ generated in AGM immediately migrate to the FL. Factors including OSM that are secreted from hematopoietic cells induce maturation of hepatic cells as a metabolic organ. The hepatic cells, in turn, stimulate expansion and maturation of AGM HSCs to FL HSCs with reduced proliferation potential. The fetal hepatic cells provide a hematopoietic microenvironment suitable for expansion of AGM HSCs but not of FL HSCs. Accordingly, HSCs migrate from the liver to bone marrow, the final destination of HSCs.
A possible model for coordinated development of HSCs and liver.
LTR HSCs with CD34+/c-Kit+ generated in AGM immediately migrate to the FL. Factors including OSM that are secreted from hematopoietic cells induce maturation of hepatic cells as a metabolic organ. The hepatic cells, in turn, stimulate expansion and maturation of AGM HSCs to FL HSCs with reduced proliferation potential. The fetal hepatic cells provide a hematopoietic microenvironment suitable for expansion of AGM HSCs but not of FL HSCs. Accordingly, HSCs migrate from the liver to bone marrow, the final destination of HSCs.
We are grateful to Dr Masaru Okabe of Osaka University for providing us with GFP transgenic mice and to Kirin and Ajinomoto for cytokines. The whole-body irradiation of mice was done at Cs-137 γ-ray Irradiation Facilities for Biological Research, Research Center for Nuclear Science and Technology, University of Tokyo, with kind assistance by Mr Hoshio Eguchi.
Supported in part by Grants-in-Aid for Scientific Research and Special Coordination Funds from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Core Research for Evolutional Science and Technology (CREST) of Japan Science and Technology; and the Organization for Pharmaceutical Safety and Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Atsushi Miyajima, Institute of Molecular and Cellular Biosciences, the University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-0032, Japan; e-mail: miyajima@ims.u-tokyo.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal