Chemokines and chemokine receptors are key mediators for regulating cell traffic and positioning in both homeostatic and inflammatory conditions. It is also presumed that chemokines and their receptors are likely to play a critical role in the localization of malignant hematopoietic cells in their target organs. This study analyzed chemokine and chemokine receptor expression in several Hodgkin disease (HD)–derived cell lines and in HD tumors. All HD-derived cell lines expressed functional CCR7 and CXCR4 receptors. CCR7 up-regulation was mediated by constitutive NF-κB activity. Lymphoid tissues in HD revealed differential expression levels of CCR7, CXCR4, and CXCR5, depending on the distinct subtypes of HD. HD of the classical subtypes, predominantly located in the interfollicular zone, showed strong CCR7 and CXCR4 expression and moderate CXCR5 expression. In contrast, the nodular lymphocyte-predominant HD (NLP) subtype, regularly associated with follicular structures, exhibited no CCR7 reactivity but abundant CXCR4 staining. Their respective chemokine ligands showed marked expression by reactive cells within the tumors of classical HD and outside of the tumor nodules in NLPHD. Functionally, such differential chemokine receptor expression might contribute to specific localization and confinement of neoplastic cells within the target organs in the distinct HD entities.
Introduction
The chemokine superfamily consists of chemoattractant cytokines, which are subdivided into 4 major groups on the basis of the relative positions of the first 2 Cys residues (C, CC, CXC, and CXXC). Chemokines exert their attractant properties by binding to 7 transmembrane-domain G protein–coupled receptors differentially expressed on leukocyte subsets. The chemokines and their receptors participate in many pathophysiologic conditions, such as inflammation and autoimmunity. Independent of their roles as proinflammatory agents, they appear to control normal hematopoietic and lymphocyte homeostasis, including lymphoid development and lymphocyte homing into and out of secondary and tertiary lymphoid organs. Chemokine-directed migration of B and T cells into such secondary lymphatic organs is a prerequisite for the development of an antigen-specific immune response.1-4 In addition, chemokines and their receptors play an important role in angiogenesis, tumor growth, and metastasis.5 6
Hodgkin disease (HD) is a lymphoid malignancy characterized by the presence of a minority of neoplastic cells, the mononucleated Hodgkin (H) and the multinucleated Reed-Sternberg (RS) cells, surrounded by abundant inflammatory cells. Within the inflammatory background, the majority of infiltrating reactive cells are T lymphocytes, eosinophils, histiocytes, and plasma cells.7,8 Recent studies have demonstrated that HD is associated with abnormal cytokine production, which may contribute to reduced T-cell immunity and inefficient antitumor responses despite a vast majority of infiltrating reactive immune cells. The cytokine imbalance of HD is characterized by elevated local levels of interleukin-1 (IL-1), IL-3, IL-5, IL-6, IL-7, IL-8, lymphotoxin-α, tumor necrosis factor-α, and granulocyte-macrophage colony-stimulating factor, which may account for the constitutive symptoms of HD.9-13 More recently, it has been shown that chemokines are present in HD tissues and that these locally produced chemokines may also be implicated in accumulation of the abundant reactive infiltrates in classical HD (cHD) and nodular lymphocyte-predominant (NLP) HD.14-16 The expression of the chemokines TARC and MDC by Hodgkin/Reed-Sternberg (HRS) cells may explain the influx of lymphocytes with an anergic and/or Th2-like subtype by binding to the CC chemokine receptor CCR4, specifically expressed by Th2 cells. In addition, HRS cells induce fibroblasts to secrete eotaxin, another potent chemoattractant for T cells and eosinophils.17 However, there is limited information on chemokine receptor expression patterns in HRS cells and their involvement in the migration and dissemination of tumor cells into lymph nodes.
In this study, we investigated the expression patterns of a number of chemokines and chemokine receptors in HD-derived cell lines and in HRS cells from patients with HD.
HD comprises 2 distinct entities: the rare NLPHD and the common cHD form. NLPHD and cHD differ mainly in histologic features, such as cytology of the tumor cells and composition of the reactive infiltrates, immunophenotypic and genetic characteristics of the tumor cells, and distribution of the neoplastic cells within the targeted lymph node.7,18 19 Tumor cells of NLPHD (L&H cells) frequently reside within follicular structures containing follicular dendritic cells, whereas the tumor cells of cHD (HRS cells) are predominantly found in the interfollicular zone or, less frequently, in the follicular mantle zone of partially infiltrated nodes. Thus, confining tumor cells in distinct lymphoid compartments may be essentially mediated by selective expression of chemokine receptors on neoplastic cells of a distinct HD entity. To test this hypothesis, we examined the expression of the CXC chemokine receptors CXCR4, CXCR5, and CCR7 and their respective chemokine ligands stromal cell–derived factor-1 (SDF-1; CXCL12), B-lymphocyte chemoattractant-1 (BCA; BLC, CXCL13), and the 2 ligands for CCR7 Epstein-Barr virus–induced molecule-1 ligand chemokine (ELC; Exodus-3, MIP-3β, CCL19), and secondary lymphoid organ chemokine (SLC; Exodus-2, 6Ckine, CCL21) in primary HD tissues and in some related lymphoma types. Our findings demonstrate that chemokine and chemokine receptor expression patterns differ between the distinct entities of HD and define a role for differential chemokine receptor expression in the infiltration of HRS cells into their targeted lymphoid organs.
Materials and methods
Cell lines and culture
The Hodgkin lymphoma–derived cell lines L428, L540, L591, L1236, HD-MyZ,20 HLMH-2, and KM-H221 were kindly provided by Dr B. Dörken (Max-Delbrück-Center, Berlin, Germany). The erythroid leukemia cell line K562, the pre–B-cell line Reh, and the mature B-cell line Namalwa were obtained from American Type Culture Collection (Rockville, MD). All cell lines were maintained in RPMI 1640 medium (Seromed-Biochrom, Hamburg, Germany), 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, and penicillin-streptomycin.
RNase protection assay
The hCR5 template set from Pharmingen (Becton Dickinson, Heidelberg, Germany) contained DNA templates for CCR1, CCR2, CCR2a, CCR2b, CCR3, CCR4, CCR5, CCR8, reduced glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and L32. The hCR6 template contained DNA templates for CXCR1, CXCR2, CXCR3, CXCR4, BLR1 (CXCR5), BLR2 (CCR7), V28, GAPDH, and L32. RNase protection assay (RPA) was performed according to the manufacturer's instructions using α32P-UTP (Amersham Pharmacia Biotech, Freiburg, Germany). The RNase-protected fragments were run on denaturing polyacrylamide–urea sequencing gel, which was then absorbed to filter paper and dried under vacuum. Autoradiography was subsequently performed using Kodak XAR-S film with a Transcreen LE intensifying screen (Eastman Kodak, Rochester, NY).
Northern blot analysis
Total RNA was prepared from all samples using the Trizol reagent (Gibco BRL, Karlsruhe, Germany) according to the supplied protocol. Isolation of mRNA was performed with the micro mRNA purification kit (Amersham Pharmacia Biotech) according to the manufacturer's instructions. Northern blot hybridization analysis was carried out according to standard methods.22 For hybridization, we used α32P-labeled cloned CXCR5, CCR7, BCA, ELC, SLC, SDF-1, or GAPDH cDNA probes of human origin. The labeling reaction was performed with a random primer labeling kit (Gibco BRL).
Flow cytometry
The following antibodies were used for cell-surface staining of HD-derived cell lines: mouse anti–human CCR1, rat anti–human CCR3, mouse anti–human CXCR3, mouse anti–human CCR5, mouse anti–human CCR6 (R&D Systems, Wiesbaden, Germany); rat anti–human CXCR4 (clone 2B11),23 rat anti–human CXCR5 (clone 8B2),24rat anti–human CCR7 (clone 3D12),25 26 phycoerythrin (PE)–conjugated goat anti–rat IgG, and PE-conjugated donkey anti–mouse IgG (Dianova, Hamburg, Germany). Irrelevant isotype-matched antibodies were used as controls. Flow cytometry analysis was performed using CellQuest software on a FACScan (Becton Dickinson).
Chemotaxis assay
Chemotaxis assays were performed in 5-μm–pore Transwell plates (Costar, Cambridge, MA) for 3 to 4 hours at 37°C. Briefly, chemotactic factors diluted in assay medium were added to the 12-well tissue culture plates in a final volume of 500 μL. Collagen-coated transwells were inserted into each well, and 1 × 105cells of the HD-derived cell lines were added to the top chamber in a final volume of 100 μL. Migrated cells were collected and counted in a Neubauer chamber. Recombinant human ELC, SLC, and BCA (R&D Systems) were used at a concentration of 100 nM, and recombinant SDF-1α (TEBU, Frankfurt, Germany) was used at a concentration of 10 nM.
Electrophoretic mobility shift assay
Whole-cell extracts were prepared as described previously.27 After washing of the cells with phosphate-buffered saline, lysis buffer was added (20 mM HEPES, pH 7.9, 350 mM NaCl, 0.5 mM EDTA, 0.1 mM EGTA, 1 mM MgCl2, 10% glycerol, 1% Nonidet P-40, 1 mM dithiothreitol, and a cocktail of protease and phosphatase inhibitors). After 10 minutes of incubation at 4°C, the lysate was centrifuged for 5 minutes at 14 000 rpm in a microfuge. Electrophoretic mobility shift assay (EMSA) was performed as described previously.28
Virus construction, preparation, and infection
Construction and preparation of Ad5-IκBΔN and Ad5-control have been described previously.29 For adenoviral infection, 1 × 107 cells were pelleted and resuspended in RPMI 1640 medium (supplemented with 10% FCS) at a concentration of 1 × 107 cells per milliliter. Viruses were added (multiplicity of infection of 300), and cells were incubated for 2 hours at 37°C and 5% CO2. After infection, cells were again pelleted and resuspended at a concentration of 3 × 105 cells per milliliter.
Tissue specimens
Formalin-fixed and paraffin-embedded biopsy specimens of the following diseases were taken from the archives of the Institute of Pathology, Klinikum Benjamin Franklin: 2 cases of hyperplastic tonsils, 18 cases of nodular sclerosis (NS) HD, 11 cases of mixed cellularity (MC) HD, and 8 cases of NLPHD. All diagnoses were established according to the revised European-American Classification of Lymphoid Neoplasms.30
Immunohistochemistry
Four-micrometer sections of paraffin-embedded tissue blocks were stained by the immunoalkaline phosphatase method.31 The primary monoclonal reagents used in this study were rat anti–human CXCR4,23 rat anti–human CXCR5,24 and rat anti–human CCR725 hybridoma culture supernatants at a dilution of 1:10. All antibodies required high-pressure cooking to optimally visualize the antigens in paraffin sections.
In situ hybridization
The cDNA probes were prepared by subcloning ELC, SLC, BCA, or SDF-1α gene cDNA fragments in the runoff transcription vector pGEM1 (Promega Biotec, Heidelberg, Germany). After linearization, runoff antisense transcripts with incorporation of 35S-labeled UTP and CTP were generated using T7 RNA polymerases (Promega Biotec). In situ hybridization (ISH) was performed on routinely fixed paraffin-embedded tissue sections using standard laboratory protocols.32 The slides were exposed for 3 to 6 weeks.
Results
Chemokine receptor RNA expression on HD-derived cell lines
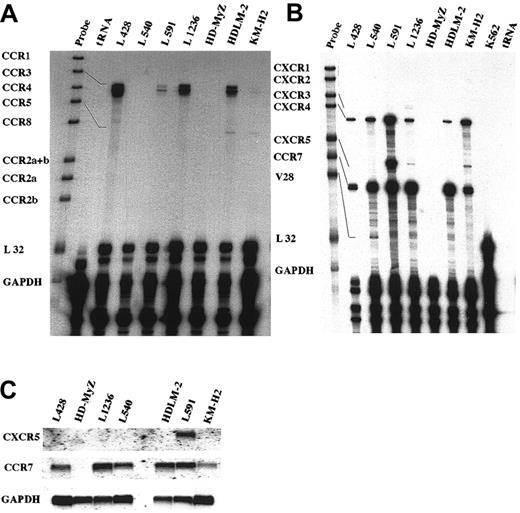
Seven different HD-derived cell lines were analyzed for chemokine receptor RNA expression by RPA and Northern blot (Figure1). The majority of these tumor lines were found to be related to lymphocytes21 with one exception, the HD cell line HD-MyZ. HD-MyZ displays myelomonocytic features and also differs immunophenotypically from HRS cells in being CD30−, CD15−, and CD68−.20 Besides these substantial deviations, HD-MyZ did not express any chemokine receptors tested in this study. The human erythroid leukemia cell line K562 was used as a negative control. RPA was performed with 2 template sets containing probes for CCR1, CCR2, CCR2a, CCR2b, CCR3, CCR4, CCR5, CCR8, and the housekeeping genes GAPDH and L32 (template set hCR5) (Figure 1A); and CXCR1, CXCR2, CXCR3, CXCR4, BLR1 (CXCR5), BLR2 (CCR7), V28, GAPDH, and L32 (template set hCR6) (Figure 1B). We found that CCR7 was highly expressed throughout all HD cell lines except for HD-MyZ, whereas CXCR5 was mainly expressed on L591 and to a weaker extent on L1236 and KM-H2. CXCR4 was highly expressed on 6 of 7 cell lines; CXCR3 and CCR3 RNA expression was quite heterogeneous; and only weak expression was detected for V28 (fractalkine) and CCR5 RNA in 1 or 2 cell lines. No RNA expression was detected for the remaining CC and CXC chemokine receptors (Figure 1A,B). Northern blot analysis was performed with cDNA fragments of CXCR5 and CCR7 as radiolabeled probes. Among all 7 HD-derived cell lines, a specific signal for CCR7 was detected on 6 of 7 cell lines, whereas CXCR5 RNA was significantly expressed only on the Epstein-Barr virus–positive L591 cell line (Figure 1C).
Differential chemokine receptor RNA expression in HD-derived cell lines.
(A,B) RNase protection assay was performed using the hCR5 template set (A) containing DNA templates for CCR1, CCR2, CCR2a, CCR2b, CCR3, CCR4, CCR5, CCR8, GAPDH, and L32; and the hCR6 template (B) containing DNA templates for CXCR1, CXCR2, CXCR3, CXCR4, BLR1 (CXCR5), BLR2 (CCR7), V28, GAPDH, and L32. Seven HD cell lines were studied, along with an erythroid leukemia cell line as a negative control (Figure 2B). (C) Expression of the chemokine receptors CXCR5 and CCR7 in HD-derived cell lines. Northern blot analysis was performed using total RNA (20 μg) isolated from the HD cell lines L428, HD-MyZ, L1236, L540, HDLM-2, L591, and KM-H2. The blot was hybridized with CXCR5- and CCR7-specific cDNA probes and with GAPDH cDNA as a housekeeping probe.
Differential chemokine receptor RNA expression in HD-derived cell lines.
(A,B) RNase protection assay was performed using the hCR5 template set (A) containing DNA templates for CCR1, CCR2, CCR2a, CCR2b, CCR3, CCR4, CCR5, CCR8, GAPDH, and L32; and the hCR6 template (B) containing DNA templates for CXCR1, CXCR2, CXCR3, CXCR4, BLR1 (CXCR5), BLR2 (CCR7), V28, GAPDH, and L32. Seven HD cell lines were studied, along with an erythroid leukemia cell line as a negative control (Figure 2B). (C) Expression of the chemokine receptors CXCR5 and CCR7 in HD-derived cell lines. Northern blot analysis was performed using total RNA (20 μg) isolated from the HD cell lines L428, HD-MyZ, L1236, L540, HDLM-2, L591, and KM-H2. The blot was hybridized with CXCR5- and CCR7-specific cDNA probes and with GAPDH cDNA as a housekeeping probe.
Chemokine receptor surface expression on HD-derived cell lines
Using a number of monoclonal antibodies directed against the chemokine receptors CXCR3, CXCR4, CXCR5, CCR3, CCR5, CCR6, and CCR7, we analyzed surface expression of these receptors on HD-derived cell lines. In line with our RNA expression data, we found high expression of CXCR4 on all 6 cell lines (except HD-MyZ), high expression of CCR7 on 4 cell lines and moderate signals on 2 cell lines, and high expression of CXCR5 on L591 (Figure2). CXCR3 (Figure 2) and CCR3 (data not shown) exhibited high to moderate surface expression on some cell lines, whereas none of these cell lines exhibited CCR5 or CCR6 immunoreactivity (data not shown).
Flow cytometry analysis of cell-surface expression of chemokine receptors in Hodgkin lymphoma–derived cell lines.
Chemokine receptor expression was analyzed in the HD cell lines L428, L540, L591, L1236, HDLM-2, and KM-H2 with antibodies directed against CXCR4, CXCR5, CCR7, and CXCR3 (solid lines) or isotype antibodies (dotted lines). One representative experiment of 4 to 6 independent experiments is shown.
Flow cytometry analysis of cell-surface expression of chemokine receptors in Hodgkin lymphoma–derived cell lines.
Chemokine receptor expression was analyzed in the HD cell lines L428, L540, L591, L1236, HDLM-2, and KM-H2 with antibodies directed against CXCR4, CXCR5, CCR7, and CXCR3 (solid lines) or isotype antibodies (dotted lines). One representative experiment of 4 to 6 independent experiments is shown.
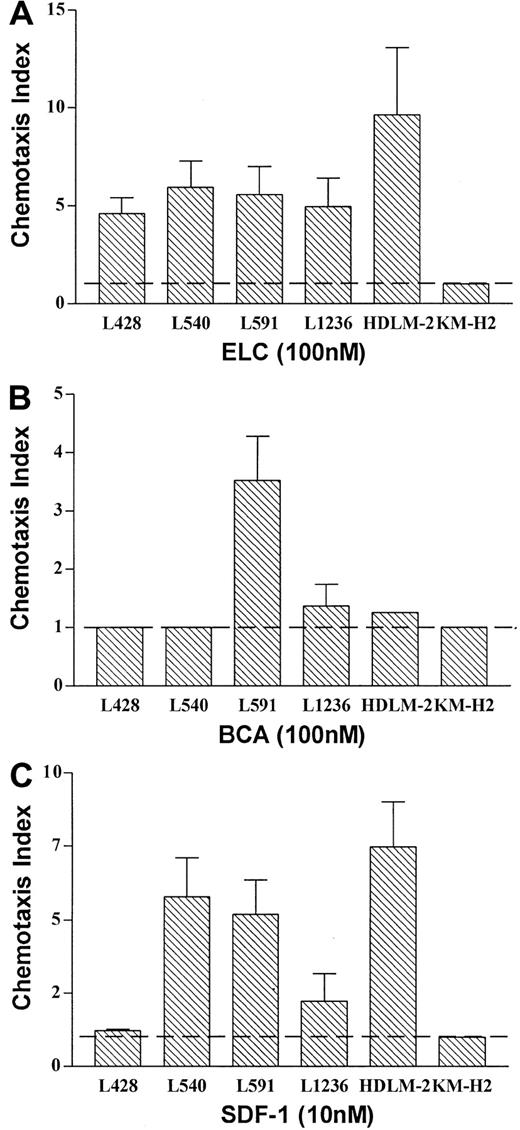
Chemokine receptors expressed on HD-derived cell lines represent fully functional receptors involved in chemotaxis
To test for the functionality of the observed chemokine receptor expression, we performed a chemotaxis assay. All cell lines expressing a distinct chemokine receptor showed migratory capacity in response to the respective chemokine ligand, with one exception: The HD cell line KM-H2 did not migrate toward SDF-1, although CXCR4 was expressed at significant levels (Figure 2; FACS analysis). Thus, HD cell lines expressing CCR7, CXCR5, and CXCR4 have the capacity to migrate toward the respective ligands ELC (Figure3A) and SLC (data not shown), BCA (Figure3B) and SDF-1 (Figure 3C), whereas cell lines expressing only CCR7 and/or CXCR4 migrate toward ELC/SLC and/or SDF-1, but not toward BCA (Figure 3A-C).
Chemokine receptors expressed on Hodgkin lymphoma–derived cell lines represent fully functional receptors involved in chemotaxis.
(A-C) The number of HD cells that migrated at indicated concentrations of ELC (A), BCA (B), and SDF-1 (C) is given relative to the number of HD cells that migrated at medium control, which was set arbitrarily at 1 (chemotaxis index). Results represent mean values of 3 to 6 independent experiments, and errors are shown as SD.
Chemokine receptors expressed on Hodgkin lymphoma–derived cell lines represent fully functional receptors involved in chemotaxis.
(A-C) The number of HD cells that migrated at indicated concentrations of ELC (A), BCA (B), and SDF-1 (C) is given relative to the number of HD cells that migrated at medium control, which was set arbitrarily at 1 (chemotaxis index). Results represent mean values of 3 to 6 independent experiments, and errors are shown as SD.
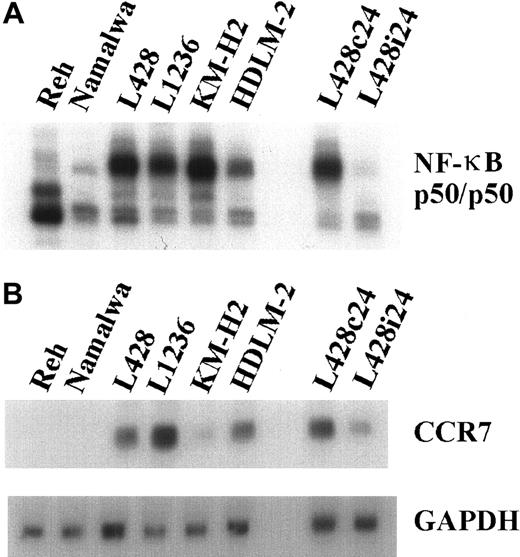
CCR7 expression on HD-derived cell lines is NF-κB dependent
To define the molecular basis of chemokine receptor overexpression, we investigated whether constitutive NF-κB activity, which has been recognized as a common feature in HD cell lines and in primary HRS cells,33 is involved in their transcriptional regulation. Constitutive NF-κB binding activity was down-modulated by adenovirus-mediated expression of the superrepressor IκBΔN in L428 cells (Figure 4A). CCR7 mRNA expression was analyzed in L428 cells infected with Ad5-IκBΔN or Ad5-control and in different control and HD cell lines (Figure 4B). NF-κB inhibition caused a significant reduction of CCR7 mRNA (Figure 4B). Hence, high expression of CCR7, typical for HRS cells, is dependent on constitutive NF-κB activity. In contrast, CXCR4 expression is NF-κB independent (data not shown).
CCR7 expression is NF-κB dependent.
(A) NF-κB binding activity. Whole-cell extracts of HD cell lines and control cells were analyzed by EMSA using H2K binding site probe for NF-κB. (B) RNA was extracted 24 hours after infection from L428 cells infected with Ad5-control (L428c24) or Ad5-IκBΔN (L428i24), and from different control and HD cell lines. Northern blot analysis was performed using an α32P-labeled CCR7 cDNA probe (upper panel). As a control, the stripped blot was reprobed with an α32P-labeled GAPDH cDNA probe (lower panel).
CCR7 expression is NF-κB dependent.
(A) NF-κB binding activity. Whole-cell extracts of HD cell lines and control cells were analyzed by EMSA using H2K binding site probe for NF-κB. (B) RNA was extracted 24 hours after infection from L428 cells infected with Ad5-control (L428c24) or Ad5-IκBΔN (L428i24), and from different control and HD cell lines. Northern blot analysis was performed using an α32P-labeled CCR7 cDNA probe (upper panel). As a control, the stripped blot was reprobed with an α32P-labeled GAPDH cDNA probe (lower panel).
Expression patterns of chemokine receptors in HD
To verify our in vitro data, we examined the expression patterns of the chemokine receptors CXCR4, CXCR5, and CCR7 on primary HD tissues.
Chemokine receptor expression was analyzed in paraffin sections of the MC, NS, and NLP HD subtypes. Immunostaining of hyperplastic tonsils served as a control for monoclonal antibody immunoreactivity and revealed CXCR4 (Figure5A) and CXCR5 (Figure 5E) expression, as expected, in B cells of the follicular mantle zone and a proportion of germinal center cells. CCR7 was expressed on dendritic cells in the interfollicular zone both in tonsils (data not shown) and dermatopathic lymphadenopathy (Figure 6A), with occasional faint staining of interfollicular lymphocytes. Tumor cells of a follicular B-cell lymphoma revealed CXCR4 (Figure 5B) and CXCR5 (Figure 5F) reactivity, confirming earlier data,34 35 but no CCR7 reactivity (Figure 6B).
Immunohistology of CXCR4 and CXCR5.
Immunohistochemical detection on paraffin-embedded sections using the 2B11/CXCR4 (A-C) and 8B2/CXCR5 (E-G) monoclonal antibodies. Most follicular mantle cells and some germinal center cells express CXCR4 in a hyperplastic tonsil (A). The neoplastic cells of some follicular lymphomas (B) and a case of cHD (C) are strongly CXCR4 positive. (D) In situ hybridization reveals specific transcripts for SDF-1 in a case of cHD. (E-G) Immunohistology of CXCR5. Most cells in the mantle zone and some germinal center cells of a hyperplastic tonsil show CXCR5 expression (E). Some tumor cells of a cHD (G) and tumor cells of a follicular lymphoma (F) are positive. (H-J) In situ hybridization with a probe specific for BCA showed positive accessory cells in a germinal center of a hyperplastic tonsil (H). Reactive cells in cases of cHD (I) and NLPHD (J) are labeled, whereas the neoplastic cells are negative (arrows). Original magnification, × 20 (A), × 40 (B, E), × 100 (F), × 200 (C, D, G-J).
Immunohistology of CXCR4 and CXCR5.
Immunohistochemical detection on paraffin-embedded sections using the 2B11/CXCR4 (A-C) and 8B2/CXCR5 (E-G) monoclonal antibodies. Most follicular mantle cells and some germinal center cells express CXCR4 in a hyperplastic tonsil (A). The neoplastic cells of some follicular lymphomas (B) and a case of cHD (C) are strongly CXCR4 positive. (D) In situ hybridization reveals specific transcripts for SDF-1 in a case of cHD. (E-G) Immunohistology of CXCR5. Most cells in the mantle zone and some germinal center cells of a hyperplastic tonsil show CXCR5 expression (E). Some tumor cells of a cHD (G) and tumor cells of a follicular lymphoma (F) are positive. (H-J) In situ hybridization with a probe specific for BCA showed positive accessory cells in a germinal center of a hyperplastic tonsil (H). Reactive cells in cases of cHD (I) and NLPHD (J) are labeled, whereas the neoplastic cells are negative (arrows). Original magnification, × 20 (A), × 40 (B, E), × 100 (F), × 200 (C, D, G-J).
Immunohistology of CCR7.
Immunohistochemical detection on paraffin-embedded sections using the 3D12/CCR7 (A-E) monoclonal antibody. In a case of dermatopathic lymphadenopathy, the dendritic cells in the interfollicular zone are strongly positive, whereas the follicles remain unlabeled (A). In a case of follicular lymphoma, CCR7 expression is restricted to dendritic cells, whereas the neoplastic cells are negative (B). The neoplastic cells of 2 cases of cHD are strongly positive for CCR7 (C,D). In contrast, the neoplastic cells in NLPHD (arrowheads) are negative, whereas a single cell (probably a dendritic cell) is stained (E). (F-J) Radioactive in situ hybridization with a probe specific for ELC or SLC transcripts. In a hyperplastic tonsil, ELC is observed only in the interfollicular zone (F). In cHD, reactive cells within the tumor infiltrate express ELC (G) and SLC (H). The nodules in NLPHD are negative for both chemokines (I,J), but SLC-specific signals (I) are found in the internodular areas and ELC-specific signals are observed outside the nodules (J). All chemokines are expressed in either the nonneoplastic leukocytic infiltrates of HD or stromal cells, but not in the HRS cells (arrows). Original magnification, × 20 (F, I), × 40 (A), × 100 (B, D), × 200 (C, E, G, H, J).
Immunohistology of CCR7.
Immunohistochemical detection on paraffin-embedded sections using the 3D12/CCR7 (A-E) monoclonal antibody. In a case of dermatopathic lymphadenopathy, the dendritic cells in the interfollicular zone are strongly positive, whereas the follicles remain unlabeled (A). In a case of follicular lymphoma, CCR7 expression is restricted to dendritic cells, whereas the neoplastic cells are negative (B). The neoplastic cells of 2 cases of cHD are strongly positive for CCR7 (C,D). In contrast, the neoplastic cells in NLPHD (arrowheads) are negative, whereas a single cell (probably a dendritic cell) is stained (E). (F-J) Radioactive in situ hybridization with a probe specific for ELC or SLC transcripts. In a hyperplastic tonsil, ELC is observed only in the interfollicular zone (F). In cHD, reactive cells within the tumor infiltrate express ELC (G) and SLC (H). The nodules in NLPHD are negative for both chemokines (I,J), but SLC-specific signals (I) are found in the internodular areas and ELC-specific signals are observed outside the nodules (J). All chemokines are expressed in either the nonneoplastic leukocytic infiltrates of HD or stromal cells, but not in the HRS cells (arrows). Original magnification, × 20 (F, I), × 40 (A), × 100 (B, D), × 200 (C, E, G, H, J).
Analysis of HD tissues showed differential expression of chemokine receptors in the different subtypes of this disease. In line with our in vitro studies of HD-derived cell lines, CCR7 and CXCR4 were frequently and strongly expressed in the HRS cells of cHD (Figure 5C; Figure 6C,D; and Table 1), whereas CXCR5 expression was less profound and appeared more prominent in the NS subtype (Figure 5G and Table 1). In contrast, the L&H cells of NLPHD were almost entirely devoid of CCR7 expression (Figure 6E and Table 1) and showed variable CXCR4 levels (Table 1). CXCR5 expression by L&H cells of NLPHD was difficult to evaluate because almost all reactive cells within the tumor nodules were labeled.
Chemokine receptor expression in Hodgkin disease
| Entity . | Case no. . | Proportion of positive tumor cells . | ||
|---|---|---|---|---|
| CCR7 . | CXCR4 . | CXCR5 . | ||
| HD-MC | 1 | +++ | ++ | (+) |
| HD-MC | 2 | +++ | +++ | NA |
| HD-MC | 3 | +++ | ++ | Negative |
| HD-MC | 4 | ++ | NA | NA |
| HD-MC | 5 | ++ | E | Negative |
| HD-MC | 6 | + | +++ | (+) |
| HD-MC | 7 | (+) | +++ | NA |
| HD-MC | 8 | + | +++ | (+) |
| HD-MC | 9 | (+) | ++ | Negative |
| HD-MC | 10 | E | +++ | NA |
| HD-MC | 11 | NA | E | E |
| HD-NS | 12 | +++ | +++ | ++ |
| HD-NS | 13 | +++ | +++ | NA |
| HD-NS | 14 | +++ | + | +++ |
| HD-NS | 15 | +++ | ++ | ++ |
| HD-NS | 16 | +++ | NA | NA |
| HD-NS | 17 | +++ | +++ | ++ |
| HD-NS | 18 | +++ | +++ | ++ |
| HD-NS | 19 | ++ | +++ | +++ |
| HD-NS | 20 | ++ | ++ | ++ |
| HD-NS | 21 | ++ | +++ | (+) |
| HD-NS | 22 | + | ++ | ++ |
| HD-NS | 23 | + | +++ | NA |
| HD-NS | 24 | + | (+) | (+) |
| HD-NS | 25 | + | + | NA |
| HD-NS | 26 | + | +++ | (+) |
| HD-NS | 27 | + | +++ | ++ |
| HD-NS | 28 | (+) | (+) | ++ |
| HD-NS | 29 | (+) | ++ | (+) |
| HD-NLP | 30 | (+) | ++ | + |
| HD-NLP | 31 | Negative | Negative | NA |
| HD-NLP | 32 | Negative | + | ? |
| HD-NLP | 33 | Negative | Negative | ++ |
| HD-NLP | 34 | Negative | + | + |
| HD-NLP | 35 | Negative | ++ | NA |
| HD-NLP | 36 | Negative | ++ | NA |
| Entity . | Case no. . | Proportion of positive tumor cells . | ||
|---|---|---|---|---|
| CCR7 . | CXCR4 . | CXCR5 . | ||
| HD-MC | 1 | +++ | ++ | (+) |
| HD-MC | 2 | +++ | +++ | NA |
| HD-MC | 3 | +++ | ++ | Negative |
| HD-MC | 4 | ++ | NA | NA |
| HD-MC | 5 | ++ | E | Negative |
| HD-MC | 6 | + | +++ | (+) |
| HD-MC | 7 | (+) | +++ | NA |
| HD-MC | 8 | + | +++ | (+) |
| HD-MC | 9 | (+) | ++ | Negative |
| HD-MC | 10 | E | +++ | NA |
| HD-MC | 11 | NA | E | E |
| HD-NS | 12 | +++ | +++ | ++ |
| HD-NS | 13 | +++ | +++ | NA |
| HD-NS | 14 | +++ | + | +++ |
| HD-NS | 15 | +++ | ++ | ++ |
| HD-NS | 16 | +++ | NA | NA |
| HD-NS | 17 | +++ | +++ | ++ |
| HD-NS | 18 | +++ | +++ | ++ |
| HD-NS | 19 | ++ | +++ | +++ |
| HD-NS | 20 | ++ | ++ | ++ |
| HD-NS | 21 | ++ | +++ | (+) |
| HD-NS | 22 | + | ++ | ++ |
| HD-NS | 23 | + | +++ | NA |
| HD-NS | 24 | + | (+) | (+) |
| HD-NS | 25 | + | + | NA |
| HD-NS | 26 | + | +++ | (+) |
| HD-NS | 27 | + | +++ | ++ |
| HD-NS | 28 | (+) | (+) | ++ |
| HD-NS | 29 | (+) | ++ | (+) |
| HD-NLP | 30 | (+) | ++ | + |
| HD-NLP | 31 | Negative | Negative | NA |
| HD-NLP | 32 | Negative | + | ? |
| HD-NLP | 33 | Negative | Negative | ++ |
| HD-NLP | 34 | Negative | + | + |
| HD-NLP | 35 | Negative | ++ | NA |
| HD-NLP | 36 | Negative | ++ | NA |
HD indicates Hodgkin disease; MC, mixed cellularity; NS, nodular sclerosis; NLP, nodular lymphocyte-predominant; +++, 50% to 100% positive tumor cells; ++, 20% to 50% positive tumor cells; +, 10% to 20% positive tumor cells; (+), less than 10% positive tumor cells; E, single positive tumor cells; NA, not analyzed.
Chemokine expression in HD tumors
Because specific migration of tumor cells may depend not only on their chemokine receptor expression profile, but also on the respective chemokines being released in the target organs, we evaluated the expression of ELC, SLC, SDF-1, and BCA in HD and control tissues. In the lymphoid tissue of hyperplastic tonsils, which served as a positive control for probe specificity, ELC (Figure 6), SLC, and SDF-1 (data not shown) were strongly expressed in nonlymphoid cells in the interfollicular zone but were absent within secondary follicles. BCA staining was confined to secondary follicles (Figure 5H). These findings are in agreement with previous published data.3 25
Analysis of HD subtypes revealed differential chemokine expression in reactive cells, whereas neoplastic cells were consistently negative. Thus, ELC was found in the tumor infiltrates of 14 of 15 cHD cases (Figure 6G), whereas the tumor nodules of 6 of 7 NLPHD cases were entirely or almost entirely negative for this chemokine (Figure 6J). Similar results were obtained for SLC, which was essentially confined to internodular areas in NLPHD (Figure 6I), but present in reactive cells in the tumor infiltrates of cHD (Figure 6H). SDF-1 transcripts were observed in 4 of 5 cHD cases in the tumor infiltrate and also in the fibrous septa of the NS subtype of cHD (Figure 5D), whereas in NLPHD, focal labeling occurred outside the tumor nodules (data not shown). For BCA, strong labeling was found in cHD (Figure 5I) and variable positivity in NLPHD (Figure 5J).
Discussion
Although differential chemokine expression by HRS cells and/or surrounding inflammatory cells has been established, little is known about chemokine receptor expression profiles on HRS cells and variants in cHD and NLPHD. What enables neoplastic cells to migrate and disseminate into secondary lymphoid organs? And once there, what holds them in place to mediate the release of growth and survival cytokines as well as chemokines responsible for mediating the local characteristic inflammatory infiltrate? We envisage the following scenario: Differential overexpression of chemokine receptors enables trafficking and homing of neoplastic cells into lymphoid organs. In addition, we hypothesize that the interaction between the chemokine receptors and locally released chemokines confines HRS cells within the lymphoid microenvironment and supports retention of these cells within the lymphoid organ.
To substantiate our presumptions, we characterized receptor expression profiles of homeostatic chemokine receptors normally expressed on T cells or B cells. In particular, we focused on CCR7, which is expressed on the vast majority of peripheral blood T lymphocytes, all resting T cells within secondary lymphoid organs, mature dendritic cells, and mature B cells25,26,36,37; CXCR4, which is normally expressed on T cells, pre–B cells, and mature B cells38,39; and CXCR5, which is expressed on mature and unstimulated B cells and a subset of T cells.40
As a first step, we analyzed chemokine receptor expression in 7 HD-derived cell lines. The majority of the tumor cell lines commonly expressed high levels of CXCR4 and CCR7, as demonstrated by gene expression as well as surface staining (Figures 1 and 2). CXCR5 was significantly expressed only on cell line L591 (Figures 1 and 2), whereas CXCR3 and CCR3 exhibited high to moderate surface expression levels on some other cell lines. We were not able to detect mRNA specific for the chemokines SDF-1, ELC, SLC, and BCA, which represent the relevant ligands for CXCR4, CCR7, and CXCR5, in HD cell lines (data not shown). These findings are in accordance with recently reported data from reverse transcriptase polymerase chain reaction analysis, which demonstrated absent or only weak products for SDF-1, SLC, ELC, and BCA in similar HD cell lines.15 Thus, we conclude that HD cell lines differentially express high levels of CXCR4, CCR7, and, less frequently, CXCR5, but they do not simultaneously produce the respective ligands. Previous studies have reported that several chemokines and chemokine receptors are differentially expressed on leukemic B and T cells as well as in distinct subtypes of B- and T-cell lymphoma.34,35,41-45 These reports indicate that chemokine and chemokine receptor expression may have important roles in the migration and dissemination of malignant hemopoietic cells. In this regard, increased CCR7 expression by adult T-cell leukemia (ATL) cells apparently enhances ATL cell infiltration of lymphoid organs.44 Just recently, Müller et al46reported that CXCR4 and CCR7 are highly expressed in breast cancer cells, malignant breast tumors, and metastases. Neutralizing CXCR4 in a mouse tumor model significantly decreased tumor progression and metastasis.46
To rule out that chemokine receptor expression on HD cells may be due to endogenous expression of certain genes in long-term cell cultures and to further define a potential role of chemokine receptors in the pathophysiology of HD, we investigated the presence of these same receptors in primary Hodgkin lymphoma tissues.
Our results show that the 2 distinct entities of Hodgkin lymphoma, cHD and NLPHD, also differ in chemokine and chemokine receptor expression. Tumor cells of the cHD but not of the NLPHD subtype exhibited high CCR7 expression (Figure 6 and Table 1). In contrast, CXCR4 was present in tumor cells of both diseases, although more prominently in cHD. CXCR5 was variably expressed at low abundance in cHD and NLPHD (Figure 5 and Table 1). These findings add a further distinction to the already considerable differences between cHD and NLPHD.7,18 19
In contrast to the expression pattern observed in HD, we found high expression levels of CXCR5, variable CXCR4 expression, and no detectable CCR7 reactivity in patients with follicular B-cell lymphoma (Figures 5 and 6). Earlier studies on chemokine receptor expression in B-cell lymphomas by Jones et al35 demonstrated that CXCR5 is widely expressed in B-cell lymphomas of all histologic subtypes. Although recent publications provided substantial evidence for the B-cell origin of HRS (cHD) and L&H cells (NLPHD),47,48chemokine receptor expression profiles strongly differed between HRS cells, L&H cells, and non-Hodgkin lymphoma B cells. Variable developmental and/or activation stages of the neoplastic cells may account for such differences. Results derived from gene microarray analysis49 have shown that the lymphocyte activation antigen CD30 up-regulates multiple gene products critical for lymphocyte homing; that is, CD30 signals strongly induce CCR7 gene expression. These results provide one potential mechanism for the observed CCR7 overexpression in HRS cells, which generally express CD30, and the lack of CCR7 expression in L&H cells and non-Hodgkin lymphoma B cells, which are CD30−. In addition, analysis of the CCR7 promoter sequence revealed 2 potential binding sites for NF-κB,50 which suggests that CCR7 could be a direct NF-κB–dependent target gene. In this report, we show that CCR7 up-regulation in HD-derived cell lines was indeed dependent on constitutive NF-κB activity (Figure 4). Because the characteristic CD30 expression in cHD might be involved in aberrant NF-κB activity, CCR7 up-regulation seems to be a consequence of 2 impaired pathways that are partially interconnected.33
What is the potential biologic significance of these findings? ELC and SLC, the ligands of CCR7, are physiologically expressed in the T-cell zone of secondary lymphoid organs and have been shown to mediate homing of T cells and dendritic cells to this compartment. A similar loop appears to be involved in the homing of HRS cells because these 2 chemokines are consistently found in the tumor infiltrate of cHD, and the tumor cells themselves express CCR7. In contrast, tumor nodules of NLPHD are almost completely devoid of both chemokines and their receptors. This fits with the histology of NLPHD, which is characterized by nodular structures related to B-cell follicles, because these latter structures do not display CCR7 immunoreactivity under physiologic conditions. However, in cases where L&H cells are located outside the tumor nodules, they did not express CCR7, and HRS cells occasionally lying within the follicular mantle zone were not devoid of this receptor (results not shown). This indicates that within the microenvironment of Hodgkin lymphoma, other chemokine–receptor interactions are likely to participate in the distribution of tumor cells. CXCR4 and its ligand SDF-1 may also be involved in the homing of HRS cells of cHD, whereas the chemokine BCA and its chemokine receptor CXCR5, typically expressed in follicular structures and on follicular B cells, apparently play a minor role in this process. Considering the frequent association of the L&H cells with the follicular dendritic cell meshworks, one might expect stronger CXCR5 expression on these cells. This hypothesis was difficult to verify because most cells in the tumor nodules of NLPHD expressed CXCR5, and therefore evaluation of its expression by L&H cells was not feasible. A possible explanation for low CXCR5 expression on HRS cells may be defective expression of the transcription factor Oct2 and its coactivator BOB1 in HRS cells51 because CXCR5 is a target gene for Oct2 and BOB1.52
CCR7 expression in HRS cells is consistent with the frequent homing of HRS cells to the interfollicular zone in partially involved lymph nodes and corresponds with the absence of these tumor cells in germinal centers, the anatomic compartment from which these cells are thought to be derived. The intensity of CCR7 immunoreactivity on HRS cells was striking and resembled that of mature dendritic cells, which are known to up-regulate CCR7 upon maturation. The staining intensity was much stronger than that observed on all reactive lymphoid cells encountered in our investigations. Although HRS cells are described to be of lymphoid origin, they share some features with dendritic cells, including the expression of restin, fascin, and TARC.53-56At the moment, there is no satisfactory explanation for these interesting similarities, and further investigation is required.
In conclusion, high expression of chemokines may cause an influx of reactive cells into the lymphoid tissue and thereby contribute to the characteristic lymphocyte infiltrate. Alternatively, binding of these chemokines to their respective receptors on HRS cells in HD could promote regulation of tumor localization, chemotaxis, or adhesion of tumor cells to nodal microenvironments. Overexpression of chemokine receptors, in particular CCR7, on HRS cells in combination with significant expression of the respective chemokine ligands by surrounding reactive cells might represent a hallmark of cHD and define distinct Hodgkin lymphoma entities.
We are grateful to Dr I. Anagnostopoulos for assistance with immunohistology and to D. Breitfeld and E. Berg for excellent technical assistance. We are indebted to Dr A. Rehm for critical reading of the manuscript and continuous discussions. We thank Prof Dr B. Dörken for the HD-derived cell lines and Dr G. Müller for his help with the preparation of the figures.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martin Lipp, Max-Delbrück-Center for Molecular Medicine, Robert-Rössle-Str 10, D-13092 Berlin, Germany; e-mail: mlipp@mdc-berlin.de.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal