Abstract
To determine whether infection by a model virus is capable of initiating dendritic cell (DC) differentiation, human CD14+peripheral blood monocytes were infected with replication-defective type 5 adenovirus. Under serum-free conditions, this resulted in differentiation of a majority of cells toward a DC phenotype within 36 to 48 hours, without the need for cytokine-induced predifferentiation. Infection induced DC morphology and altered the expression of surface markers, including loss of CD14, de novo induction of CD83 and CD25, and strongly augmented expression of CD86, CD80, CD40, and HLA-DR and HLA class I molecules. Differentiated cells maintained immunophenotype without loss of viability for at least 2 days after removal of the differentiation agent and cytokines. A greatly enhanced capacity to stimulate T-lymphocyte alloproliferation and increased expression of the DC-associated transcription factor RelB were observed. Virus without transgene was found to induce changes similar to transgene-expressing viruses. RelB up-regulation and DC immunophenotype were sensitive to the antioxidant N-acetylcysteine, suggesting a critical role for nuclear factor κB. RNAse protection assays revealed elevated levels of messenger RNA for a number of chemokines and cytokines associated with DCs. Finally, during differentiation, adenovirus-infected monocytes were shown to secrete chemokines and cytokines, including tumor necrosis factor-α (TNF-α). Furthermore, a TNF-α–neutralizing antibody inhibited the expression of some DC surface markers, indicating a contributing role for this cytokine in the adenovirus-induced differentiation of DC from monocytes. These findings have implications for the biology of monocytes as precursors to DCs and also for the use of recombinant adenovirus in vaccines or gene therapy.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that sensitize T lymphocytes to alloantigens and nominal antigens.1,2 Because of their role in priming T-cell responses, DCs are a focus of efforts to develop immunotherapeutic strategies aimed at treating malignancies and chronic infections. DCs can be derived in vitro from CD14+ monocytes with the use of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4).3 Cells so treated down-regulate CD14 expression and differentiate into immature DCs. If tumor necrosis factor-α (TNF-α), bacterial lipopolysaccharide (LPS), CD40 ligand, or monocyte-conditioned medium is also added, this differentiation process is completed, and concomitant CD83 expression is observed.4-7 The complete differentiation process can take between 1 and 2 weeks. We recently reported that culture of human CD14+ monocytes in a commercial serum-free medium (but not serum-containing medium) supplemented with GM-CSF and either bacterial LPS, TNF-α, or calcium ionophore led to a much more rapid (2- to 4-day) acquisition of the CD83+ DC phenotype.8This shorter time frame is consistent with reports of in vivo monocyte-derived DC differentiation in animal models.9
Because of our previous success in inducing rapid DC differentiation under serum-free conditions using LPS, a compound likely to signal Gram-negative bacterial infection in vivo, we have now turned our attention to other classes of pathogens, in this case a modified type 5 adenovirus. As a group, adenoviruses have a relatively broad tissue tropism, and replication-defective deletion mutants were initially considered to have a relatively low pathogenic potential.10 Because of these perceived features, recombinant, replication-defective adenoviruses have become workhorses for the introduction of transgenes into a variety of cultured cell types for investigational purposes, as well as vehicles for recombinant vaccines and, perhaps most importantly, a delivery system for transgenes in human gene therapy settings.
Adenovirus has been previously shown to infect CD14+monocytes cultured in GM-CSF11 as well as immature monocyte-derived DCs.12 It has been reported that adenovirus infection of immature DCs under tested conditions was completely nonperturbing: adenovirus infection did not cause any apparent activation or further maturation of DCs.13-15However, work by others suggests that adenovirus infection of immature DCs can promote varying degrees of activation.16-18
In the studies reported here, we demonstrate that under serum-free conditions, replication-defective type 5 adenovirus initiates the rapid differentiation of CD83+ DCs from CD83−CD14+ monocytes. The present studies differ from previous work because adenovirus is shown to act very rapidly upon native CD14+ monocytes, rather than upon immature, CD14−CD83low DCs whose “predifferentiation” had already been induced by extensive culture with GM-CSF/IL-4. The adenovirus-induced DC differentiation that we report is achieved within 36 to 48 hours of infection, regardless of whether monocytes are infected within 2 hours of plating or after 2 days of culture. This adenovirus-induced differentiation is accompanied by secretion of TNF-α, the neutralization of which profoundly inhibits acquisition of a surface phenotype associated with DCs. These findings have implications not only for the biology of monocytes as precursors to DCs, but also for the use of recombinant adenoviruses as vaccines or as vectors for gene therapy.
Materials and methods
Replication-defective recombinant adenoviruses
Regions E1- and E3-deleted recombinant serotype 5 adenoviruses, containing a green fluorescent protein (GFP) gene (adenovirus-GFP) (Quantum Biotechnologies, Montreal, QC, Canada) or firefly luciferase gene (adenovirus-Luc),19 each driven by a cytomegalovirus immediate early promoter, were grown on the 293 adenovirus propagation line and concentrated to 3 × 1010 plaque-forming units (PFUs) per milliliter by centrifugation on 2 successive, discontinuous CsCl gradients. Viral titers were also determined by measuring the optical density of dissociated virus (1 absorbance unit at 260 nm equals 1012 particles per milliliter). Depending on the preparation, the particle-to-PFU ratio was on the order of 50-100:1, which is typical for adenovirus.12 CsCl-purified control E1/E3–deleted adenovirus 5 vector encoding no transgene or transpromoter (adenovirus-null, 1 × 1013 viral particles per milliliter) was purchased from Quantum Biotechnologies. Heat inactivation of virus was achieved by 30 minutes' incubation in a 56°C water bath. Wherever possible, tissue-culture grade reagents were used during virus preparation to minimize potential endotoxin contamination.
Monoclonal antibody and fluorescence-activated cell sorting analysis
Fluorescent-activated cell sorting (FACS) protocols including monoclonal antibodies (mAbs) specific for human CD80, CD86, CD14, CD33, CD11c, CD33, CD83, CD25, CD40, HLA-A, -B, -C, and HLA-DR, as well as isotype-matched control mAbs, were identical to those used in previously published studies.20 21 A gate (R1) was defined in all FACS analyses to exclude, on the basis of size and propidium iodide staining, all nonviable cells and debris. The instrumentation employed in flow cytometry studies was a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA) running CellQuest (Clearwater, FL) analysis software.
Human peripheral blood monocyte and allogenic mixed lymphocyte reaction cultures
Human CD14+ peripheral blood monocytes (92% to 94% purity) were obtained from healthy donors by leukapheresis and countercurrent centrifugal elutriation according to National Institutes of Health guidelines for human subjects and were either cultured immediately or cryopreserved as described previously.22Lymphocyte-rich fractions were also collected and cryopreserved. Monocytes were plated at a density of 2.5 × 106 cells per well in 24-well tissue-culture plates (Costar, Corning, NY) in 2 mL per well macrophage serum-free medium (MΦ-SFM) (Life Technologies, Gaithersburg, MD) supplemented with 50 ng/mL recombinant human GM-CSF (Immunex, Seattle, WA), which is necessary for maintaining cell viability, as described previously.21 This combination of MΦ-SFM and GM-CSF, which constitutes basal culture medium for all monocytes and DCs used in these studies, is henceforth referred to in the text simply as SFM/G. Where indicated, cultures were supplemented with additional agents prior to the addition of adenovirus or other differentiation-inducing stimuli. These agents includedN-acetylcysteine (NAC) (Sigma, St Louis, MO), a TNF-α–neutralizing mAb 5N,23 or polymyxin B (Sigma). For allosensitization studies, T cells, purified from lymphocyte-rich elutriation fractions by means of T-cell isolation columns (R&D Systems, Minneapolis, MN), were plated in 96-well plates in RPMI medium as described previously.8 Gamma-irradiated (20 Gy) monocytes or monocyte-derived DCs were added at varying T cell–to–APC ratios and cocultured for 96 hours at 37°C in 5% CO2. Cells were then pulsed with 1 μCi (.038 MBq) per well [3H]-thymidine ([3H]-TdR) (3.2 TBq/mmol) (Amersham Pharmacia Biotech, Arlington Heights, IL) and harvested 18 hours later. The [3H]-TdR incorporation was assessed by liquid scintillation spectroscopy.
Induction of DC differentiation with LPS or replication-defective adenovirus
Monocytes cultured overnight in SFM/G were treated with 50 ng/mLEscherichia coli O26:B6 LPS (Sigma) for 3 additional days, a treatment previously shown to induce rapid DC differentiation in about half of the cells.8 For viral infections of freshly purified monocytes, cells were plated in 200 μL SFM/G. For monocytes cultured in 2 mL SFM/G for 24 to 48 hours prior to infection, supernatants were carefully removed until the culture volume was 200 μL. Adenovirus suspensions were added to the cells and gently mixed. Infection was allowed to proceed for 2 hours at 37°C; then, fresh SFM/G was added to bring the cultures to 2 mL per well. Infected cells were typically cultured an additional 36 to 48 hours to achieve peak adenovirus-induced differentiation. Since maximal adenovirus-induced differentiation could usually be completed 1 day earlier than with LPS treatment, this staggered introduction of differentiating agents synchronized the development of DCs after 4 total days of culture, the time when most immunophenotypical, morphological, molecular, and functional properties were evaluated.
Microscopy
Monocytes treated with LPS, infected with adenovirus, or left untreated, as described above, were harvested by careful removal of medium, gentle washing of plates with Ca2+Mg2+–free phosphate-buffered saline, and 20 minutes' incubation with enzyme-free dissociation buffer (Life Sciences, Gaithersburg, MD) to remove residual adherent cells. Cytospin preparations were made onto glass slides, stained with Wright solution, and subjected to light photomicroscopy, as described previously.8
RNAse protection assay
The multiprobe RNAse protection assay (Pharmingen, San Diego, CA) was performed according to the manufacturer's directions, with the following modifications:
Hybridization.
33P–uridine 5′ triphosphate (70 to 80 μCi [2.6 to 3 MBq] per reaction) was used to synthesize the probe, and 0.5 to 1.0 × 106 cpm was added to each hybridization reaction. After synthesis and addition of yeast transfer RNA (tRNA) and EDTA (final volume, 50 μL), the reaction mixture was placed on a G25 microspin column (Amersham Pharmacia Biotech), and the probe was purified by centrifugation for 2 minutes at 3000 rpm in a microcentrifuge.
RNAse inactivation.
A master cocktail was prepared containing (per RNA sample) 200 μL RNAse inactivation reagent (Ambion, Austin, TX), 50 μL ethanol, 5 μg yeast tRNA, and 1 μL Ambion GycoBlue coprecipitate. Then, 250 μL aliquots of this cocktail were pipetted into 1.5-mL microcentrifuge tubes, and the individual RNAse-treated samples were added to each tube. After mixing, the samples were kept at −70°C for 30 minutes, followed by centrifugation for 15 minutes at 14 000 rpm at room temperature. The supernatants were poured off; a sterile cotton swab was used to remove excess liquid from the tube; and the pellet was resuspended in 3 μL Pharmingen sample buffer.
Preparation of whole-cell extracts and immunoblotting
Cells were pelleted and lysed in a buffer containing 10 mM Tris (pH 7.4), 220 mM NaCl, 30 mM sodium pyrophosphate, 50 mM NaF, 5 μM ZnCl2, 1% Triton X-100, and protease inhibitor mixture (Roche Molecular Biochemicals, Indianapolis, IN) supplemented with additional protease inhibitor α2-macroglobulin (1.25 U/mL) (Roche Molecular Biochemicals). The extract was incubated on ice for 10 minutes and centrifuged at 15 000g for 15 minutes at 4°C; the supernatant constituted the whole-cell extract. Protein concentrations were measured by using the Bio-Rad (Hercules, CA) protein assay with the use of a bovine serum albumin standard. Then, 5 to 10 μg protein were resolved by 10% Tricine sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Invitrogen, Carlsbad, CA) and transferred to Immobilon-P (Millipore, Bedford, MA). The membrane was probed with anti-RelB antiserum 1319 and antiactin mAb (Chemicon, Temecula, CA).8 Immunoreactive proteins were revealed with an enhanced chemiluminescent system (Amersham Pharmacia Biotech).
Cytokine and chemokine quantitation
Enzyme-linked immunosorbent assay kits (R&D Systems) were used to quantify several cytokines and chemokines in 24- to 48-hour culture supernatants of adenovirus-transduced monocytes. Detection limits were as follows: for IL-6, IL-1α, and IL-1β, 8 pg/mL; RANTES and TNF-α, 16 pg/mL; for IL-10, 47 pg/mL; for IL-12 p40/70, 15 pg/mL; and for macrophage inflammatory protein-1α (MIP-1α) and MIP-1β, 62 pg/mL. Assays were performed by the Lymphokine Testing Laboratory, SAIC, National Cancer Institute at Frederick, MD.
Results
Infection of human CD14+ monocytes with adenoviral vectors under serum-free conditions results in the rapid acquisition of DC immunophenotype
Starting populations of human monocytes were 92% to 94% CD33+, CD11c+, CD14+, and negative for the DC activation marker, CD83 (Figure1A-B). As reported previously,8 21 these cells were negative for the costimulatory molecule CD80 and low/negative for CD40, but showed moderate levels of CD86, HLA-DR, and HLA class I antigens (Figure1B).
Adenovirus-GFP–induced DC immunophenotype in human CD14+monocytes.
Fresh monocytes or monocytes cultured for various times in SFM/G were infected with adenovirus-GFP at a multiplicity of infection (MOI) of 200. Cells were harvested 72 to 94 hours later and analyzed by FACS. Monocytes that had never been cultured (“not cultured”) were also examined. Results are representative of 3 experiments with different donors. (A) Demonstration of the purity of starting populations of monocytes. Uncultured elutriated monocytes stained with fluorescein isothiocyanate–conjugated anti-CD33 (x-axis) and phycoerythrin (PE)–conjugated anti-CD11c (y-axis) mAbs. (B) Multipanel histogram analysis of monocytes that were treated in one of the following ways: not cultured; infected with adenovirus-GFP 2 hours after culture; infected 2 hours after culture, washed free of all cytokines at 48 hours, and recultured in fresh SFM (without any added cytokines) for 48 hours; infected with adenovirus-GFP 24 hours after culture; or cultured in SFM/G only. All cultured cell groups were maintained for a total of 96 hours irrespective of time of infection or washout and replacement of ambient culture medium. Open traces represent isotype-matched control antibody staining; filled traces indicate marker-specific antibody. (C) Analysis of CD25 expression after adenovirus infection. Monocytes were cultured 24 hours in SFM/G, infected with adenovirus-GFP, and harvested after an additional 72 hours culture. Light dotted and dashed lines represent anti-CD25–stained uncultured cells and SMF/G-cultured cells, respectively. Heavy solid line represents anti-CD25–stained adenovirus-infected cells.
Adenovirus-GFP–induced DC immunophenotype in human CD14+monocytes.
Fresh monocytes or monocytes cultured for various times in SFM/G were infected with adenovirus-GFP at a multiplicity of infection (MOI) of 200. Cells were harvested 72 to 94 hours later and analyzed by FACS. Monocytes that had never been cultured (“not cultured”) were also examined. Results are representative of 3 experiments with different donors. (A) Demonstration of the purity of starting populations of monocytes. Uncultured elutriated monocytes stained with fluorescein isothiocyanate–conjugated anti-CD33 (x-axis) and phycoerythrin (PE)–conjugated anti-CD11c (y-axis) mAbs. (B) Multipanel histogram analysis of monocytes that were treated in one of the following ways: not cultured; infected with adenovirus-GFP 2 hours after culture; infected 2 hours after culture, washed free of all cytokines at 48 hours, and recultured in fresh SFM (without any added cytokines) for 48 hours; infected with adenovirus-GFP 24 hours after culture; or cultured in SFM/G only. All cultured cell groups were maintained for a total of 96 hours irrespective of time of infection or washout and replacement of ambient culture medium. Open traces represent isotype-matched control antibody staining; filled traces indicate marker-specific antibody. (C) Analysis of CD25 expression after adenovirus infection. Monocytes were cultured 24 hours in SFM/G, infected with adenovirus-GFP, and harvested after an additional 72 hours culture. Light dotted and dashed lines represent anti-CD25–stained uncultured cells and SMF/G-cultured cells, respectively. Heavy solid line represents anti-CD25–stained adenovirus-infected cells.
Dose-response studies indicated that maximum infectivity with adenovirus-GFP (as assessed by transgene expression), coupled with maximal induction of DC phenotype (without significant loss of viability), was achieved at an MOI of 100 to 200 (data not shown). These results are consistent with reports of others using immature DCs,13 16-18 and an MOI of 200 was therefore used in all our subsequent experiments.
Infection with a GFP-encoding adenovirus in this dose range for 48 to 96 hours resulted in dramatic changes in surface immunophenotype consistent with differentiation of monocytes into DCs. Infected cells decreased CD14 expression while strongly up-regulating surface CD80, CD86, CD40, and HLA-DR. HLA class I levels rose slightly above those seen in uncultured cells (Figure 1B). Markers of activated DCs, CD83 (Figure 1B) and CD25 (Figure 1C), were also highly induced in adenovirus-infected cultures. Similar results were obtained regardless of whether monocytes were cultured in SFM/G for only 2 hours or for 1 to 2 days prior to infection. It is clear that the observed differentiation, seen in 20 separate experiments with 14 different donors, was triggered by adenovirus and not by the GM-CSF–containing SFM/G. When monocytes were cultured for 96 hours in SFM/G in the absence of virus, most cells increased expression of CD80 and CD40, but they showed decreased expression of CD86 and HLA class I. Most importantly, they remained CD14+ and CD83−. GM-CSF was not necessary for virus-induced DC differentiation, although elimination of GM-CSF at the time of infection resulted in much lower cell viability (data not shown). Thus, adenovirus infection of CD14+ monocytes under serum-free conditions results in the rapid acquisition of DC immunophenotype.
One characteristic of fully differentiated cells is the ability to maintain phenotype after removal of the differentiating agent(s) and cytokines. To test the adenovirus-infected cells, they were washed following 48 hours exposure to virus and were cultured for an additional 48 hours in SFM (without GM-CSF). No change in immunophenotype and no loss of viability were observed in the differentiated cells (Figure 1B).
We also found that adenovirus-induced differentiation is not attributable to the expression of a transgene: virus without transgene induced profound alterations in immunophenotype similar to those seen with adenovirus-GFP and adenovirus-Luc (Figures2 and 7). The possibility that endotoxin contamination makes a major contribution to adenovirus-mediated DC differentiation has been minimized, since heat inactivation of virus (which does not affect LPS activity) strongly inhibited adenovirus-induced DC immunophenotype (Figure 2). In addition, polymyxin B, an inactivator of LPS, did not significantly affect adenovirus-induced DC differentiation (Figure 2).
Adenovirus-induced acquisition of DC phenotype in the absence of endotoxin contamination.
Adenovirus-induced acquisition of DC phenotype is not a result of endotoxin contamination. Monocytes were cultured in SFM/G for 24 hours and were then either infected with adenovirus-Luc (with and without polymyxin B), infected with heat-inactivated (HI) adenovirus-Luc, or treated with 10 ng/mL LPS (with or without polymyxin B). Monocytes were incubated with 200 ng/mL polymyxin B for 1 hour before the indicated treatments. All cells were harvested, stained, and subjected to FACS analysis 36 hours after infection or treatment. Results are representative of 6 separate experiments with different donors.
Adenovirus-induced acquisition of DC phenotype in the absence of endotoxin contamination.
Adenovirus-induced acquisition of DC phenotype is not a result of endotoxin contamination. Monocytes were cultured in SFM/G for 24 hours and were then either infected with adenovirus-Luc (with and without polymyxin B), infected with heat-inactivated (HI) adenovirus-Luc, or treated with 10 ng/mL LPS (with or without polymyxin B). Monocytes were incubated with 200 ng/mL polymyxin B for 1 hour before the indicated treatments. All cells were harvested, stained, and subjected to FACS analysis 36 hours after infection or treatment. Results are representative of 6 separate experiments with different donors.
Since in previous studies8 we showed that rapid DC differentiation induced by LPS or TNF-α was inhibited by the presence of serum, we asked whether serum might also attenuate adenovirus-induced differentiation. We found that it does. When SFM was supplemented with 10% fetal calf serum (FCS), the intensity of expression of the DC activation marker, CD83, was strongly inhibited (up to 85%), even though transgene expression was approximately equal between serum-free and serum-containing groups (data not shown). There was little or no inhibitory effect on CD86 expression. Thus, the presence of serum appears to minimize adenovirus-induced DC differentiation from CD14+ monocytes. It is therefore possible that lot-to-lot variations in this inhibitory property of serum may have contributed to the conflicting reports in the literature regarding the modulatory effect of adenovirus on DC maturation.
Infection with adenovirus in SFM/G had no major effect on cell viability. In most experiments, viability ranged from 80% to 90%, whether measured at 48, 72, or 96 hours after infection. In summary, infection of human monocytes with adenovirus vectors under serum-free conditions results in the rapid (48-hour) and virtually complete differentiation of the cells into DCs, without need for cytokine-induced predifferentiation.
Adenovirus-infected monocytes acquire DC morphology
Virus infection also dramatically affected the appearance and behavior of the cells in culture. Cells cultured in SFM/G were moderately adherent in culture, with little tendency to aggregate. In contrast, adenovirus-infected cells formed large, loosely adherent clusters (Figure 3), which are typical of DCs matured in vitro.5 24 Judging from expression of GFP as determined by FACS, at least 70% of the monocytes were virus infected (Figure 3). GFP expression was also apparent by Western blot analysis (data not shown) and fluorescent microscopy. The FACS-based comparison of GFP expression with CD83 expression shows that even the cells that expressed little or no GFP had elevated CD83 expression. Since there are many more noninfectious than infectious (ie, GFP-expressing) particles in the virus preparation (as determined on the 293 adenovirus propagation line), the result may mean that viral infectivity is not required to trigger differentiation of the cells. Alternatively, the result may indicate that the differentiating effects of infectious adenovirus may not be direct. Instead, the effects may stem from the induced secretion of a soluble factor(s), a point to which we will return later.
Analysis of GFP expression in adenovirus-GFP–infected monocytes.
Monocytes were cultured overnight in SFM/G or infected with adenovirus-GFP at an MOI of 200. At 48 hours, cells were harvested, stained with either PE-labeled isotype-matched control antibodies or PE-labeled anti-CD83 mAb (x-axis), and subjected to multicolor FACS analysis to detect relative levels of expression of GFP (y-axis). In addition, phase contrast and fluorescent photomicrographs of both uninfected and infected cultures were taken to demonstrate GFP expression and virus-induced cell clustering.
Analysis of GFP expression in adenovirus-GFP–infected monocytes.
Monocytes were cultured overnight in SFM/G or infected with adenovirus-GFP at an MOI of 200. At 48 hours, cells were harvested, stained with either PE-labeled isotype-matched control antibodies or PE-labeled anti-CD83 mAb (x-axis), and subjected to multicolor FACS analysis to detect relative levels of expression of GFP (y-axis). In addition, phase contrast and fluorescent photomicrographs of both uninfected and infected cultures were taken to demonstrate GFP expression and virus-induced cell clustering.
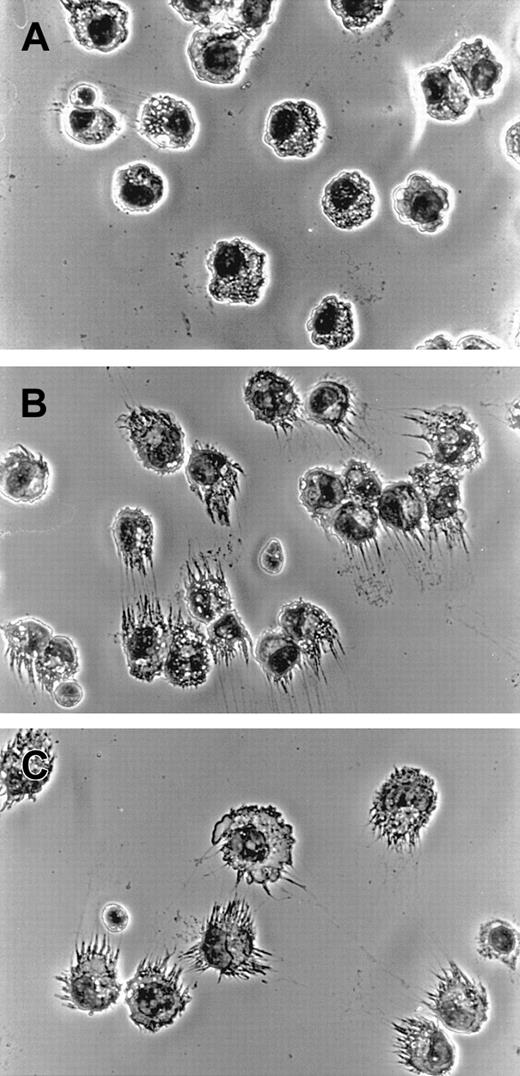
When fixed and stained cells were microscopically examined at higher magnifications, morphological changes to individual cells induced by adenovirus infection became apparent. Cells from adenovirus-infected cultures displayed elaborate cellular processes consistent with DCs (Figure 4B), very similar to what was seen when LPS was used to drive differentiation in parallel (Figure4C). In contrast, cells cultured for the same length of time in SFM/G displayed the unremarkable morphology of monocytes (Figure 4A), demonstrating that culture without adenovirus infection did not cause any apparent differentiation of cells.
DC morphology in adenovirus-infected monocytes.
Monocytes were cultured in SFM/G for 24 hours and then either infected with adenovirus-Luc at an MOI of 200 or treated with 50 ng/mL LPS. At 72 hours later, cells were harvested, and cytospin preparations were made onto glass slides, Wright stained, and subjected to photomicrography. Original magnification, × 400. Results are representative of 3 separate experiments with different donors. (A) Monocytes cultured in SFM/G. (B) Adenovirus-infected monocytes. (C) LPS-treated cells.
DC morphology in adenovirus-infected monocytes.
Monocytes were cultured in SFM/G for 24 hours and then either infected with adenovirus-Luc at an MOI of 200 or treated with 50 ng/mL LPS. At 72 hours later, cells were harvested, and cytospin preparations were made onto glass slides, Wright stained, and subjected to photomicrography. Original magnification, × 400. Results are representative of 3 separate experiments with different donors. (A) Monocytes cultured in SFM/G. (B) Adenovirus-infected monocytes. (C) LPS-treated cells.
Virus-infected cells acquire enhanced allostimulatory activity
We tested the ability of virus-infected cells to stimulate T lymphocytes at low APC numbers in the allogenic mixed lymphocyte reaction (MLR), since DCs have been shown to be extremely efficient APCs in this assay. As expected, uncultured monocytes had a very poor allostimulatory capacity (Figure 5). As reported previously,8 monocytes cultured in SFM/G displayed a somewhat greater allosensitizing capacity, probably owing to the culture-enhanced levels of CD80 and CD40. Stimulation of proliferation by these cells, however, was reduced to nearly background levels at APC–to–T-cell ratios of 1:15 625. In contrast, adenovirus-GFP–infected monocytes were highly allostimulatory compared with other groups, with the capacity to induce considerable proliferation of allogenic T cells even at a 1:15 625 (stimulator-to-responder) ratio, which corresponds to a mere 13 cells added per well (Figure 5). Cells differentiated with adenovirus containing no transgene showed similar, albeit slightly lower, APC capacity (not shown). DCs differentiated with LPS also showed enhanced capacity to induce allogenic T-cell proliferation, but appeared generally inferior to adenovirus-derived cells. As expected, heat-inactivated adenovirus showed a much inhibited capacity to enhance MLR activity of cultured cells (Figure 5), consistent with the generally modest immunophenotypical modulations (Figure 2). We conclude that infection of normal CD14+ monocytes with adenovirus vectors yields cells with enhanced allostimulatory capacity even when very small numbers of APCs are added to T-cell coculture, a functional capacity characteristic of DCs.
Enhanced allostimulatory ability in adenovirus-infected monocytes.
Graded numbers of gamma-irradiated (20 Gy) monocytes or monocyte-derived DCs were added to 96-well tissue-culture plates with purified allogenic T lymphocytes (2 × 105 per well). Cells were cocultured for 96 hours and pulsed with 1 μCi (.038 MBq) [3H]-TdR for an additional 18 hours; then, [3H]-TdR incorporation in harvested cells was assessed by liquid scintillation spectrometry. Results are representative of 3 separate experiments with different donors. (○) indicates uncultured monocytes; (■), monocytes cultured 72 hours in SFM/G; (▵), monocytes cultured overnight in SFM/G, infected with adenovirus-GFP, and incubated for 48 hours; (▴), monocytes that received similar treatment with heat-inactivated (HI) adenovirus-GFP; (✙), monocytes that were cultured overnight in SFM/G and treated with LPS for 48 hours.
Enhanced allostimulatory ability in adenovirus-infected monocytes.
Graded numbers of gamma-irradiated (20 Gy) monocytes or monocyte-derived DCs were added to 96-well tissue-culture plates with purified allogenic T lymphocytes (2 × 105 per well). Cells were cocultured for 96 hours and pulsed with 1 μCi (.038 MBq) [3H]-TdR for an additional 18 hours; then, [3H]-TdR incorporation in harvested cells was assessed by liquid scintillation spectrometry. Results are representative of 3 separate experiments with different donors. (○) indicates uncultured monocytes; (■), monocytes cultured 72 hours in SFM/G; (▵), monocytes cultured overnight in SFM/G, infected with adenovirus-GFP, and incubated for 48 hours; (▴), monocytes that received similar treatment with heat-inactivated (HI) adenovirus-GFP; (✙), monocytes that were cultured overnight in SFM/G and treated with LPS for 48 hours.
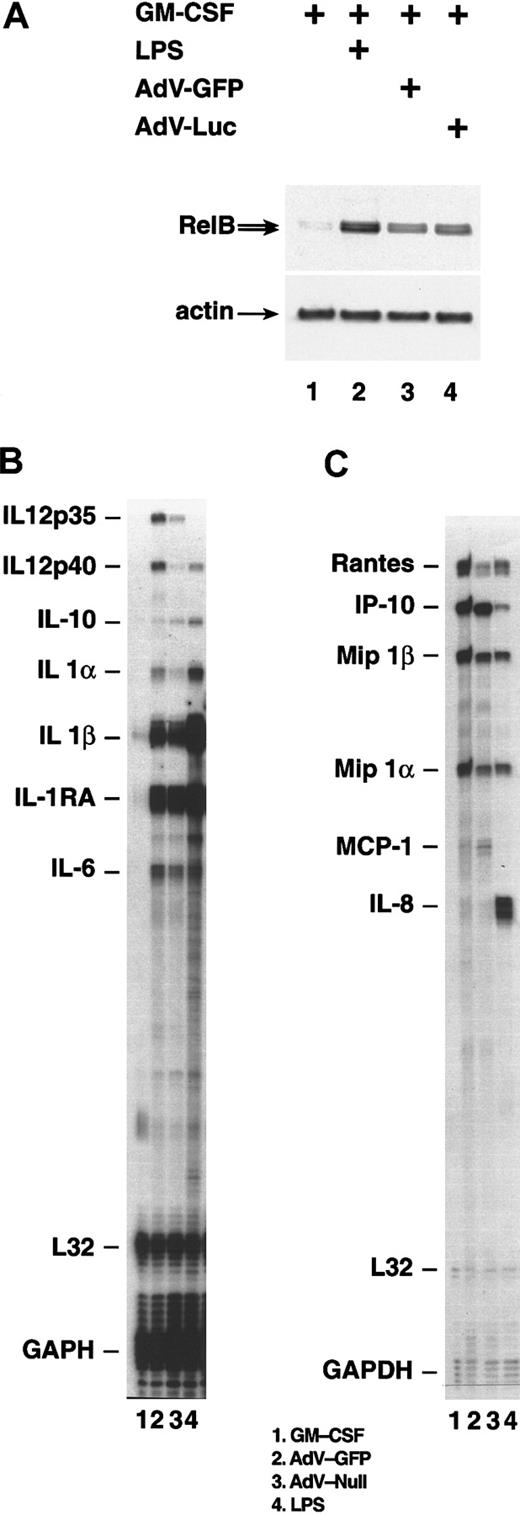
Adenovirus infection induced expression of RelB
The nuclear factor (NF)–κB family member RelB is associated with, and essential for, normal development of myeloid DCs.25-30 To test whether infection of monocytes with adenovirus vectors resulted in enhanced RelB expression, immunoblots from lysates of infected cells were probed with an antibody specific for RelB (Figure 6A). Very little RelB was detected in control cells cultured in SFM/G (lane 1), but the protein was highly induced in both LPS- and adenovirus-treated cells (lanes 2-4). Thus, adenovirus infection causes the induction of the transcription factor RelB, which is associated with DC maturation.
Gene expression following infection with adenovirus vectors.
After 24 hours in SFM/G, monocytes were uninfected, were infected with an adenovirus at an MOI of 200, or were treated with 50 ng/mL LPS. (A) Monocytes harvested 48 hours after infection with adenovirus-GFP, infection with adenovirus-Luc, or treatment with LPS. Whole-cell lysates were analyzed by immunoblotting with the use of antiserum specific for RelB. The membrane was subsequently reprobed with an actin-specific antibody. (B) (C) Monocytes harvested 24 hours after infection with adenovirus-GFP or adenovirus-null. Total RNA was analyzed by RNAse protection assay. Results comparing SFM/G–, LPS–, and adenovirus-GFP–treated cells are representative of 5 separate experiments with different donors. Comparison of adenovirus-GFP and adenovirus-null was performed once.
Gene expression following infection with adenovirus vectors.
After 24 hours in SFM/G, monocytes were uninfected, were infected with an adenovirus at an MOI of 200, or were treated with 50 ng/mL LPS. (A) Monocytes harvested 48 hours after infection with adenovirus-GFP, infection with adenovirus-Luc, or treatment with LPS. Whole-cell lysates were analyzed by immunoblotting with the use of antiserum specific for RelB. The membrane was subsequently reprobed with an actin-specific antibody. (B) (C) Monocytes harvested 24 hours after infection with adenovirus-GFP or adenovirus-null. Total RNA was analyzed by RNAse protection assay. Results comparing SFM/G–, LPS–, and adenovirus-GFP–treated cells are representative of 5 separate experiments with different donors. Comparison of adenovirus-GFP and adenovirus-null was performed once.
Cytokine and chemokine profiles of adenovirus-infected cells
DCs synthesize a large number of cytokines and chemokines that exert profound autocrine and paracrine effects. To test whether monocytes infected with adenovirus vectors resemble DCs in their pattern of cytokine/chemokine expression, we performed RNAse protection assays, using total RNA isolated 24 hours after infection. The results showed that infection with adenovirus vectors was nearly as efficient as LPS treatment in inducing expression of cytokines and chemokines typical of DCs.31-33 Both adenovirus infection and LPS treatment induced high levels of messenger RNA (mRNA) encoding IL-1β, as well as appreciable increases of mRNA for IL-1α, IL-6, IL-10, and IL-12 p40 (Figure 6B). Adenovirus also induced low-level expression of IL-12 p35 mRNA, which was not seen in LPS-treated cells. Control cells cultured in SFM/G expressed none of these mRNAs at detectable levels.
We also monitored the secretion of several cytokine proteins. Whereas LPS induced secretion of low to moderate levels of IL-10, IL-12, and IL-1β, these cytokines were below the level of detection in supernatants of virus-infected monocyte cultures. However, adenovirus did induce measurable IL-6 secretion, though at a much lower level (about 10 × lower) than seen with LPS-treated cells (data not shown).
Chemokine genes up-regulated by both virus and LPS include RANTES, MIP-1α, and MIP-1β, all of which are commonly expressed by DCs, and interferon-inducible protein (IP)–10, which can be expressed by a variety of cell types and is known to be induced by adenovirus infection31,33-37 (Figure 6C). IL-8 expression, which is characteristic of DCs,31,33 34 was induced by LPS and by adenovirus initially at 3 hours after infection (data not shown), but its mRNA fell nearly to background level by 24 hours after infection. In contrast, LPS-treated cells maintained high IL-8 mRNA levels at 24 hours. MIP-1α, MIP-1β, and RANTES were secreted at high levels by adenovirus-infected cells; in most experiments, these levels were comparable to those induced by LPS (Table1). Thus, adenovirus infection of monocytes induces cytokine and chemokine expression characteristic of DCs.
Chemokine production by adenovirus-infected or LPS-treated human monocytes
| Treatment . | MIP-1α . | MIP-1β . | RANTES . |
|---|---|---|---|
| Donor 1 | |||
| SFM/G | < .062 | < .062 | < .016 |
| Plus adenovirus-GFP | 40 | 79 | 14 |
| Plus LPS | 74 | 181 | 61 |
| Donor 2 | |||
| SFM/G | < .062 | < .062 | < .016 |
| Plus adenovirus-GFP | 15 | 46 | 12 |
| Plus LPS | 30 | 163 | 16 |
| Treatment . | MIP-1α . | MIP-1β . | RANTES . |
|---|---|---|---|
| Donor 1 | |||
| SFM/G | < .062 | < .062 | < .016 |
| Plus adenovirus-GFP | 40 | 79 | 14 |
| Plus LPS | 74 | 181 | 61 |
| Donor 2 | |||
| SFM/G | < .062 | < .062 | < .016 |
| Plus adenovirus-GFP | 15 | 46 | 12 |
| Plus LPS | 30 | 163 | 16 |
Human CD14+ monocytes were cultured for 36 hours in macrophage serum-free medium and granulocyte-macrophage colony-stimulating factor and then were infected with adenovirus-GFP (200 plaque-forming units per cell) or treated with lipopolysaccharide (50 ng/mL). At 48 hours later, supernatants were assayed for the indicated chemokines. Results are expressed in nanograms per milliliter.
MIP indicates macrophage inflammatory protein; SFM/G, macrophage serum-free medium and granulocyte-macrophage colony-stimulating factor; GFP, green fluorescent protein; and LPS, lipopolysaccharide.
Adenovirus-induced differentiation is inhibited by NAC and by neutralization of TNF-α
It has been reported that infection of humans, mice, and rats with adenovirus or adenovirus-based vectors leads within hours to the production of proinflammatory cytokines, including TNF-α.38-41 We previously showed that much of the DC-differentiating effect of LPS on monocytes was a result of induced secretion of TNF-α.8 Therefore, the possibility that adenovirus vector–infected monocytes might produce TNF-α was investigated. We found that TNF-α was readily detectable in culture supernatants at 24 hours after infection, although at a much lower level (> 10 × ) than seen in LPS-treated cultures.
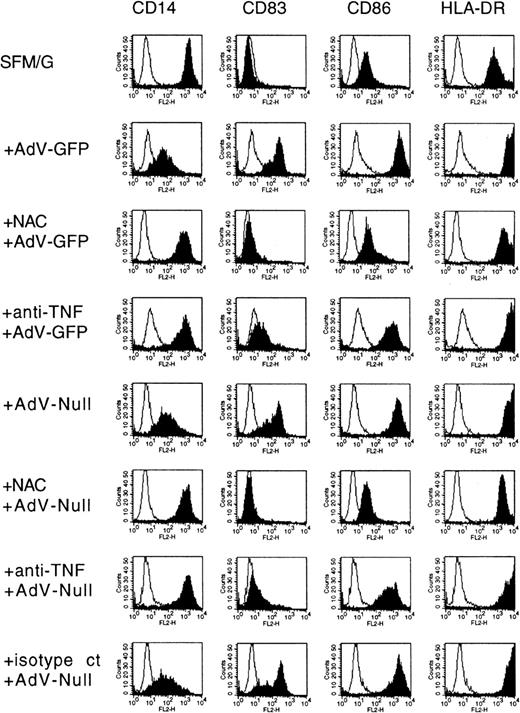
We then investigated whether virus-induced differentiation of some or all of the cells was dependent on the presence of TNF-α. To accomplish this, infection and subsequent postinfection culture were carried out in the presence of a TNF-α–neutralizing mAb.8 The result was that virus-induced changes in the immunophenotype were largely inhibited in the presence of a TNF-α–neutralizing antibody (Figure 7, compare rows 2 and 4, and rows 5 and 7). CD14 down-regulation as well as up-regulation of CD83 was strongly blocked, with apparent, albeit modest, inhibition of CD86 and HLA-DR expression. An isotype-matched control antibody had no effect (row 8). It should be stressed that CD83 expression was inhibited on both GFP+ and GFP−populations. If infectious virus (as measured on the 293 adenovirus propagation line) is required to trigger differentiation, then this result indicates that GFP+ cells produce TNF-α, which in turn mediates their own differentiation as well as differentiation of GFP− cells. However, we mentioned earlier the possibility that differentiation may be triggered not only by infectious virus, but by the more numerous noninfectious particles as well. According to this scenario, both GFP+ and GFP− cells would produce TNF-α, which mediates differentiation of all of the cells.
Effects of expressed transgene, neutralizing TNF-α antibody, and NAC on adenovirus-induced monocyte differentiation.
Human monocytes were cultured for 36 hours in SFM/G and then infected with adenovirus-GFP or adenovirus-null, with or without 50 mM NAC (pretreatment for 45 minutes) or 10 μg/mL TNF-α–neutralizing mAb 5N (pretreatment for 45 minutes) or 10 μg/mL isotype-matched control antibody (isotype ct). Cells were harvested 48 hours later and analyzed by FACS. Results are representative of 5 separate experiments with different donors.
Effects of expressed transgene, neutralizing TNF-α antibody, and NAC on adenovirus-induced monocyte differentiation.
Human monocytes were cultured for 36 hours in SFM/G and then infected with adenovirus-GFP or adenovirus-null, with or without 50 mM NAC (pretreatment for 45 minutes) or 10 μg/mL TNF-α–neutralizing mAb 5N (pretreatment for 45 minutes) or 10 μg/mL isotype-matched control antibody (isotype ct). Cells were harvested 48 hours later and analyzed by FACS. Results are representative of 5 separate experiments with different donors.
Our previous study showed that NAC, an antioxidant and a nonspecific inhibitor of NF-κB, completely suppresses LPS-induced differentiation of monocytes to DC-like cells. We found the same to be true for adenovirus infection. When infection and subsequent culture were carried out in the presence of 50 mM NAC, down-regulation of CD14 and up-regulation of CD83 and CD86 were effectively blocked (Figure 7, compare rows 2 and 3, and rows 5 and 6). Only HLA-DR up-regulation was partially resistant. The inhibition of differentiation was not due to toxicity of the NAC, for the viability of the cells was not affected for at least 3 days. It is unlikely that this inhibition was due to a failure of viral particles to enter the cell, since application of NAC at 2 hours after infection was as effective as application at the time of infection (data not shown). In both cases, there was little or no conversion of monocytes to the DC immunophenotype. Thus, like LPS-induced differentiation, adenovirus-induced differentiation is sensitive to the antioxidant NAC.
Discussion
Our previous studies showed that human CD14+ monocytes cultured in serum-free medium can rapidly differentiate into DCs upon treatment with TNF-α, LPS, or calcium ionophore.8 21Here we show that fresh CD14+ monocytes infected with replication-defective adenovirus under serum-free conditions rapidly (within only 48 hours) differentiate into CD83+CD25+ DCs, without the requirement for a cytokine-induced predifferentiation step. Differentiated cells maintain immunophenotype without loss of viability for at least 2 days after removal of the differentiation agent and cytokines. This demonstration is distinguished from previous studies because we clearly show that virus infection causes actual differentiation of CD83+ DCs from CD83−CD14+ monocytes, rather than simply causing the further maturation of GM-CSF/IL-4–derived CD83lowCD14− immature DCs. Although we also employ GM-CSF in our culture medium, which is necessary to maintain high cell viability of monocytes in SFM, treatment with this cytokine alone cannot be construed as a DC differentiation–inducing regimen by the assays employed in these studies. This is because cells cultured for up to 4 days in GM-CSF–containing SFM remain CD83−CD14+, do not up-regulate major histocompatibility complex (MHC) molecules, do not acquire DC morphology, do not produce cytokines or chemokines associated with DCs, and do not show a dramatically enhanced capacity to stimulate allogenic T-cell proliferation at low APC numbers, a hallmark of true DC function. On the other hand, cells examined only 48 hours after adenovirus infection display stable immunophenotype, morphology, and cytokine/chemokine profile as well as the enhanced APC function characteristic of DCs. This was the typical response: monocytes from 12 of 14 tested donors differentiated into DCs when infected with adenovirus. Thus, under serum-free conditions, adenovirus type 5 potently induces monocyte-to-DC differentiation, an effect that we showed to be unrelated to expression of a transgene.
Both TNF-α and NF-κB play vital roles in the differentiation process. We found that a TNF-α–neutralizing antibody markedly inhibited virus-induced differentiation, with especially profound effects on CD83 and CD86 up-regulation. Our previous work showed that LPS-induced DC differentiation is also dependent on TNF-α.8 Thus, both LPS and adenovirus induce the monocytes to secrete TNF-α (though at a much higher level in response to LPS than to adenovirus), and TNF-α in turn triggers differentiation toward a DC phenotype.
Transcription factors belonging to the NF-κB family, particularly RelB, are closely associated with DC differentiation triggered by a variety of agents, including LPS, TNF-α, and calcium ionophore.8,30,42-44 The present studies show that the same appears to be true for adenovirus-induced differentiation, a result that is consistent with the observation that NF-κB is activated in mouse liver minutes after viral infusion.41Not only did our Western blot analysis demonstrate enhanced expression of RelB, but in RNAse protection assays, we also observed the up-regulation of numerous genes known to be positively regulated by NF-κB. These genes include IL-1α, IL-1β, IL-1Ra, IL-6, IL-12 p40, RANTES, MIP-1α, MIP-1β, IP-10, and IL-8.45 In addition, the TNF-α gene itself is under NF-κB control.46 Further evidence of NF-κB involvement comes from the effect of NAC on DC differentiation. NAC inhibits NF-κB activation in a variety of cell types in response to a wide range of inducers, including viruses.43,47-49 In our experiments, NAC blocked both RelB up-regulation and adenovirus-induced differentiation. Recently, Morelli et al17 showed that NF-κB is not just associated with, but actually required for, at least some aspects of DC maturation. They observed that an adenovirus vector encoding dominant-negative IκBα, which prevents activation of NF-κB, blocked the virus-induced up-regulation of CD86 and MHC-II in murine bone marrow–derived DCs.
Currently, published reports regarding the effect of adenovirus infection on the maturation of DCs are somewhat conflicting. Some studies indicate that adenovirus infection of immature DCs is completely nonperturbing and does not initiate further activation or maturation of DCs in the absence of other agents.13-15,50In contrast, others have convincingly demonstrated maturation-inducing effects of adenovirus infection for both immature human and murine DCs.16-18 We clearly show that adenovirus infection under serum-free conditions induces actual differentiation of CD14+CD83− CD25− monocytes into CD14−CD83+CD25+ DCs rather than merely inducing a maturation of immature DCs. The differentiating effects of adenovirus we observed were very rapid (36 to 48 hours) and arguably complete, with a wide spectrum of phenotypical, morphological, functional, and molecular features of DCs being induced directly from CD14+ monocytes. Culture in SFM/G alone induced none of these effects. In medium supplemented with FCS, this process was strongly inhibited, a finding that may help to explain the conflicting reports in the literature.
Our results have several important implications. First, our results show that serum-free culture can be used for simultaneous adenovirus-mediated gene transfer and rapid induction of differentiation of DCs from monocytes. This method speeds and simplifies the process of introducing target proteins to APCs for DC-based vaccine strategies and thus represents an appreciable advance.
Second, the vigorous response of monocytes to replication-defective adenovirus offers an additional cautionary statement on the use of such vectors in gene therapy trials where the purpose is to install transgenes into human tissues while provoking minimal inflammatory responses. The proinflammatory cytokine/chemokine response to adenovirus that we have described in vitro is consistent with in vivo murine studies, reported by others,36,41,51 that were likewise independent of transgene expression and viral replication.36 51 The strong proinflammatory response induced by adenovirus vectors suggest that the in vivo response of monocytes (and presumably other cell types) to infection with adenovirus could have potentially negative consequences in human gene therapy settings, unless these qualities can be engineered out of the virus.
Finally, these studies suggest that CD14+ monocytes have an innate capacity to sense the presence of viruses in vitro and respond by rapidly differentiating into potent DCs. There is good reason to suppose that this can also happen in vivo. Respiratory epithelial cells, when infected with a variety of viruses that cause respiratory tract infections, secrete not only GM-CSF, but also considerable amounts of RANTES and MIP-1α,52 which possess monocyte chemoattractant properties. Monocytes would therefore be expected to leave the bloodstream and infiltrate sites of virus infection. Since cells lining the respiratory passages of adenovirus-infected individuals have been estimated to contain as many as 10 000 virions per infected cell,10 53 monocytes that have migrated to infected tissue beds can be expected to come in contact with high levels of virus. It therefore seems credible that the conditions described in our in vitro system, including a serum-free environment, with exposure to GM-CSF and adenovirus, exist in local epithelial tissues during viral infection.
Several questions regarding the adenovirus-induced differentiation of DCs from monocytes remain to be resolved and form the focus of our ongoing studies. For instance, the precise mechanism by which monocytes are signaled to the presence of adenovirus is not clear. Since the virus we used is competent to enter human monocytes and transcribe the inserted sequences but is defective for replication, and since psoralen/UV– inactivation had no affect on adenovirus-mediated differentiation,17 it is likely that the signal(s) is delivered prior to the transcription of any virus-encoded products. It is also not clear whether the absence of IL-12 secretion by the apparently fully differentiated infected cells represents an appropriate physiologic response directed at the generation of robust Th2/antibody–mediated (ie, virus-neutralizing) immunity or is a viral subversion of the immune response that limits Th1-type cell–mediated immunity (which could enhance viral survival). Since RANTES and MIP-1α (which are abundantly secreted by adenovirus-infected cells) have been shown to substitute for IL-12 in the generation of TH1-type T cells,37 and since IL-12 knockout mice can still mount effective TH1-type immune responses during viral infection,54 it is possible that the lack of IL-12 does not have significant functional consequences.
The studies reported here clearly demonstrate that a model virus is capable of initiating actual and rapid differentiation of DCs directly from CD14+ monocytes, lending additional credibility to the notion that monocytes represent a bona fide precursor pool for DCs that can be quickly mobilized by infectious agents. The elucidation of the mechanisms by which this occurs and the interplay between virus and host that modulates the character of the developing immune response will be of critical value to our developing understanding of host-virus relationships.
We wish to express our gratitude to Drs Susan Leitman, E. J. Read, Harvey Klein, Thomas Trischmann, and Charles Carter of the Cell Processing Section of the Transfusion Medicine Branch at the National Cancer Institute, National Institutes of Health, Bethesda, MD, for their generosity and technical support.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nancy R. Rice, National Cancer Institute at Frederick, PO Box B, Frederick, MD, 21702-1201; e-mail:ricen@ncifcrf.gov.




![Fig. 5. Enhanced allostimulatory ability in adenovirus-infected monocytes. / Graded numbers of gamma-irradiated (20 Gy) monocytes or monocyte-derived DCs were added to 96-well tissue-culture plates with purified allogenic T lymphocytes (2 × 105 per well). Cells were cocultured for 96 hours and pulsed with 1 μCi (.038 MBq) [3H]-TdR for an additional 18 hours; then, [3H]-TdR incorporation in harvested cells was assessed by liquid scintillation spectrometry. Results are representative of 3 separate experiments with different donors. (○) indicates uncultured monocytes; (■), monocytes cultured 72 hours in SFM/G; (▵), monocytes cultured overnight in SFM/G, infected with adenovirus-GFP, and incubated for 48 hours; (▴), monocytes that received similar treatment with heat-inactivated (HI) adenovirus-GFP; (✙), monocytes that were cultured overnight in SFM/G and treated with LPS for 48 hours.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.600/6/m_h80222029005.jpeg?Expires=1767704189&Signature=RxcvSLl22bj2jSJ88J9K9ZQ4ZZHcOUAzO6M-yLsKmfjCNW61y91EOUXFuKMaPctaADp55PBjScMvbRgtu3hzrME5LRaaFnGDjdANTIac8t6Bui~qes7bHn5TygMRBq~emprq4UfPkbcOSdUhc9FjfpdKqg7BVsEyDE-YeDK4YAqv1czR6oD6XoO-ML1ZPilxbGKpRrz613Jc5O7MpijlijPvV28xZGWEMgFYf1Jbj82Hv73u2JfHHVJLG4Ykwz34rrbx-KrWfzeBZQK2kzo~oCiE~oC0TkuJlMXwHC0x5IUqeNN3U9JZ9xw2g9SymFFq4aCThZItom2RWv3gcJ1rjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal