Abstract
Red cell development depends on the binding of erythropoietin (EPO) to receptors expressed by erythroid colony-forming units (CFUe) and the subsequent activation of receptor-bound Janus kinase (Jak2). Jak2 then mediates the phosphorylation of receptor tyrosine sites and the recruitment of 25 or more Src homology 2 domain-encoding proteins and associated factors. Previous studies have shown that an EPO receptor form containing Jak2-binding domains plus a single phosphotyrosine343 (PY343)–STAT5-binding site provides all signals needed for erythroid cell development. However, roles for PY343 and STAT5 remain controversial, and findings regarding PY-null receptor activities and erythropoiesis in STAT5-deficient mice are disparate. To study activities of a PY-null EPO receptor in primary cells while avoiding compensatory mechanisms, a form retaining domains for Jak2 binding and activation, but lacking all cytoplasmic tyrosine sites, was expressed in transgenic mice from aGATA1 gene-derived vector as a human epidermal growth factor receptor- murine EPO receptor chimera (EE-T-Y343F). The bio-signaling capacities of this receptor form were investigated in CFUe from thiamphenicol-treated mice. Interestingly, this PY-null EPO receptor form supported CFUe development (in the absence of detectable STAT5 activation) at efficiencies within 3-fold of those levels mediated by either an EE-T-Y343 form or the endogenous EPO receptor. However, EE-T-Y343F–dependent Ter119+ erythroblast maturation was attenuated. In tests of cosignaling with c-Kit, EE-T-Y343F nonetheless retained full capacity to synergize with c-Kit in promoting erythroid progenitor cell proliferation. Thus, EPO receptor PY-dependent events can assist late erythropoiesis but may be nonessential for EPO receptor–c-Kit synergy.
Introduction
Red blood cell production depends on the interaction of erythropoietin (EPO) with its single transmembrane receptor. In EPO−/− or EPO receptor−/−mice, definitive erythropoiesis is blocked at the erythroid colony-forming unit (CFUe) stage.1 EPO is secreted from kidney and fetal liver in response to hypoxia,2 targets erythroid lineage- and stage-specific EPO receptor-positive cells primarily in marrow,3 and is clinically important in the treatment of anemia.4 As a class 1 cytokine receptor,5 the EPO receptor initiates EPO-dependent signal transduction events by activating Janus kinase (Jak) 2, which binds preformed EPO receptor dimers at a conserved Box1 motif.6,7 Activated Jak2 is then thought to phosphorylate the EPO receptor at 8 cytoplasmic tyrosine sites, and primary Src homology 2 domain-encoding proteins are recruited. Analyses using truncated and tyrosine-mutated murine EPO (mEPO) receptors have assigned several proteins to specific phosphotyrosine (PY) sites (reviewed in references 8 and 9), including signal transducers and activators of transcription (STAT)5a and 5b at PY343, PY401, SHP1, and SHP2 tyrosine phosphatases at PY429/431 and PY401, respectively, Ship1 inositol 5-phosphatase at PY401, PY429, PY431,10 p85 regulatory subunit of phosphatidylinositol 3 (PI-3) kinase at PY479, APS adaptor at PY343, Grb2 adaptor at PY464, Lyn tyrosine kinase at PY464, and Cis1 and Cis3/SOCS3 suppressors of cytokine signaling at PY401.11 Additional signal transduction factors that have been shown to bind activated EPO receptor complexes include the tyrosine kinases Syk and Tec, phospholipase C-γ, the adaptors Shc, Cbl, CrkL, IRS-2, and Gab1/2, and the nucleotide exchange factors mSOS, Vav, and C3G.8,9Finally, signals generated by this collection of factors also integrate with signals from several coexpressed receptors, including those for stem cell factor (SCF), interleukin-3, and insulinlike growth factor-1.8 9
Despite the complexity of this EPO receptor signal transduction network, studies of EPO receptor PY or carboxyl terminal truncation mutants in fetal liver and transgenic mice interestingly have demonstrated that either PY479- or PY343-directed pathways alone can efficiently support CFUe development.12-14 Within the PY479 route, PI-3 kinase, Akt kinase, and FKHRL1 Forkhead transcription factor have been implicated as important downstream effectors,15 and PY343 has been shown to target STAT5 andbcl-x.16,17 Regarding PY343 and STAT5, however, their roles in EPO receptor signaling and erythropoiesis are controversial. In several cell line models, PY343 has been documented as dispensable18,19or essential for efficient EPO receptor-dependent growth, survival, andbcl-x gene transcription.17,20-22 In addition, in STAT5a−/− and STAT5b−/− mice, no defects in embryonic or adult erythropoiesis were revealed in one analysis,23 while a second described marked fetal anemia and reduced hematocrit levels in 50% of neonates.16,24These disparate findings might reflect cell-line–specific effects or compensatory or non–cell autonomous complications in vivo. To investigate the signaling capacities of a PY-null EPO receptor form in primary CFUe while avoiding compensatory mechanisms potentiated in knock-out and knock-in systems, a minimal receptor form retaining domains for Jak2 binding and activation but lacking all 8 cytoplasmic tyrosine sites was expressed in transgenic mice from a GATA1gene vector25,26 as a human epidermal growth factor (hEGF)–mEPO receptor chimera (EE-T-Y343F). To allow for analyses of the signaling capacities of this minimal EPO receptor form at an appropriate stage of erythropoiesis, a thiamphenicol (TAP)–based system also was used to generate developmentally synchronized erythroid progenitor cells from these mice.14,27 28 Analyses reveal that a Jak2-activating, PY-null EPO receptor form supports CFUe development in the absence of detectable STAT5 activation. However, attenuated bioactivity of this PY-null EPO receptor form (compared with either a PY343-containing form or the endogenous EPO receptor) also is described and is linked to a stage-specific defect in erythroblast maturation. Finally, in analyses of EPO receptor and c-Kit cosignaling, PY343-containing and PY-null EPO receptor forms were observed to efficiently support SCF-dependent synergy toward CFUe proliferation. Possible mechanisms underlying this apparent EPO receptor PY-independent synergy are discussed.
Materials and methods
EPO receptor constructs and expression in transgenic mice
A cDNA encoding the minimal hEGF receptor–mEPO receptor chimera EE-T-Y343F29 was subcloned into the GATA1gene–derived vector pA2GATA.25,26 Then a KpnI to ClaI fragment (17 kb) was purified and injected into pronuclei. These preparations were implanted into pseudopregnant B6D2F1 females, and founders were identified by Southern blot analysis using BglII and a 32P-labeled, 770-bp BglII to XbaI mEPO receptor cDNA fragment.30 Transgenic mice expressing the chimera EE-T-Y343 (from the above pA2GATA vector) were generated as previously described.14 In assays of chimeric receptor expression, marrow cells were cultured under conditions shown by Panzenbock et al31 to promote the selective expansion of CFUe. At 72 hours of culture, cells were washed in 140 mM NaCl, 2.7 mM KCl, 1.2 mM KH2PO4, 8.1 mM Na2HPO4, pH 7.4, and 0.1% bovine serum albumin and were incubated at 4°C stepwise with the murine immunoglobulin (Ig)G Fc fragment (5 μg/mL, 1.5 hours) and a monoclonal antibody to the hEGF receptor extracellular domain (EGFR.1; PharMingen, San Diego, CA) (3.3 μg/mL, 1.5 hours). Cells then were washed, incubated for 30 minutes on ice with a phycoerythrin-conjugated anti–murine IgG F(ab')2 antibody (Jackson, West Grove, PA), washed again, and analyzed quantitatively by flow cytometry as described previously.14
Erythroid progenitor cell preparations and assays of CFUe development
Erythroid progenitor cells were prepared from the spleens of mice treated with TAP.27 On day 1, TAP (15 mg per gram-weight in 0.4 mL water) was implanted subcutaneously, and mice were phlebotomized (80 μL) for 3 subsequent days. On day 6, TAP was removed; at 72 hours after TAP withdrawal, splenocytes were isolated as previously described.14 For in vitro differentiation assays, erythroid splenocytes were plated at 1 × 106cells/mL in 0.1% methylcellulose (Methocult M3234; Stem Cell Technologies, Vancouver, BC, Canada), 15% fetal bovine serum, 1% delipidated bovine serum albumin, 200 μg/mL iron-saturated transferrin, 0.1 mM β-mercaptoethanol, 3 μg/mL insulin, and 150 ng/mL mSCF in Iscoves modified Dulbecco medium. Either hEPO (5 U/mL; Amgen, Thousand Oaks, CA) or hEGF (5 ng/mL; R&D Systems, Minneapolis, MN) was then added. Ter119 antigen expression was assayed using a phycoerythrin-conjugated antibody (1.5 μg/0.15 mL assay) (PharMingen). Hemoglobinized colonies were stained with 0.1 vol freshly combined solution of 1 part 1.5% benzidine in 90% acetic acid plus 6 parts 5% hydrogen peroxide. For assays of SCF-, hEGF-, and EPO-stimulated proliferation, erythroid splenocytes (5 × 106 cells/mL in 10% fetal bovine serum; OptiMEM I medium; Life Technologies, Gaithersburg, MD) were exposed to cytokines and were cultured for 48 hours. Rates of methyl–[3H]-thymidine incorporation then were determined as described.28
Northern blot and electrophoretic mobility shift assays
Splenocytes were isolated from TAP-treated mice at 72, 96, and 120 hours after TAP withdrawal. RNA then was isolated using TRIzol reagent (Life Technologies) and was analyzed by Northern blotting32 using the following32P-labeled cDNA probes: EPO receptor (1.5 kbXhoI fragment of pXMwtER), βmaj-globin (1.1-kb BglII to XbaI fragment of pGEM7-βmaj-globin), c-Kit (1.5-kbBamHI to NheI fragment of pREP4ΔEB-c-Kit), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (0.8-kbKpnI to XhoI fragment of pSP-GAPDH).32 In electrophoretic mobility shift assays, splenocytes from TAP-treated mice were cultured for 5 hours in the absence of cytokines and then were exposed for 7.5 minutes to either hEGF (15 ng/mL) or EPO (10 U/mL). STAT5 DNA-binding activities in nuclear extracts then were determined as described17 using a 32P-labeled interferon γ–activated site (5′-TGC TTC TTG GAA TT-3′) from the β-casein promoter. Unlabeled competing STAT5 or irrelevant NFκB (5′-AGC TAA GGG ACT TTC CGC TGG GGA CTT TCC AGG-3′) elements were used at a 50-fold molar excess.
Results
Chimeric PY-null and PY343-containing EPO receptor forms and expression in transgenic mice
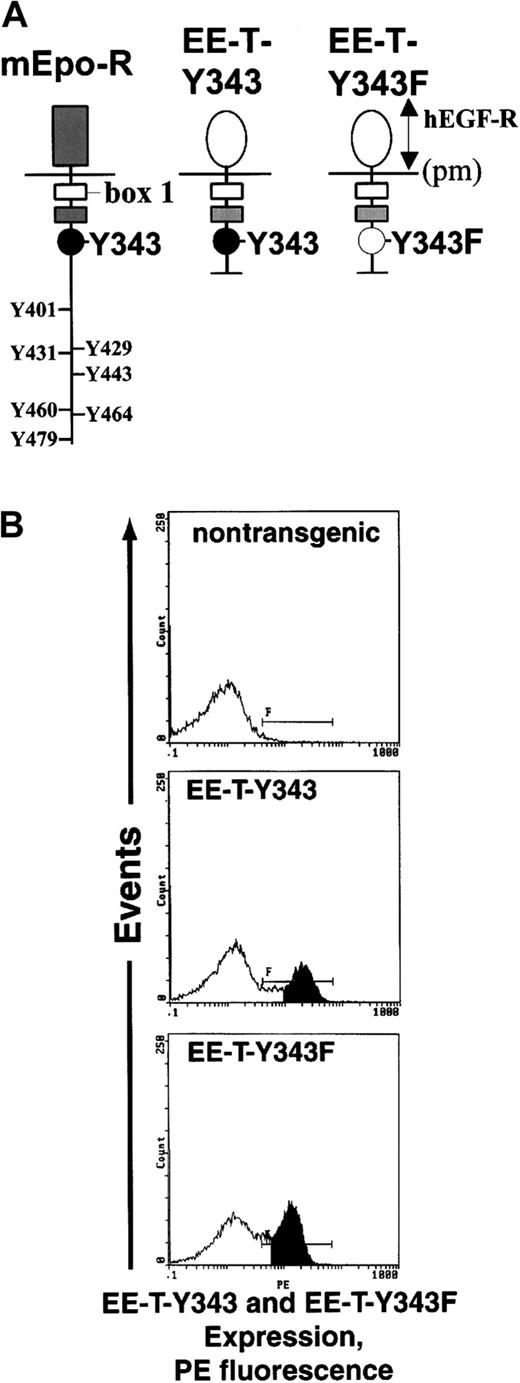
Investigations first sought to compare the abilities of 2 minimal chimeric EPO receptor forms, EE-T-Y343 and EE-T-Y343F, to support CFUe development during adult erythropoiesis. In these chimeras (Figure1, upper panel), the extracellular domain of the mEPO receptor was replaced with that of the hEGF receptor to provide for direct immunoassay of expression levels, hEGF-dependent conditional activation, and direct comparisons of signaling capacities with coexpressed endogenous EPO receptors in primary cells. Within the EPO receptor cytoplasmic region, Box1 (and 2) motifs—including a domain for Jak2 association—were retained, but the carboxyl terminus was truncated to remove 7 of 8 tyrosine residues. In EE-T-Y343, a well-defined PY343 site for STAT5 binding also was retained,33-36 whereas in EE-T-Y343F this site was mutated to phenylalanine. Previously, our laboratory37 and Pacifici et al38 have shown in cell lines that high proliferative activity is retained by chimeras fused at C620 of the hEGF receptor to P225 of the mEPO receptor and that, for a corresponding full-length chimera, hEGF dose-response curves closely parallel those for EPO and the wild-type EPO receptor. Expression of EE-T-Y343 and -Y343F receptors in transgenic mice was accomplished using a GATA1 gene–derived vector. This vector, pA2GATA, contains enhancer, promoter, and intron elements that together restrict expression to erythroid and megakaryocytic progenitor cells.25,26 To confirm comparable levels of surface expression for each chimeric EPO receptor form in the lines of transgenic mice selected for study, erythroid progenitor cells were expanded from marrow and were analyzed by flow cytometry using an hEGF receptor antibody (Figure 1, lower panels). In each case, receptor densities were estimated to be between 2000 and 5000 receptors per cell.14
EE-T-Y343 and EE-T-Y343F chimeras and expression in transgenic mice.
(A) Diagrammed are the wild-type mEPO receptor (mEpo-R) and the derived carboxyl-terminal deletion constructs EE-T-Y343 and EE-T-Y343F. In each chimera, the extracellular dimerization domain is that of the hEGF receptor (hEGF-R), and the EPO receptor cytoplasmic Box1 domain for Jak2 binding is retained (pm, plasma membrane). In EE-T-Y343, a Y343 site for STAT5 binding is retained, whereas in EE-T-Y343F this residue is mutated to phenylalanine. (B) Levels of EE-T-Y343 and EE-T-Y343F receptor expression in transgenic mice were assayed by flow cytometry of marrow cells after their culture for 72 hours in the presence of mSCF and EPO plus dexamethasone, β-estradiol, and insulinlike growth factor-1 under conditions shown by Panzenbock et al31 to selectively promote CFUe proliferation. As a control, marrow cells from nontransgenic mice were also analyzed. PE indicates phycoerythrin.
EE-T-Y343 and EE-T-Y343F chimeras and expression in transgenic mice.
(A) Diagrammed are the wild-type mEPO receptor (mEpo-R) and the derived carboxyl-terminal deletion constructs EE-T-Y343 and EE-T-Y343F. In each chimera, the extracellular dimerization domain is that of the hEGF receptor (hEGF-R), and the EPO receptor cytoplasmic Box1 domain for Jak2 binding is retained (pm, plasma membrane). In EE-T-Y343, a Y343 site for STAT5 binding is retained, whereas in EE-T-Y343F this residue is mutated to phenylalanine. (B) Levels of EE-T-Y343 and EE-T-Y343F receptor expression in transgenic mice were assayed by flow cytometry of marrow cells after their culture for 72 hours in the presence of mSCF and EPO plus dexamethasone, β-estradiol, and insulinlike growth factor-1 under conditions shown by Panzenbock et al31 to selectively promote CFUe proliferation. As a control, marrow cells from nontransgenic mice were also analyzed. PE indicates phycoerythrin.
Development of an optimized adult erythroid progenitor cell system for studies of chimeric receptor function
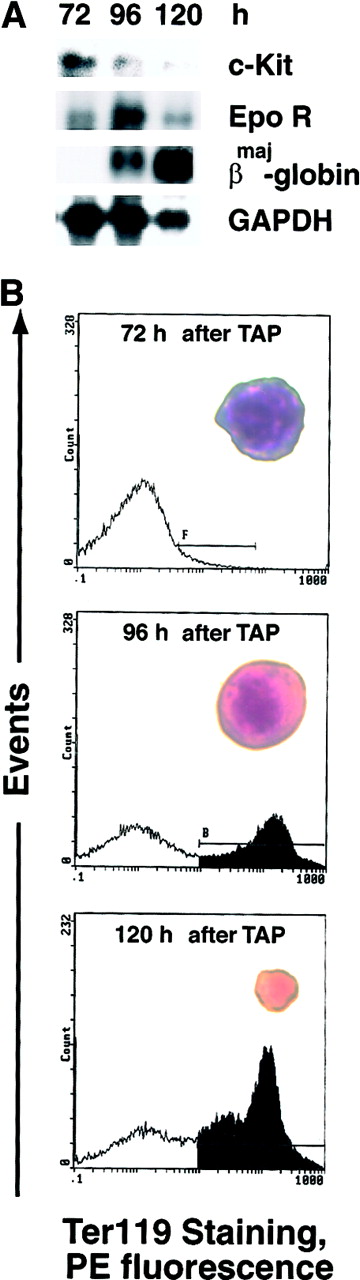
One potential complication associated with the above approach to EPO receptor signal dissection is the predicted expression of chimeric receptors at an early stage of erythroid development. A system was developed, therefore, to bypass this limitation and to provide high frequencies of developmentally synchronized CFUe. This involved the treatment of mice with TAP at doses that block the development of early erythroid progenitor cells in marrow (especially erythroid burst-forming units) and that induce a synchronous wave of splenic erythropoiesis after TAP withdrawal. As described originally by Nijhof et al27 and recently optimized by our laboratory,28 this regimen of TAP treatment yields (at 72 hours after TAP withdrawal) a relatively high-frequency cohort of CFUe. In Figure 2 (upper panel), these developing cells are profiled by Northern blot analysis of endogenous c-Kit, EPO receptor, and βmaj globin transcripts. Recently, we also optimized conditions for the efficient in vitro development of these erythroid cells.28 In Figure 2 (lower panel), their synchronous EPO-dependent development as Ter119+ cells39 is shown together with morphologic profiles (insets) of early CFUe, late CFUe, and maturing erythroblasts.
Developmental synchrony of erythroid progenitor cells from TAP-treated mice.
Mice were treated for 5 days with TAP to block the development of erythroid burst-forming units. TAP then was removed, erythropoiesis was allowed to advance, and at 72, 96, and 120 hours after TAP withdrawal, levels of c-Kit, EPO receptor and βmaj-globin transcripts in erythroid splenocytes were analyzed by Northern blotting (A). In addition, splenocytes isolated at 72 hours after TAP withdrawal were cultured in the presence of mSCF (150 ng/mL) and EPO (5 U/mL). At 24-hour intervals, expression of Ter119 antigen was assayed as an index of late differentiation (B). For each population, morphologies also were examined (insets, × 1000).
Developmental synchrony of erythroid progenitor cells from TAP-treated mice.
Mice were treated for 5 days with TAP to block the development of erythroid burst-forming units. TAP then was removed, erythropoiesis was allowed to advance, and at 72, 96, and 120 hours after TAP withdrawal, levels of c-Kit, EPO receptor and βmaj-globin transcripts in erythroid splenocytes were analyzed by Northern blotting (A). In addition, splenocytes isolated at 72 hours after TAP withdrawal were cultured in the presence of mSCF (150 ng/mL) and EPO (5 U/mL). At 24-hour intervals, expression of Ter119 antigen was assayed as an index of late differentiation (B). For each population, morphologies also were examined (insets, × 1000).
Activation of STAT5 by EE-T-Y343, but not EE-T-Y343F, in primary CFUe
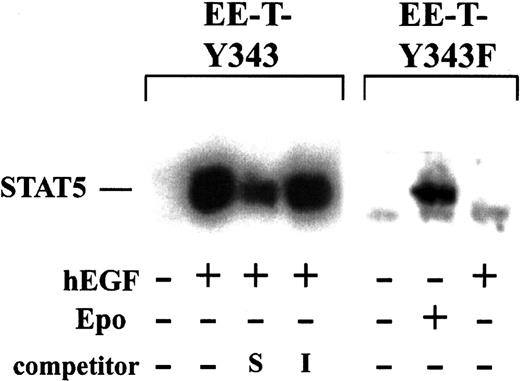
In primary CFUe isolated from TAP-treated transgenic mice, the abilities of the chimeric EPO receptor forms EE-T-Y343 and EE-T-Y343F to activate STAT5 were first assayed (Figure3). Erythroid splenocytes at the CFUe stage were isolated from mice at 72 hours after TAP withdrawal, cultured for 5 hours in the absence of cytokines, and exposed for 7.5 minutes to hEGF (±15 ng/mL). Nuclear extracts then were prepared, and STAT5 DNA-binding activity was assayed by electrophoretic mobility shift using a 32P-labeled interferon γ–activated site from the β-casein promoter.17 In these primary cells, STAT5 DNA-binding activity was activated efficiently by hEGF via EE-T-Y343 and was inhibited specifically by a 50-fold excess of this unlabeled cassette (but not by an irrelevant element from the NFκB gene promoter). In contrast, hEGF failed to activate STAT5 in cells from EE-T-Y343F mice, whereas EPO-activation of STAT5 by endogenous receptors was obvious in these cells. In parallel experiments, cells exposed to hEGF (or EPO) also were placed in CFUe differentiation assays, and the responsiveness of cells from EE-T-Y343 and EE-T-Y343F mice was confirmed (data not shown, and see below).
Efficient activation of STAT5 by EE-T-Y343, but not by EE-T-Y343F, in primary erythroid progenitor cells.
EE-T-Y343 and EE-T-Y343F mice were treated with TAP, and, at 72 hours after TAP withdrawal, erythroid splenocytes were prepared. Cells were cultured for 5 hours in the absence of cytokines and were exposed to EPO (±10 U/mL) or hEGF (±15 ng/mL) for 7.5 minutes. Nuclear extracts were prepared, and levels of STAT5 DNA-binding activity were assayed by electrophoretic mobility shift using a32P-labeled element from the β-casein promoter. Extracts prepared from hEGF-stimulated EE-T-Y343 splenocytes were preincubated with 50-fold molar excess of self (S, β-casein) or irrelevant (I, NFκB) unlabeled oligonucleotide cassettes.
Efficient activation of STAT5 by EE-T-Y343, but not by EE-T-Y343F, in primary erythroid progenitor cells.
EE-T-Y343 and EE-T-Y343F mice were treated with TAP, and, at 72 hours after TAP withdrawal, erythroid splenocytes were prepared. Cells were cultured for 5 hours in the absence of cytokines and were exposed to EPO (±10 U/mL) or hEGF (±15 ng/mL) for 7.5 minutes. Nuclear extracts were prepared, and levels of STAT5 DNA-binding activity were assayed by electrophoretic mobility shift using a32P-labeled element from the β-casein promoter. Extracts prepared from hEGF-stimulated EE-T-Y343 splenocytes were preincubated with 50-fold molar excess of self (S, β-casein) or irrelevant (I, NFκB) unlabeled oligonucleotide cassettes.
EE-T-Y343F supports CFUe formation
Next, the abilities of EE-T-Y343 and EE-T-Y343F receptor forms to support CFUe development to hemoglobinized colonies were studied. The outcomes of previous analyses of bioactivities of PY-null EPO receptor forms in retrovirally transduced CFUe from fetal liver12 40predicted that little activity would be exerted by EE-T-Y343F. However, this EPO receptor form proved to support the formation of hemoglobinized colonies at significant levels. Representative colony-forming assays are shown in Figure4A, and they illustrate that colony sizes were essentially normal but were decreased in frequency compared with the number of colonies formed on EPO activation of the endogenous wild-type EPO receptor or on hEGF-activation of the PY343-containing chimera EE-T-Y343 in EE-T-Y343 mice. Analyses of 5 independent mice confirmed an overall 3-fold relative deficiency in the ability of EE-T-Y343F to support hemoglobinized colony formation (Table 1). For EE-T-Y343, activity overall was slightly above that of the endogenous EPO receptor. In this assay system, essentially no hemoglobin-positive cells formed in the absence of EPO or hEGF (and hEGF exerted no detectable effects on erythroid progenitor cells from nontransgenic mice). Therefore, any possible production of EPO by erythroid progenitor cells (or possible elevated EPO levels in TAP-treated mice) was insufficient to affect assay outcomes.
CFUe development is supported by EE-T-Y343F but is attenuated at a Ter119+ stage of differentiation.
(A) Splenocytes were isolated from EE-T-Y343 and EE-T-Y343F mice at 72 hours after exposure to TAP. Cells then were cultured in the presence of EPO (5 U/mL) plus mSCF (150 ng/mL); mSCF with no EPO; or hEGF (5 ng/mL) plus mSCF. At 48 hours of culture, hemoglobinized colonies were stained with benzidine. Results shown are representative of 5 independent experiments (and of all mice assayed). (B) Erythroid splenocytes from TAP-treated EE-T-Y343F mice (upper subpanels) and EE-T-Y343 mice (lower subpanels) were cultured in the presence of mSCF plus EPO or hEGF. Ter119 antigen expression and relative size (FALS, forward angle light scatter) were assayed by flow cytometry. For all EE-T-Y343F mice studied, a defect in the production of more mature, small Ter119+ cells in response to hEGF was observed (arrow). No similar defect was observed in the formation of mature Ter119+ cells from these mice in the presence of EPO or in cells from EE-T-Y343 mice in the presence of hEGF or EPO. Results shown are representative of 5 independent experiments.
CFUe development is supported by EE-T-Y343F but is attenuated at a Ter119+ stage of differentiation.
(A) Splenocytes were isolated from EE-T-Y343 and EE-T-Y343F mice at 72 hours after exposure to TAP. Cells then were cultured in the presence of EPO (5 U/mL) plus mSCF (150 ng/mL); mSCF with no EPO; or hEGF (5 ng/mL) plus mSCF. At 48 hours of culture, hemoglobinized colonies were stained with benzidine. Results shown are representative of 5 independent experiments (and of all mice assayed). (B) Erythroid splenocytes from TAP-treated EE-T-Y343F mice (upper subpanels) and EE-T-Y343 mice (lower subpanels) were cultured in the presence of mSCF plus EPO or hEGF. Ter119 antigen expression and relative size (FALS, forward angle light scatter) were assayed by flow cytometry. For all EE-T-Y343F mice studied, a defect in the production of more mature, small Ter119+ cells in response to hEGF was observed (arrow). No similar defect was observed in the formation of mature Ter119+ cells from these mice in the presence of EPO or in cells from EE-T-Y343 mice in the presence of hEGF or EPO. Results shown are representative of 5 independent experiments.
hEGF-dependent formation of hemoblobinized CFUe from EE-T-Y343 and EE-T-Y343F transgenic mice
| Receptor form (mouse no.) . | hEGF-dependent CFUe/ CFUe formed in EPO (%) . |
|---|---|
| EE-T-Y343F (64) | 33 |
| EE-T-Y343F (76) | 43 |
| EE-T-Y343F (59) | 60 |
| EE-T-Y343F (83) | 22 |
| EE-T-Y343F (62) | 9 |
| EE-T-Y343F (mean ± SE, n = 5) | 33 ± 9 |
| EE-T-Y343 (mean ± SE, n = 5) | 110 ± 21 |
| Receptor form (mouse no.) . | hEGF-dependent CFUe/ CFUe formed in EPO (%) . |
|---|---|
| EE-T-Y343F (64) | 33 |
| EE-T-Y343F (76) | 43 |
| EE-T-Y343F (59) | 60 |
| EE-T-Y343F (83) | 22 |
| EE-T-Y343F (62) | 9 |
| EE-T-Y343F (mean ± SE, n = 5) | 33 ± 9 |
| EE-T-Y343 (mean ± SE, n = 5) | 110 ± 21 |
Stage-specific defect in the development of Ter119+erythroid progenitor cells from EE-T-Y343F mice
Whether the decreased ability of EE-T-Y343F to support the production of hemoglobinized cells might be revealed as a stage-specific defect next was investigated. Here, flow cytometry was used to monitor the EPO receptor-dependent decrease in the size of maturing Ter119+ erythroblasts in vitro (in pilot experiments, maturation was shown to be essentially complete by 48 hours of culture). For CFUe from EE-T-Y343 mice, this highly reproducible profile of development was observed at 48 hours of culture in the presence of either EPO (5 U/mL) or hEGF (5 ng/mL). In contrast, CFUe from EE-T-Y343F mice were reduced in their ability to develop in the presence of hEGF (but not EPO) from an immature population of Ter119+ large cells to a compartment of smaller, mature erythroblasts (Figure 4B). Outcomes from 3 independent experiments were uniform, and they underscore this stage-specific attenuation in CFUe development as supported by the PY-null EPO receptor form EE-T-Y343F (Table 2).
Attenuated hEGF-dependent maturation of CFUe from EE-T-Y343F transgenic mice
| Receptor form (mouse no.) . | Ratio of mature to immature Ter119+ cells/ ratio formed in EPO . |
|---|---|
| EE-T-Y343F (62) | 0.41 |
| EE-T-Y343F (76) | 0.44 |
| EE-T-Y343F (83) | 0.49 |
| EE-T-Y343 (29) | 1.6 |
| EE-T-Y343 (98) | 1.1 |
| EE-T-Y343 (99) | 1.2 |
| Receptor form (mouse no.) . | Ratio of mature to immature Ter119+ cells/ ratio formed in EPO . |
|---|---|
| EE-T-Y343F (62) | 0.41 |
| EE-T-Y343F (76) | 0.44 |
| EE-T-Y343F (83) | 0.49 |
| EE-T-Y343 (29) | 1.6 |
| EE-T-Y343 (98) | 1.1 |
| EE-T-Y343 (99) | 1.2 |
Capacities of EE-T-Y343 and EE-T-Y343F to synergize with endogenous c-Kit
The efficient expansion of BFUe and early CFUe is known to depend on synergistic cosignaling between the EPO receptor and c-Kit.41,42 As shown in Figure 1, splenic erythroid progenitor cells, when isolated from TAP-treated mice 68 to 72 hours after TAP withdrawal, correspond closely to early CFUe and express c-Kit transcripts at relatively high levels. Using this population of erythroid progenitor cells from TAP-treated EE-T-Y343 and EE-T-Y343F transgenic mice, independent versus combined effects of SCF, EPO, and hEGF exposure on proliferation were assayed quantitatively based on stimulated rates of [3H]-thymidine incorporation. This format was developed based on the known ability of c-Kit to transduce primarily proliferative signals in this lineage,43 the consideration that SCF was included in the above CFUe differentiation assays (see Figures 3, 4), and models in which EPO receptor–c-Kit synergy has been proposed to involve demonstrable trans-phosphorylation of the EPO receptor by c-Kit.44
In these experiments (Figure 5), proliferative effects of EPO, hEGF, and SCF alone first were tested independently over a range of concentrations. In addition, synergy was assessed by including SCF at half-maximal dose (50 ng/mL) or less while varying the concentrations of EPO and hEGF. With regard to signaling by the endogenous EPO receptor and c-Kit, maximal proliferative responses in cells from all mice tested depended on coexposure to EPO and SCF, and this response was approximately 5-fold above either EPO or SCF alone (and on average was 2-fold above their additive effects). With regard to activities of chimeric receptors, in keeping with the results of the above CFUe development–differentiation assays, the proliferative activity of EE-T-Y343 in the presence of hEGF (and the absence of SCF) was greater than that of the endogenous EPO receptor, whereas that of EE-T-Y343F was lower (Figure 5, open symbols). Interestingly, however, when SCF was included in hEGF dose-response assays, not only EE-T-Y343 but also EE-T-Y343F proved to mediate synergy with c-Kit at comparably high capacities. Maximal synergy (1.8-fold above additive effects) between EE-T-Y343 and c-Kit was observed at lower doses of hEGF and was decreased to 1.3-fold at the highest dose of hEGF, whereas synergy between EE-T-Y343F and c-Kit increased from 1.4- to 2.2-fold from lower to higher concentrations of hEGF. This increased ability of EE-T-Y343F to synergize with c-Kit at higher hEGF doses may reflect the lower overall proliferative activity of the EE-T-Y343F receptor (in the presence of hEGF alone) and suggests that SCF–c-Kit may be more important in the hEGF-dependent expansion of erythroid progenitors from EE-T-Y343F mice than from EE-T-Y343 mice. Taken together, these experiments reveal first that EE-T-Y343 and EE-Y343F receptor forms are able to signal, albeit with comparably limited potency, in the absence of c-Kit activation. Second, they also reveal that each receptor form retains a relatively high capacity to cointegrate proliferative signals relayed by c-Kit.
EE-T-Y343 and EE-T-Y343F retain high capacities to cosignal in combination with c-Kit.
Erythroid progenitor cells were prepared from TAP-treated mice at 70 hours after TAP withdrawal (c-Kit+, EPO receptor+, Ter119− stage) and were used in proliferation response assays to assess the abilities of these minimal EPO receptor forms to cosignal with endogenous c-Kit. Specifically, cells at 5 × 106 cells per milliliter were exposed to EPO, hEGF, or SCF at the concentration indexed, and proliferation was assayed based on rates of cytokine-induced methyl–[3H]-thymidine ([3H]dT) incorporation. Stem cell factor (+SCF) was included at a concentration of 50 ng/mL (50% or lower maximum dose as shown in parallel and independent SCF-only response profiles).
EE-T-Y343 and EE-T-Y343F retain high capacities to cosignal in combination with c-Kit.
Erythroid progenitor cells were prepared from TAP-treated mice at 70 hours after TAP withdrawal (c-Kit+, EPO receptor+, Ter119− stage) and were used in proliferation response assays to assess the abilities of these minimal EPO receptor forms to cosignal with endogenous c-Kit. Specifically, cells at 5 × 106 cells per milliliter were exposed to EPO, hEGF, or SCF at the concentration indexed, and proliferation was assayed based on rates of cytokine-induced methyl–[3H]-thymidine ([3H]dT) incorporation. Stem cell factor (+SCF) was included at a concentration of 50 ng/mL (50% or lower maximum dose as shown in parallel and independent SCF-only response profiles).
Discussion
Possible roles for PY343 and STAT5 during EPO-dependent erythropoiesis are the subject of controversy. One aim of the current study was to test the extent to which a PY-null EPO receptor form might support the development of adult primary CFUe in the absence of the detectable activation of STAT5. Investigations revealed an ability of the PY-null receptor form EE-T-Y343F to support late-stage adult erythropoiesis but to be compromised in this activity compared with STAT5-activating EE-T-Y343 or endogenous EPO receptor forms. This outcome is consistent with the results of recently published receptor knock-in experiments in which a PY-null EPO receptor form also was observed to support erythropoiesis in vivo yet consistently indicated less than wild-type activity.45 By comparison, the current model system circumvents compensatory events that may be realized during the development of knock-in mice and is well controlled for extrinsic factors.
The ability of EE-T-Y343F to support CFUe development at significant levels raises several interesting questions. One is the issue of how Jak2-initiated signals are transduced in the absence of receptor tyrosine sites. One possibility is that certain known factors may somehow be activated by EE-T-Y343F. PI-3 kinase comprises one candidate because this factor has been shown, in at least certain systems, to be activated detectably by PY-null EPO receptor forms46,47 and because LY294002 inhibition of this kinase limits CFUe development.48 Alternatively, EPO receptor PY-independent signals may be sufficient to mediate erythroid cell development. Although factors that couple the EPO receptor and Jak2 to PY-independent downstream events are unknown, our laboratory17 and Quelle et al33 have demonstrated (in cell lines) an EPO receptor PY-independent route to c-myc gene transcription. In FDC cells, we also have recently shown that a similar route may be used during the EPO-induced transcription of dipeptidyl-peptidase I precursor and calcyclin-binding protein genes.17
Also of significant interest is the currently described reduced activity of the EE-T-Y343F receptor form in promoting CFUe production and its linkage to a stage-specific defect in Ter119+proerythroblast maturation. This defect, compared with the efficient activity of EE-T-Y343, could be attributable to possible differences in the steady state expression levels of these 2 transgenic receptors (levels of EE-T-Y343 expression were detectably higher than EE-T-Y343F; see Figure 1). However, estimates of receptor densities showed each chimera to be expressed on the order of several thousand receptors per cell. In addition (and as discussed below), the EPO receptor form EE-T-Y343F also retained essentially full activity in cosignaling with c-Kit, and defects in EE-T-Y343F activity persisted even when hEGF concentrations were sharply limited. Because EE-T-Y343F failed to detectably activate STAT5 and because PY343 and STAT5 have been proposed to couple EPO receptor signals to Bcl-xL,16,17 one mechanism underlying this defect might involve a decreased survival rate for maturing EE-T-Y343F proerythroblasts. However, additional targets for STAT5 likely exist within the EPO receptor-signaling network that normally might also affect maturation or survival. For example, using FDC cells and initially a limited cDNA micro-array, we recently described one such example target gene whose activation by the EPO receptor depends on PY343—proliferation-associated protein I.17Although these considerations underscore roles for the EPO receptor and PY343 in progenitor cell survival, the apparent inability of Bcl-xL or Bcl-2 to rescue CFUe development in fetal liver or transgenic mice in the absence of EPO signaling49suggests that more than simple survival signals might be activated by the endogenous EPO receptor and by fully bio-active forms such as EE-T-Y343.
Finally, we have analyzed the extent to which EE-T-Y343 and EE-T-Y343F might act in synergy with c-Kit. As revealed originally by the phenotypes of mice with naturally occurring mutations in eitherc-Kit or SCF–Kit ligand genes, c-Kit signaling is essential for normal erythropoiesis.50 In addition, the ability of c-Kit and the EPO receptor to act in synergy also has been established in experiments describing the effects of their coadministration in vivo51 and their coinclusion in assays of BFUe and CFUe formation.41,42 In cell line models, stimulation of c-Kit has been shown to result in tyrosine phosphorylation of the EPO receptor, and a model for receptor interaction has been proposed in which c-Kit may directly bind and phosphorylate EPO receptor complexes.44 In addition, our laboratory17 and others42 have provided evidence that the mitogen-activated protein kinases Erk1 and Erk2 at least in part may function as downstream integrators of EPO receptor and c-Kit signals. In the current studies, the PY-null form EE-T-Y343F retained the capacity to synergize with c-Kit. This result underscores the likelihood that an EPO receptor PY-independent axis contributes to EPO receptor–c-Kit cosignaling. The currently described ability to prepare useful numbers of developmentally synchronized EPO receptor+, c-Kit+ CFUe from EE-T-Y343 and EE-T-Y343F mice also should significantly assist molecular dissections of c-Kit–activated pathways that specifically enhance EPO-dependent erythropoiesis.
We thank Dr Cindy E. McKinney for expert efforts in the production of transgenic mice.
Supported by National Institutes of Health grant RO1-DK40242
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Don M. Wojchowski, Department of Veterinary Science, 115 Henning Building, The Pennsylvania State University, University Park, PA 16802; e-mail: dmw1@psu.edu.


![Fig. 5. EE-T-Y343 and EE-T-Y343F retain high capacities to cosignal in combination with c-Kit. / Erythroid progenitor cells were prepared from TAP-treated mice at 70 hours after TAP withdrawal (c-Kit+, EPO receptor+, Ter119− stage) and were used in proliferation response assays to assess the abilities of these minimal EPO receptor forms to cosignal with endogenous c-Kit. Specifically, cells at 5 × 106 cells per milliliter were exposed to EPO, hEGF, or SCF at the concentration indexed, and proliferation was assayed based on rates of cytokine-induced methyl–[3H]-thymidine ([3H]dT) incorporation. Stem cell factor (+SCF) was included at a concentration of 50 ng/mL (50% or lower maximum dose as shown in parallel and independent SCF-only response profiles).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/3/10.1182_blood.v99.3.898/4/m_h80322044005.jpeg?Expires=1769188832&Signature=mkzFEqHESAFsJCbAdlH5ekHPm7JyMZz4LX0flfwQ7bynL7Sj-QwX408f0FUebQbLDcAp4bPpHP7KZwR1x97Z8yJ9WXX3u9j9CordjrlCMc0jGhRnUUfd3ieHhBVk~jmrmT1ByZW-dJqNC7ajldG3VSDHhWy~ArN8XbP478FLCluuD5-YZqJG6c9GN5d03Ya31IVPtg~DMU8uY9fKJjvqI3-ooME3La~lcKHJtnSg0ju4JWskoXyY8izPGO8WcQSB6QuaanzjM4QtlgAeGdUhoJnrSi0JIZNnPxeSAwfAMhMZkZjEvlAgbnuPytPfBlOaX4oEJQqt84HE-WEpGYCIKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal