Angiogenesis and mechanisms of vascular repair are currently gaining a lot of attention as new avenues for cardiovascular and renal therapy. The present concept is that cells participating in repair can be either vessel-wall–derived or circulation-derived. In vitro experiments1-4 showed that human CD34+ cells were able to differentiate into endothelial cells, suggesting potential involvement of stem cells in processes such as angiogenesis and vascular repair. In agreement, labeled CD34+ cells have been shown to be incorporated into the vessel wall in experimental models of neovascularization. Participation of circulating endothelial cells in human vascular repair was recently shown by Lagaaij et al5 in kidneys. They identified acceptor endothelial cells in allografted donor kidneys. The number of acceptor endothelial cells was increased after vascular rejection, suggesting a role of these cells in vascular repair. The source of these circulating endothelial cells, however, still remains unclear. Here we present evidence that human endothelial injury repair involves bone-marrow–derived re-endothelialization.
A 28-year-old woman suffering from malignant hypertension was admitted to our hospital for a renal biopsy. Six months earlier, she underwent a bone marrow transplantation because of a relapsing acute myeloid leukemia M2 (AML-M2). After total body irradiation (2 consecutive 600 cGy doses) and cyclophosphamide conditioning (2 days at 60 mg/kg), she received T-cell–depleted (< 1 × 105 T cells per kilogram) bone marrow (5.3 × 109 cells) from her elder brother. After 4 months her serum creatinin levels and blood pressure started to rise, reaching 448 μM and 180/110 mm Hg, respectively, on the day of admission.
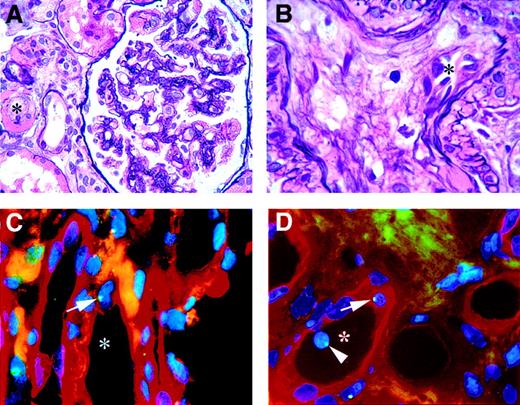
Histology of the biopsy showed dilated capillary loops, swollen foamy endothelial cells, widened subendothelial space, and some double contours of glomerular basement membranes (Figure 1). The media of some arterioles were interrupted, and there were several arteriolar hyalinosis lesions with occasional fibrin. These histologic findings and the clinical course are indicative of thrombotic microangiopathy in accordance with the presence of malignant hypertension and the total body radiation 6 months earlier.
Kidney biopsy.
Histology (A-B) and immunohistochemistry and FISH (C-D). (A-B) Hematoxylin and eosin silver staining, showing the characteristics of thrombotic microangiopathy. (A) Reduced vascular lumen (*) of arteriole next to a glomerulus with reduced capillary lumina and ischaemic winding of some basement membranes. (B) Renal arteriole with a reduced vascular lumen (*) and mucoid intima degeneration. (C-D) The participation of male donor bone marrow cells in vascular endothelial repair and maintenance in a female kidney. Endothelial cells are stained with Ulex Europaeus TRITC, and the green dots in the blue nuclei (DAPI) are FISH-stained Y chromosomes. (C) Vessel (*) with male endothelial cell (arrow). (D) Vessel (*) with male endothelial cell (arrow) and a circulating male cell (arrowhead). Original magnification A, × 630; B-D, × 1000.
Kidney biopsy.
Histology (A-B) and immunohistochemistry and FISH (C-D). (A-B) Hematoxylin and eosin silver staining, showing the characteristics of thrombotic microangiopathy. (A) Reduced vascular lumen (*) of arteriole next to a glomerulus with reduced capillary lumina and ischaemic winding of some basement membranes. (B) Renal arteriole with a reduced vascular lumen (*) and mucoid intima degeneration. (C-D) The participation of male donor bone marrow cells in vascular endothelial repair and maintenance in a female kidney. Endothelial cells are stained with Ulex Europaeus TRITC, and the green dots in the blue nuclei (DAPI) are FISH-stained Y chromosomes. (C) Vessel (*) with male endothelial cell (arrow). (D) Vessel (*) with male endothelial cell (arrow) and a circulating male cell (arrowhead). Original magnification A, × 630; B-D, × 1000.
The hallmark of thrombotic microangiopathy is endothelial damage, generally followed by spontaneous repair. Because the patient underwent a sex-mismatched bone marrow transplantation, we were able to determine the origin of the endothelial cells in the kidney by fluorescent in situ hybridization (FISH) for the Y chromosome. For an endothelial-cell–specific marker, we used Ulex Europaeus Agluttin I (UEA I) lectin and used DAPI as a nuclear counterstain. The triple staining revealed nuclear staining for the Y chromosome in several Ulex-positive cells lining the inside of the vessel, incorporated into the endothelial surface. This shows involvement of bone marrow (BM)–derived progenitor cells in endothelial repair.
This is the first report of participation of BM-derived endothelial cells in human renal vessels. Our finding suggests that endothelial progenitor cells play a role in renal endothelial repair. Not only does this provide insights into the mechanism of renal vascular repair and maintenance, but it also provides a basis for development of new strategies for treatment of the vascular lesions of thrombotic microangiopathy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal