Abstract
The γ genes provide the major contribution to beta-like globin chain production in the fetal liver of humans. However, the expression of γ genes in the fetus is a recent evolutionary trend seen only in the primate lineage. In a previous study, it was shown that galago and human γ genes retain their characteristic stage-specific expression patterns in transgenic mice (galago γ is expressed exclusively in the embryo, whereas human γ is expressed in the fetus). In that experiment, human and galago γ genes were linked to hypersensitive site 3 (HS3) of the locus control region. To rule out the possibility that HS3 is required for these differential expression profiles, additional transgenic lines were tested in which human or galago γ genes were linked to HS2. Once again, the galago γ gene was embryonic and the human γ gene was fetal, indicating that the stage specificity of these genes is driven by elements located within the 4-kb fragments that contain the human and galago γ genes proper.
Introduction
Two developmental switches in gene activity characterize the human beta-like globin cluster. At the end of embryonic life, the ε gene is silenced, and the γ genes are up-regulated. A second switch after birth involves the silencing of γ genes and the activation of δ and β genes. The embryonic-to-fetal (ε-to-γ) switch is particularly interesting because it is not seen in most other mammals. In nonprimate mammals and in nonsimian primates (such as galago), the γ gene exhibits an embryonic expression pattern and is silent in the fetal liver.1,2 Thus, a single globin switch (from embryonic ε and γ expression to adult δ and β expression) is observed in these species. The recruitment of the γ gene to a fetal expression profile occurred approximately 58 to 40 million years ago (MYA),2 sometime after the separation of nonsimian and simian primates but before the divergence of the 2 major groups of simian primates—the catarrhines (Old World Monkeys, apes, and humans) and the platyrrhines (New World Monkeys). All species of catarrhines and platyrrhines studied exhibit fetal γ expression patterns.2
Thus, the galago and the lemur are the species most closely related to human that still express γ in the embryonic and not the fetal time period. Previously, we showed that the distinct characteristic expression patterns of galago and human γ genes can be reproduced in transgenic mice.3 Using constructs in which the hypersensitive site 3 (HS3) region of the human locus control region (LCR) was linked to the human ε gene plus either the human or the galago γ genes, we found that the galago γ gene was expressed at high levels in the embryonic yolk sac and was silenced in the fetal liver, whereas the human γ gene was expressed at high levels in the fetal liver.3 These results suggest that elements responsible for the distinct expression patterns of the human and galago γ genes are linked to the genes themselves. However, the question of whether HS3 was required to observe these distinct expression patterns was not addressed.
Here, we confirm that HS2, like HS3, supports the embryonic profile of the galago γ gene and the fetal expression pattern of the human γ gene. Thus, HS2 and HS3 fragments behave in a redundant fashion; elements responsible for stage-specific expression patterns of γ are, therefore, located within the 4-kb γ fragments themselves.
Study design
Generation of transgenic mice
The HS2-εhumγ transgenic construct was described earlier.4 Here, we generated HS2-εgalγ in which galago γ sequences (spanning 10508 to 14995 of GenBank M73981, the Galago crassicaudatus β globin cluster) were substituted for the human γ sequences. The HS2 fragment (described earlier4) was derived from the human globin cluster. Purified insert DNA fragments were microinjected by the University of Michigan Transgenic Animal Core team.
DNA analysis
All restriction enzymes (SphI, ClaI,EcoRV, PstI, BamHI, NheI, and XbaI) came from New England Biolabs, Beverly, MA. Polymerase chain reaction genotyping primers, 5′-CAAATTGTTATTATTCCAGGCCACTGAATT (3′ end of the HS2 fragment) and 5′-TAGTTATTGTGAATCAAATATTTATCTT-GCAGGTGG (2 kb upstream of the ε cap site), detected an 800–base-pair (bp) product in transgenic animals. For copy number determination, genomic DNA was digested withSphI, which cuts once within the construct, and Southern blots were probed with a ClaI-EcoRV fragment from the human ε gene.5
RNA analysis
RNA was extracted as described previously3,4 from 10.5- and 12.5-day yolk sacs and from fetal liver of 12.5- and 14.5-day F2 concepti. S1 nuclease protection was used to quantitate mRNA levels. The probe for mouse ζ was described previously.4 To enable multiplexing, a human ε probe was generated from human ε exon 2 sequence; afterBamHI digestion and end labeling, the probe protects a 159-bp band. The mouse α probe was labeled at a PstI site within a NheI-BamHI genomic fragment (116-bp protected fragment).4 The galago γ probe was a 435-bpXbaI-BamHI genomic fragment labeled at theBamHI site in exon 2 (204-bp protected fragment). S1 analyses were quantitated using a Bio-Rad PhosphorImager with Multi-Analyst software (Bio-Rad, Hercules, CA).
Results and discussion
Of 3 founder animals carrying the HS2-εgalγ transgene, 2 passed the transgene to progeny and were used in these studies. Transgene copy numbers of 8 (line 1) and 57 (line 2) were determined by Southern blot analysis; transgenes were intact (data not shown).
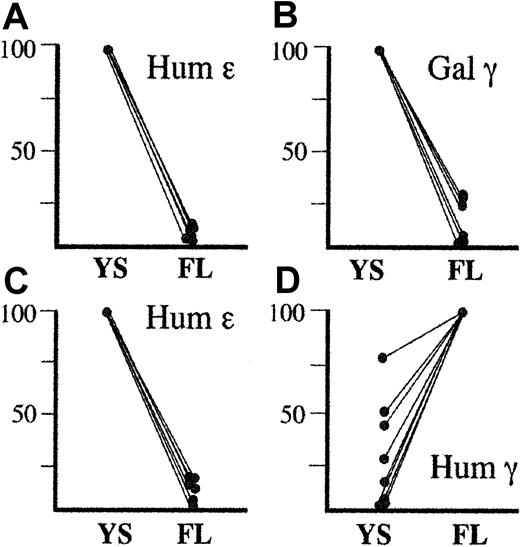
S1 nuclease assays (Figure 1) revealed that the galago γ gene was, like the human ε gene, expressed at high levels in the embryonic time period and at considerably reduced levels in the 14.5-day fetal liver, consistent with the known embryonic expression pattern of the galago γ gene. This is in contrast to the clear activation of the human γ gene in the fetal liver (Figure1).4
S1 nuclease analysis of transgene expression.
Tissues were harvested from transgenic embryos at 10.5, 12.5, and 14.5 days after conception. Yolk sac (YS) tissue was examined at 10.5 and 12.5 days, and fetal liver (FL) tissue was tested at 12.5 and 14.5 days. Bands corresponding to the galago (Gal) γ, human (Hum) γ, human ε, and mouse(Mus) α genes are labeled. Mouse ζ gene is not shown. Panels Ai and Aii are derived from analysis of transgenic lines 1 and 2 carrying the HS2-ε-galγ transgene (diagrammed at top). Panel B is derived from analysis of line 187 carrying the HS2-ε-humγ construct; these data are taken from work described earlier.4 Gels were subjected to PhosphorImager scanning, and the expression of the human ε and galago γ genes was determined as [transgene expression/(mouse ζ expression/2) + (mouse α expression/4)]/copy number.
S1 nuclease analysis of transgene expression.
Tissues were harvested from transgenic embryos at 10.5, 12.5, and 14.5 days after conception. Yolk sac (YS) tissue was examined at 10.5 and 12.5 days, and fetal liver (FL) tissue was tested at 12.5 and 14.5 days. Bands corresponding to the galago (Gal) γ, human (Hum) γ, human ε, and mouse(Mus) α genes are labeled. Mouse ζ gene is not shown. Panels Ai and Aii are derived from analysis of transgenic lines 1 and 2 carrying the HS2-ε-galγ transgene (diagrammed at top). Panel B is derived from analysis of line 187 carrying the HS2-ε-humγ construct; these data are taken from work described earlier.4 Gels were subjected to PhosphorImager scanning, and the expression of the human ε and galago γ genes was determined as [transgene expression/(mouse ζ expression/2) + (mouse α expression/4)]/copy number.
HS2 and HS3 contain the most powerful enhancers in the LCR.6,7 Although both can drive high-level expression of linked globin genes in transgenic mice, possible functional differences have been noted: (1) HS3, but not HS2, harbors a dominant chromatin opening activity8; (2) HS2 and HS3 drive different developmental patterns of γ and β expression7,9; and (3) HS3 may be essential for specific parts of the switching program. Embryonic expression of ε (but not γ) was disrupted by deletion of the core of HS3 in a human β-locus YAC.10 Because these investigations indicate that HS2 and HS3 could differ in their ability to interact with the various globin promoters, it was important to establish whether the distinct stage-specific patterns of gene expression, observed for galago and human γ genes in an earlier study,3 could also be detected when the genes were linked to HS2. Here we show that this is indeed the case, confirming that fetal versus embryonic expression of γ is attributed to changes in cis elements linked closely to the genes proper.
Although the constructs we have used in these 2 studies are relatively small (11-12 kb) and are subject to position effect, our combined experience (summarized in Figure 2) provides compelling evidence that the human and galago γ genes are indeed regulated differently in the context of the sametrans environment (the mouse fetal liver). Moreover, the data indicate that HS3 and HS2 are able to activate γ in the context of the fetal environment because the human γ gene is highly expressed in the 14.5-day fetal liver regardless of whether it is linked to HS2 or HS3. However, the fact that the galago γ gene cannot form a productive interaction with the LCR in the fetal stage suggests thatcis elements within the human γ gene facilitate productive interaction with the LCR or that cis elements within the galago γ gene prohibit productive interaction. In either case, eventual identification of these elements will provide insights into developmental regulation and promoter–LCR interactions.
Combined developmental profiles of human ε, human γ, or galago γ transgenes driven by HS2 and HS3.
Panels A and B represent expression of the human ε (A) and galago γ (B) transgenes in HS2-ε-galγ constructs (this study) and HS3-ε-galγ constructs.3 For all these lines, the human ε and galago γ genes were expressed highly in embryonic life and were silenced in fetal life. Thus, in panels A and B, transgene expression levels in embryonic life is set to 100% for each line, and the level seen in fetal life is expressed relative to the embryonic level for that line. Panels C and D represent expression of the human ε (C) and human γ (D) transgenes in HS2-ε-humγ constructs3 and in HS3-ε-humγ constructs.4 For the ε gene, embryonic levels for each line are set at 100%, and fetal levels are normalized to this level as for panels A and B. For the human γ gene, however, expression was consistently highest in the fetal liver. Thus, the fetal expression level was set at 100%, and the embryonic level in each line was normalized to this level. Although human γ gene expression is variable in embryonic life, the difference in expression profile between the human γ gene and the galago γ gene is strikingly consistent.
Combined developmental profiles of human ε, human γ, or galago γ transgenes driven by HS2 and HS3.
Panels A and B represent expression of the human ε (A) and galago γ (B) transgenes in HS2-ε-galγ constructs (this study) and HS3-ε-galγ constructs.3 For all these lines, the human ε and galago γ genes were expressed highly in embryonic life and were silenced in fetal life. Thus, in panels A and B, transgene expression levels in embryonic life is set to 100% for each line, and the level seen in fetal life is expressed relative to the embryonic level for that line. Panels C and D represent expression of the human ε (C) and human γ (D) transgenes in HS2-ε-humγ constructs3 and in HS3-ε-humγ constructs.4 For the ε gene, embryonic levels for each line are set at 100%, and fetal levels are normalized to this level as for panels A and B. For the human γ gene, however, expression was consistently highest in the fetal liver. Thus, the fetal expression level was set at 100%, and the embryonic level in each line was normalized to this level. Although human γ gene expression is variable in embryonic life, the difference in expression profile between the human γ gene and the galago γ gene is strikingly consistent.
We thank Christine Babcock for help with maintaining the mouse colony.
Supported by National Institutes of Health grants HL48802 (D.L.G.) and DK56927 (M.G., D.L.G.) and a Hematology Training Grant (NIH T32-HL07622) (D.M.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Deborah L. Gumucio, Dept of Cell and Developmental Biology, University of Michigan, 5704 Medical Science II, Ann Arbor, MI 48109-0616; e-mail: dgumucio@umich.edu.

![Fig. 1. S1 nuclease analysis of transgene expression. / Tissues were harvested from transgenic embryos at 10.5, 12.5, and 14.5 days after conception. Yolk sac (YS) tissue was examined at 10.5 and 12.5 days, and fetal liver (FL) tissue was tested at 12.5 and 14.5 days. Bands corresponding to the galago (Gal) γ, human (Hum) γ, human ε, and mouse(Mus) α genes are labeled. Mouse ζ gene is not shown. Panels Ai and Aii are derived from analysis of transgenic lines 1 and 2 carrying the HS2-ε-galγ transgene (diagrammed at top). Panel B is derived from analysis of line 187 carrying the HS2-ε-humγ construct; these data are taken from work described earlier.4 Gels were subjected to PhosphorImager scanning, and the expression of the human ε and galago γ genes was determined as [transgene expression/(mouse ζ expression/2) + (mouse α expression/4)]/copy number.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/3/10.1182_blood.v99.3.1082/5/m_h80322089001.jpeg?Expires=1767778502&Signature=eBVU8xjRJ1XDjKp2ACbOxmow0-B0Y8E9fV6RaSwkDMT-s16DkuSRfS9umcnvqSnNt1ovPcFv-fwnBTfC37fOcVN2pz3o66Me313sXtW1ynybdGz0DARTOduFaUXASq91YZam~2gBU7EKTqt8e~nMXAyqD0ZsClStc3KeAiv0Cmftso-YutqsHoZ3mNd6xD~JkkY6~3Go5ZFcXJyc4grwmp9tiZtoHIoV2pragNNC0f3ANH4bVG-kGjtL3ucqJBhDdwSg9lkQOC4fsLHKAMroO1EcJt2n6xdWA2myt8l~1dVVZMnCD~usLHUutL6n9nuTA26I4UXlCy1a1FcswkcY-Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal