Abstract
At different developmental stages, candidate human hematopoietic stem cells (HSCs) are present within the CD34+ CD38− population. By means of xenotransplantation, such CD34+CD38− cells were recently shown to engraft the hematopoietic system of fetal sheep and nonobese diabetic severe combined immunodeficient adult mice. Here it is demonstrated that, after their injection into murine blastocysts, human cord blood (CB)–derived CD34+and CD34+ CD38− cells repopulate the hematopoietic tissues of nonimmunocompromised murine embryos and that human donor contribution can persist to adulthood. It is further observed that human hematopoietic progenitor cells are present in murine hematopoietic tissues of midgestational chimeric embryos and that progeny of the injected human HSCs activate erythroid-specific gene expression. Thus, the early murine embryo provides a suitable environment for the survival and differentiation of human CB CD34+ CD38− cells.
Introduction
Hematopoietic stem cells (HSCs) are a rare cell type present in the adult bone marrow of mammals that provide the organism with lifelong hematopoiesis. During mammalian embryogenesis, a first transient wave of primitive hematopoiesis originates in the extraembryonic yolk sac. Later, the fetal liver is colonized by HSCs from the aorta gonad mesonephros (AGM) region, which is regarded as the first site of definitive hematopoiesis. Subsequently, HSCs migrate to the bone marrow, which is the hematopoietic active tissue of the adult animal.1-4 An in vivo test system for the identification and characterization of human HSCs consists of the intravenous injection of human candidate HSCs into murine nonobese diabetic severe combined immunodeficient hosts and the subsequent evaluation of human hematopoiesis in the recipient mice.5,6 By means of this assay, human HSCs were shown to be highly enriched within the CD34+CD38−fraction of adult bone marrow and cord blood.7 8
Previously, we could show that following their injection into day-3.5-old murine blastocysts, murine bone marrow–derived HSCs generate chimeric fetal and adult mice.9 Now we describe the first step toward an experimental assay system that enables us to analyze the early human hematopoietic system and the developmental potentials of human HSCs. We have injected human cord blood (CB)–derived CD34+ progenitor and CD34+CD38− stem cells into murine blastocysts and could follow the fate of injected human cells during murine embryogenesis and adulthood.
Study design
Cord blood cells were collected from healthy, full-term infants, and sodium citrate was added as an anticoagulant. For CB sampling, approved institutional procedures for obtaining informed consent according to the declaration of Helsinki were observed (Ethics Committee, Medical Faculty, University of Freiburg, Germany); the use of human CB cells for injection into murine blastocysts was approved by the responsible ethics committee (Ethics Committee, Medical Faculty, University of Würzburg, Germany). Low-density cells (less than 1.077 g/mL) were pooled from several CB samples. For CD34 enrichment, CB samples were pretreated with an antihuman Fc antibody (Pharmingen, Franklin Lakes, NJ), incubated with an anti-CD34 antibody conjugated to magnetic beads (Miltenyi Biotec, Auburn, CA) and transferred to an affinity column for positive selection. For sorting, the cells were labeled with anti-CD34-phycoerythrin (clone 581, Coulter Immunotech, Marseille, France) and anti-CD38–fluorescein isothiocyanate (clone T16, Coulter Immunotech), gated for an intermediate forward scatter and low sideward scatter profile, and then sorted for either CD34+CD38− or CD34+CD38+.10 Murine blastocysts were isolated from donor TK− C57BL/6 or NMRI mice on day 3.5 of gestation, and 70 to 100 human CB CD34+ or CD34+CD38− cells were injected into each blastocyst. Blastocysts were retransferred into foster mothers. All animal experimentation was done in accordance with approved institutional guidelines (Regierung von Unterfranken, Würzburg, Germany). Human donor contribution was determined by semiquantitative human chromosome 17-specific (17αmod) polymerase chain reaction (PCR) on genomic DNA prepared from embryonic and adult tissues.11 Importantly, the PCR signals were proportional to the amount of human genomic DNA input into the PCR reaction (data not shown).
To detect human hematopoietic progenitor cell activity in chimeric murine embryos, single-cell suspensions of yolk sac, fetal liver, and embryonic blood were prepared at day 11.5 of gestation (E11.5), and cells were seeded in MethoCult (StemCell Technologies, Vancouver, BC), complemented with recombinant human growth factors: granulocyte-macrophage colony-stimulating factors, interleukin-3; and stem cell factor (50 ng/mL each). After 10 days, single colonies of more than 50 cells were picked; murine cells were added as a source of carrier DNA; genomic DNA was prepared; and 17αmod PCR was performed.
For reverse-transcriptase–PCR (RT-PCR) analysis, total RNAs were extracted (RNAzol, Biogenesis, Poole, United Kingdom), reverse transcribed (Omniscript, Qiagen, Valencia, CA), and normalized by means of murine-specific hypoxanathinephospho-ribosyltransferase or human-specific β-actin gene expression as an internal standard.12 Normalized complementary DNAs were subjected to human ε-, γ-, and β-globin and human lysozyme-specific RT-PCR.13 14 PCR products were separated on agarose gels, subjected to Southern blotting, and hybridized with32P-labeled DNA fragments before exposure to BIOmax Kodak films (Rochester, NY).
Results and discussion
CB-derived human hematopoietic stem and progenitor cells engraft developing murine recipients following injection into blastocysts
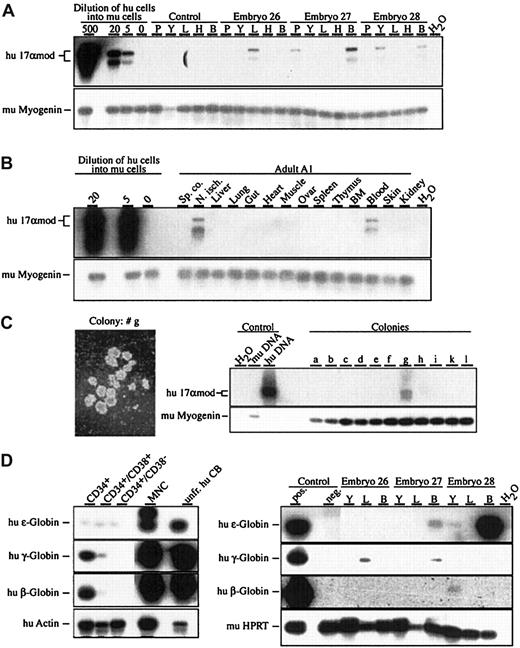
In 4 independent experiments, human CB CD34+CD38− cells were injected into a total of 98 murine blastocysts that developed to 66 midgestational embryos, of which 57 were analyzed by 17αmod PCR. We frequently detected human donor cells in embryonic tissues, isolated at E8.5, E11.5, and E16.5 (Figure 1A, Table1). At E8.5, human donor cells were detected in the yolk sac in 2 of 14 analyzed embryos and in the embryo proper in 2 of 13 embryos. One of the E8.5 embryos showed human donor contribution in the embryo proper as well as in the yolk sac. In E11.5 embryos, the highest contribution and the highest frequency of human cells were found in the embryonic blood (8 of 20). Later on, in E16.5 murine embryos, human cells could be detected in the embryonic blood (5 of 23) and in the developing fetal thymus (2 of 23). Estimation of the number of human cells present in chimeric embryonic tissues at different developmental stages by semiquantitative PCR showed that the human donor cell fraction was in the range of 5 cells per 100 000 murine cells.
Human donor contribution in developing murine embryos after injection of purified human CD34+CD38− cells into murine blastocysts.
(A) (B) Detection of human donor contribution in embryonic and adult tissues by means of 17αmod PCR on genomic DNA from developing E11.5 embryos (panel A) and an adult at 5 months of age (panel B) following blastocyst injection of CD34+CD38− cells (panel A) and CD34+ cells (panel B). Graded numbers of human cells diluted into 105 murine (mu) splenocytes were used for quantification of the PCR products. PCR specific for murine myogenin was used for normalization of genomic DNA.2 (C) Photograph of a human colony (25 × magnification) and 17αmod PCR on genomic DNA extracted from single colonies after 10 days of culture. (D) Human globin–specific RT-PCR on total RNA isolated from CB fractions and from chimeric embryonic tissues. As positive controls, RT-PCR was done on a mixture (1/500) of total RNA prepared from unfractionated cord blood and murine fetal liver samples. Abbreviations: P, placenta; Y, yolk sac; L, fetal liver; H, head; B, embryonic blood; T, thymus; BM, bone marrow; Sp. co., spinal cord; N. isch., nervus ischiadicus; hu DNA, human genomic DNA; mu DNA, murine genomic DNA; unfr. CB, unfractionated CB; MNCs, mononuclear cells. Autoradiograms of Southern blots are shown.
Human donor contribution in developing murine embryos after injection of purified human CD34+CD38− cells into murine blastocysts.
(A) (B) Detection of human donor contribution in embryonic and adult tissues by means of 17αmod PCR on genomic DNA from developing E11.5 embryos (panel A) and an adult at 5 months of age (panel B) following blastocyst injection of CD34+CD38− cells (panel A) and CD34+ cells (panel B). Graded numbers of human cells diluted into 105 murine (mu) splenocytes were used for quantification of the PCR products. PCR specific for murine myogenin was used for normalization of genomic DNA.2 (C) Photograph of a human colony (25 × magnification) and 17αmod PCR on genomic DNA extracted from single colonies after 10 days of culture. (D) Human globin–specific RT-PCR on total RNA isolated from CB fractions and from chimeric embryonic tissues. As positive controls, RT-PCR was done on a mixture (1/500) of total RNA prepared from unfractionated cord blood and murine fetal liver samples. Abbreviations: P, placenta; Y, yolk sac; L, fetal liver; H, head; B, embryonic blood; T, thymus; BM, bone marrow; Sp. co., spinal cord; N. isch., nervus ischiadicus; hu DNA, human genomic DNA; mu DNA, murine genomic DNA; unfr. CB, unfractionated CB; MNCs, mononuclear cells. Autoradiograms of Southern blots are shown.
Frequency of human donor contribution in murine embryos
| Age of recipients analyzed . | Tissues analyzed (positive/total) . | ||||||
|---|---|---|---|---|---|---|---|
| P . | Y . | EP . | L . | H . | B . | T . | |
| E8.5: | N/A | 2/14 | 2/13 | N/A | N/A | N/A | N/A |
| E11.5: | 2/20 | 3/20 | N/A | 2/20 | 1/20 | 8/20 | N/A |
| E16.5: | N/A | 0/23 | N/A | 0/23 | 0/23 | 5/23 | 2/23 |
| Age of recipients analyzed . | Tissues analyzed (positive/total) . | ||||||
|---|---|---|---|---|---|---|---|
| P . | Y . | EP . | L . | H . | B . | T . | |
| E8.5: | N/A | 2/14 | 2/13 | N/A | N/A | N/A | N/A |
| E11.5: | 2/20 | 3/20 | N/A | 2/20 | 1/20 | 8/20 | N/A |
| E16.5: | N/A | 0/23 | N/A | 0/23 | 0/23 | 5/23 | 2/23 |
P indicates, placenta; Y, yolk sac; EP, embryo proper; L, fetal liver; H, head; B, embryonic blood; T, thymus; E, gestational age (in days); N/A, not analyzed.
In a second set of experiments, CD34+ cells of human CB were injected into murine blastocysts, and 4 adult animals were analyzed at 5 to 12 months of age. PCR analysis on 14 different tissues revealed that 3 animals contained human donor cells in hematopoietic and nonhematopoietic tissues. We detected human cells in one animal in the peripheral blood and nervus ischiadicus; the second animal showed donor contribution in nervus ischiadicus and spinal cord; the third animal contained human cells in the thymus; and the fourth animal was devoid of any human donor contribution (Figure 1B and data not shown). The cellular nature of the human donor cells in chimeric tissues of adult animals is currently unknown.
Following injection, human CB CD34+CD38−cells give rise to hematopoietic progenitors in fetal liver and embryonic blood
To test for the potential of the murine embryonic environment to support human hematopoietic progenitor activity, we injected human CB CD34+CD38− cells into murine blastocysts, isolated developing embryos at E11.5, and assayed for the presence of human progenitor cells. Single-cell suspensions of individual yolk sac, fetal liver, and embryonic blood samples of 11 embryos were prepared and seeded into MethoCult containing human recombinant growth factors that allow the preferential growth of human colony-forming cells. After 10 days of in vitro culture, single colonies were picked and their origin was tested by 17αmod PCR. Of 93 colonies analyzed, 8 colonies were of human origin coming from 6 individual embryos (Figure 1C and data not shown). Four of the 8 colonies originated from fetal liver and 4 from embryonic blood; no human colony could be derived from yolk sac cells. These results show that 10 days after the injection of human CB CD34+CD38− into murine blastocysts, human progenitor activity is present in murine fetal hematopoietic tissues.
Progeny of human CD34+CD38− cells express human globin genes in developing murine embryos
To look for the presence of differentiated human hematopoietic cells in chimeric murine embryos derived from blastocysts injected with CD34+CD38−, we performed RT-PCR specific for human ε-, γ-, and β-globin and human lysozyme gene transcripts. First, using RT-PCR on total RNA, we established the transcription pattern of human ε-, γ-, and β-globin transcripts of progressively enriched progenitors of human CB samples (Figure 1D). In total RNA derived from unfractionated CB, CB mononuclear cells (MNCs), and CB CD34+ cells, high amounts of fetal and adult-type γ- β-globin transcripts were detected, whereas embryonic-type ε-globin transcripts were present in unfractionated CB and MNCs and at lower levels in CB CD34+ cells. Similarly, we examined total RNA derived from E11.5 and E12.5 yolk sac, fetal liver, and embryonic blood of chimeric embryos. Of 6 E11.5 and 10 E12.5 embryos tested, 8 embryos, 3 at E11.5 and 5 at E12.5, were positive for human globin gene expression (Figure 1D and data not shown). Transcripts of all 3 human globin genes could be detected by RT-PCR in yolk sac, fetal liver, and embryonic blood. While there was no expression of the human γ- and β-globin genes in the injected CD34+CD38− cells, we detected transcription of human ε-, γ-, and β-globin genes in murine chimeric tissues, suggesting erythroid differentiation of the injected CB CD34+CD38− cells. No transcripts of the human lysozyme gene were detected in yolk sac, fetal liver, or embryonic blood (data not shown).
Thus, despite the xenogenic situation and the developmental gap between cells of recipient blastocysts and donor cells from newborns, human CB CD34+ and CD34+CD38− cells have the capacity to survive and proliferate in the environment provided by the early murine embryo.
The importance of this finding is highlighted by the fact that human donor contribution is detected, although only 70 to 100 human cells were injected into the nonimmunocompromised hosts. The hosts did not provide a selective advantage for the injected hematopoietic progenitor and stem cells, nor have they been specifically engineered for the reception of human donor cells. Interestingly, we noted human donor contribution in the developing embryos in yolk sac, fetal liver, and, most often, in the embryonic blood. Thus, injected human HSCs or their differentiated progeny are found at the same sites during ontogeny as their murine counterparts. This indicates that the murine embryonic hematopoietic system provides at least some of the cues important for migration, survival, or differentiation of the human HSCs and their progeny.
The injection of human CB CD34+CD38− cells into murine blastocysts may possibly be further developed to generate mice with a multilineage human hematopoietic system present from early embryos onward and to further analyze the developmental potentials of human HSCs, since the murine blastocyst is permissive for the development of all cell types.
We thank Li de Lima-Hahn, Orinta Schneider, and Sonja Rotzoll for cell sorting; Alexandra Fehrenbach for blastocyst injection; and Stefanie Sick, Angela Merkel, and Bettina Mühl for excellent technical assistance. Special thanks go to Randall Cassada and Bruce Jordan for critically reading the manuscript.
Supported by the IZKF (01KS9003), Würzburg, and by a grant from the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 465) to A.M.M.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Albrecht M. Müller, Institut für Medizinische Strahlenkunde und Zellforschung (MSZ), Universität Würzburg, Versbacherstr 5, 97078 Würzburg, Germany; e-mail:albrecht.mueller@mail.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal