Abstract
The mechanisms by which transforming growth factor β (TGF-β) exerts a negative effect on cell-cycle entry in primary human hematopoietic stem/progenitor cells were examined at the molecular and cellular levels. After treatment of primary human CD34+progenitors with TGF-β there was a decrease in the levels of cyclin D2 protein and an increase in levels of the cyclin-dependent kinase inhibitor (CDKI) p15 as compared to the levels in untreated cells. The converse was true after addition of neutralizing anti–TGF-β antibody. Administration of TGF-β to CD34+ cells in the presence of cytokines prevented retinoblastoma protein (pRb) phosphorylation, which occurred in the same cells treated with cytokines alone or cytokines and anti–TGF-β antibody. Neutralization of TGF-β during 24 to 48 hours of culture with cytokines significantly increased the number of colony-forming progenitors, but did not modulate the human stem cell pool, as measured in 6- to 12-month xenotransplantation assays. Equivalent numbers of human B, T, and myeloid cells were obtained after transplantation of cells treated with or without neutralization of TGF-β.
Introduction
Transforming growth factor β (TGF-β) is a pleiotropic cytokine that exerts inhibitory effects on mesenchymal cells, including hematopoietic stem and progenitor cells. The binding of TGF-β results in recruitment and autophosphorylation of the heterodimeric form of the TGF-β receptor. Subsequently, the TGF-β type 1 receptor kinase phosphorylates Smad2 or Smad3 promoting their dissociation from the receptor, allowing their association with Smad4 for nuclear translocation. In the nucleus, Smads have been shown to activate transcription of extracellular matrix proteins such as collagen a1 and elastin while inhibiting transcription of cell-cycle–related genes such as c-myc andcdc25A (for a review, see Massague1).
The functional role of TGF-β as an inhibitor of the cell cycle has been investigated in numerous cell types, but not yet in primary human hematopoietic progenitors, at the molecular level. In mink lung epithelial cells, addition of TGF-β induces an increase in the p15Ink4 levels and a decrease in cdk4 levels, thus arresting cells in mid G1.2,3 TGF-β blocked mouse keratinocytes in late G1 by altering the transcription of cyclin A and B-myb.4 In the monocytic cell line THP-1, TGF-β enhanced the binding between TGF-β II receptor and cyclin B1. Consequently, cdc2 bound to the cyclin B1/TGF-β II receptor complex becomes phosphorylated on its threonine residues, down-modulating cdc2 kinase activity and arresting the cell cycle in the G2/M phase.5 In the murine hematopoietic progenitor cell line 32D3, TGF-β treatment resulted in G1 arrest due to decreased cdk4 kinase activity.6 Through an alternate route, TGF-β prevented cell-cycle progression via up-regulation of p27Kip1, a cyclin-dependent kinase inhibitor (CDKI) that binds and inhibits the activity of cyclin E/cdk2.7
In primary hematopoietic progenitors that express the CD34 antigen, it was previously shown that addition of TGF-β resulted in decreased proliferation8 and that neutralization of TGF-β in culture recruited the quiescent progenitors into cycle.9,10 Although numerous reports have confirmed the inhibitory effect of TGF-β on primary CD34+ hematopoietic progenitors, little is known about the molecular mechanism by which TGF-β inhibits proliferation of these cells, which is the topic of the current studies. It is of particular interest to understand the pathways regulated by TGF-β in primary CD34+ progenitors. TGF-β is secreted via autocrine as well as paracrine pathways in hematopoietic CD34+ cells.9,10 Thus, neutralization of TGF-β may alter differentiation or pluripotentiality of the primitive hematopoietic progenitors. The effect of TGF-β on cells can vary depending on the cell type and the level of maturation,11,12 so elucidation of the molecular events occurring in the exact cells of interest is crucial. It has been proposed that cycling hematopoietic stem/progenitor cells do not engraft as well as quiescent cells in bone marrow (BM) transplantation settings.13-15 Because TGF-β is a major factor in maintaining quiescence in murine and human hematopoietic stem cells, it was possible that TGF-β neutralization could have adverse effects in a transplantation setting. Our goal in the current study was to elucidate the molecular pathways by which TGF-β alters cell-cycle progression in CD34+ cells as well as the impact of TGF-β neutralization on primary human hematopoietic stem/progenitor cell transplantation and differentiation. We demonstrate that TGF-β inhibits the proliferation of CD34+ cells and that neutralization of TGF-β in primary human stem/progenitor cell culture leads to cell-cycle induction but does not lead to an induction of differentiation or a loss of the stem cell pool.
Materials and methods
Isolation and culture of human hematopoietic progenitors
Normal human BM cells were obtained from screens used to filter BM during harvest of allogeneic donors. Umbilical cord blood (UCB) samples were collected at Kaiser Permanente (Los Angeles, CA). Use of these samples was approved by the Committee on Clinical Investigations at Children's Hospital of Los Angeles. CD34+ progenitors were isolated from Ficoll mononuclear cell fractions from both BM and UCB by incubation with the monoclonal antibody HPCA-1 (Becton Dickinson, San Jose, CA), followed by goat antimouse-conjugated immunomagnetic beads (Dynal, Oslo, Norway) or by using sequential passes through 2 MiniMACS columns as directed by the manufacturer (Miltenyi, Auburn, CA). CD34+CD38− cells were isolated from human BM by pre-enrichment of CD34+ cells using MiniMACS columns, followed by fluorescence-activated cell sorting (FACS) acquisition using a stringent gate as described,16-18 to obtain a highly quiescent population. Cells were cultured in serum-containing or serum-free medium (Ex-Vivo 15, Biowhittaker, Walkersville, MD) with the cytokines interleukin (IL)-6, stem cell factor (SCF), and FLT3 ligand (50 ng/mL, Biosource, Camarillo, CA) and IL-3 (10 ng/mL, Biosource). Cells were cultured on the COOH-terminal domain of fibronectin (Retronectin, Takara, Otsu, Japan), with and without addition of neutralizing antibody to TGF-β (panspecific, R & D Systems, Minneapolis, MN) at a final concentration of 5 μg/mL. Immediately after the designated culture period, cells were taken for molecular analyses, colony-forming unit (CFU) analysis, and transplantation into immune-deficient mice. To determine the clonogenic potential after culture in the different conditions, cells were plated in methylcellulose colony-forming assay, then counted at day 21 as described.19
Cell-cycle analysis by Ki67/7-aminoactinomycin D staining
Following culture at the indicated time points, progenitors were collected using cell dissociation buffer and subjected to Ki67/7-aminoactinomycin D (AAD) staining as described by Jordan et al.20 Briefly, cells were washed in phosphate-buffered saline (PBS)/1% fetal calf serum (FCS) and fixed in PBS/0.4% formaldehyde for 30 minutes on ice. Permeabilization with PBS/0.2% Triton X-100 was performed overnight at 4°C. Cells were then washed and resuspended in PBS/1% FCS and stained with 10 μL Ki-67-fluorescein isothiocyanate (FITC; Immunotech). After washing, cells were resuspended in PBS/1% FCS/0.5 μg/mL 7-AAD overnight at 4°C. Acquisition and analyses were performed on the FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA). Freshly isolated peripheral blood T cells and phytohemagglutinin (PHA) plus IL-2–stimulated T cells were used as controls for G0 and cycling cell populations, respectively.
35S metabolic labeling
CD34+ progenitors were plated in Dulbecco modified Eagle medium (DMEM)-methionine free medium for a 4-hour starvation period in the presence of 2% dialyzed FCS, IL-3, IL-6, and SCF. Fifty microcuries 35S-methionine/cysteine with the appropriate amount of TGF-β or anti–TGF-β antibody was then added and labeling proceeded for 18 hours at 37°C. Cells collected using dissociation buffer were washed in PBS, then lysed in 500 μL immunoprecipitation buffer as described by Matsushime et al.21 In brief, cells were sonicated twice with 10 seconds each time at 4°C, then were clarified at 10 000 rpm for 5 minutes. Lysate (50 μL) was collected from each sample and frozen at −80°C for Western analysis. The remaining 450 μL was transferred to a new tube containing 25 μL agarose-coated cdk4 polyclonal antibody and incubated for 2 to 6 hours at 4°C. The immunoprecipitated samples were then spun at 10 000 for 5 minutes and the supernatants were transferred to another tube containing 25 μL agarose-coated cyclin D2 polyclonal antibody for a subsequent 4-hour immunoprecipitation at 4°C. A/G-agarose beads were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). At the end of the serial immunoprecipitation, all samples were washed twice in immunoprecipitation buffer, followed with 3 washes in buffer containing 50 mM Hepes and 1 mM dithiothreitol. Sample buffer was added and boiled samples were run on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. The gel was dried for 2 hours and exposed to autoradiogram film for 24 to 48 hours.
Immunoblotting
Cells (10 000 or 50 000) were incubated with and without soluble TGF-β or anti–TGF-β antibody in serum-free medium for the indicated times at 37°C, in 5% CO2. Pelleted cells were then lysed on ice for 10 minutes in 1% NP-40 lysis buffer (50 mM Tris-HCL, pH 7.4, 250 mM NaCl, 2 mM EDTA, 2 μg/mL aprotinin,1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM NaF, 0.5 μg/mL leupeptin, 1% NP-40). The cleared lysates were boiled in SDS sample buffer at 95°C and electrophoresed on 12% or 15% SDS-PAGE gels, then transferred onto Hybond membrane (Amersham, Arlington Heights, IL). Immunoblotting was done as described22 using antibodies to cyclin A (H-432), cyclin D2 (SC-754), cyclin D3 (SC-182), cdk4 (SC-749), cdk6 (SC-7961), cdk2 (SC-748), p15 (SC-613) from Santa Cruz Biotechnology; retinoblastoma protein (pRb; G3-245) from Pharmingen; or phospho-Ser807/811 pRb from Cell Signaling Technology.
To ensure equal loading of proteins, membranes were incubated with amido black stain (Sigma, St Louis, MO), which stains all proteins. The stain was prepared according to the manufacturer's instructions. Membranes were incubated with the stain for 4 hours, then washed in 10% acetic acid/40% methanol/50% water, with 8 to 10 washes of 30 minutes each at room temperature on a shaker. The membranes were then rinsed 3 times in water alone, dried, and photographed.
Mice
Studies used 6- to 8-week-old beige/nude/xid (bnx) homozygous mice (bg.bg/nu.nu/xid.xid, NIH-3) bred at Children's Hospital, Los Angeles. Cotransplantation of human progenitors and mesenchymal stem/progenitor cells producing IL-3 was performed as previously published.18,19 22 Sublethal conditioning was done by administering 400 rads or 150 μg/kg 5-fluoruacil 48 hours prior to injection of human cells. Mice were killed by 90% CO2/10% O2 narcosis 6 to 12 months after receiving transplanted human cells. BM was flushed from the tibiae and femurs of each mouse into PBS, dispersed with a fine needle, counted, and used for the assays described below.
FACS analysis
Single-cell suspensions recovered from the BM of mice undergoing cotransplanation were blocked by preincubation for 15 minutes on ice with unconjugated mouse immunoglobulin (MsIgG, Coulter, Hialeah, FL). Directly conjugated antibodies used to identify human-specific cell surface antigens were HLE-1 (anti-CD45, Becton Dickinson [BD]), My9-RD1 (anti-CD33, Coulter), Leu-12 (anti-CD19, BD), Leu-3a (anti-CD4, BD), and Leu-2a (anti-CD8, BD). Samples were acquired on a Becton Dickinson FACScan and analyzed using the CellQuest software package (BD). Ten thousand events were acquired for each sample. Parallel staining and FACS analyses were done on healthy human and nontransplanted bnx mouse BM controls to confirm that none of the human-specific antibodies cross-reacted with murine cells.
Statistical analyses
All analyses were done using Excel 5.0 software (Microsoft Corporation). Average values are listed with SDs. SEM was used if all values were listed in table format to provide the range. The significance of each set of values was assessed using the 2-tailed t test assuming equal variance.
Results
Effects of TGF-β addition versus neutralization on cell-cycle progression at the DNA level
To determine the effect of TGF-β on primary hematopoietic progenitors, freshly isolated CD34+ cells from BM or UCB were incubated for 24 hours in the presence of cytokines alone, cytokines with soluble TGF-β, or cytokines with addition of neutralizing anti–TGF-β antibody. The percentages of cells in G0, G1, and S/G2M phases were analyzed using Ki67/7AAD staining as described.20 There was an increase in the fraction of cells in G0 phase when TGF-β was added, as compared to cells cultured in cytokines alone and in cytokines plus anti–TGF-β antibody (Figure1, P < .05, n = 5). Cells treated with TGF-β had significantly lower levels that progressed from the G0 phase into the G1 or S/G2M phases of the cell cycle, in comparison to the other conditions (Figure 1, P < .05, n = 5).
Cell-cycle analysis of CD34+ progenitors incubated with soluble TGF-β1 or anti–TGF-β antibody.
After 48 hours of incubation, CD34+ cells isolated from UCB were collected, fixed, permeabilized, and stained with Ki67-FITC. Cells were then stained with 7-AAD overnight before acquisition analysis by FACS Calibur flow cytometry. Controls for the G0 population were peripheral blood T cells starved overnight in serum-free medium. Controls for the cycling cell population were peripheral blood T cells stimulated with PHA and IL-2 overnight. White bars indicate cells incubated with the cytokines IL-3, IL-6, and SCF alone. Black bars indicate cells incubated in the same cytokine mixture with addition of soluble TGF-β1. Gray bars indicate cells incubated in the same cytokine mixture with addition of anti–TGF-β–neutralizing antibody. *P < .05.
Cell-cycle analysis of CD34+ progenitors incubated with soluble TGF-β1 or anti–TGF-β antibody.
After 48 hours of incubation, CD34+ cells isolated from UCB were collected, fixed, permeabilized, and stained with Ki67-FITC. Cells were then stained with 7-AAD overnight before acquisition analysis by FACS Calibur flow cytometry. Controls for the G0 population were peripheral blood T cells starved overnight in serum-free medium. Controls for the cycling cell population were peripheral blood T cells stimulated with PHA and IL-2 overnight. White bars indicate cells incubated with the cytokines IL-3, IL-6, and SCF alone. Black bars indicate cells incubated in the same cytokine mixture with addition of soluble TGF-β1. Gray bars indicate cells incubated in the same cytokine mixture with addition of anti–TGF-β–neutralizing antibody. *P < .05.
Following culture of the primary CD34+ cells in cytokines plus anti–TGF-β antibody, there was consistently a higher percentage of cells in G1, as compared to culture in cytokines alone (Figure 1, P < .05, n = 5). However, there were no statistical differences in the number of cells that progressed from the G1 phase into the S/G2M phase following treatment with anti–TGF-β antibody (Figure 1). These data indicate that a portion of the CD34+ cells were prompted from the G0 to the G1 phase of the cell cycle by TGF-β neutralization, but that they did not progress further through the cycle after this treatment.
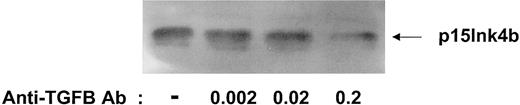
Determining the minimal amount of anti–TGF-β antibody required to decrease p15Ink4b levels
It has been shown that TGF-β can be secreted via an autocrine pathway in human CD34+ cells and that neutralizing antibody to TGF-β can overcome the inhibitory effects on cell cycle.9,10 We have previously demonstrated that neutralization of TGF-β greatly reduces levels of the CDKI p15.22 23 To determine the optimal concentration of anti–TGF-β antibody needed to neutralize the autocrine TGF-β and decrease endogenous p15 levels, CD34+ progenitors were cultured in serum-free medium for 24 hours in the presence of increasing amounts of anti–TGF-β antibody. As shown in Figure2, a minimum concentration of 0.2 ng/mL anti–TGF-β antibody was required to detect a significant decrease in p15 levels.
Optimizing the amount of anti–TGF-β antibody to decrease p15 protein levels.
UCB CD34+ progenitors were incubated for 18 hours in serum-free media alone or in the presence of increasing concentrations of anti–TGF-β antibody (ng/mL). Cell lysates were prepared and subjected to 15% SDS-PAGE, followed by immunoblotting with a polyclonal antibody to p15Ink4b.
Optimizing the amount of anti–TGF-β antibody to decrease p15 protein levels.
UCB CD34+ progenitors were incubated for 18 hours in serum-free media alone or in the presence of increasing concentrations of anti–TGF-β antibody (ng/mL). Cell lysates were prepared and subjected to 15% SDS-PAGE, followed by immunoblotting with a polyclonal antibody to p15Ink4b.
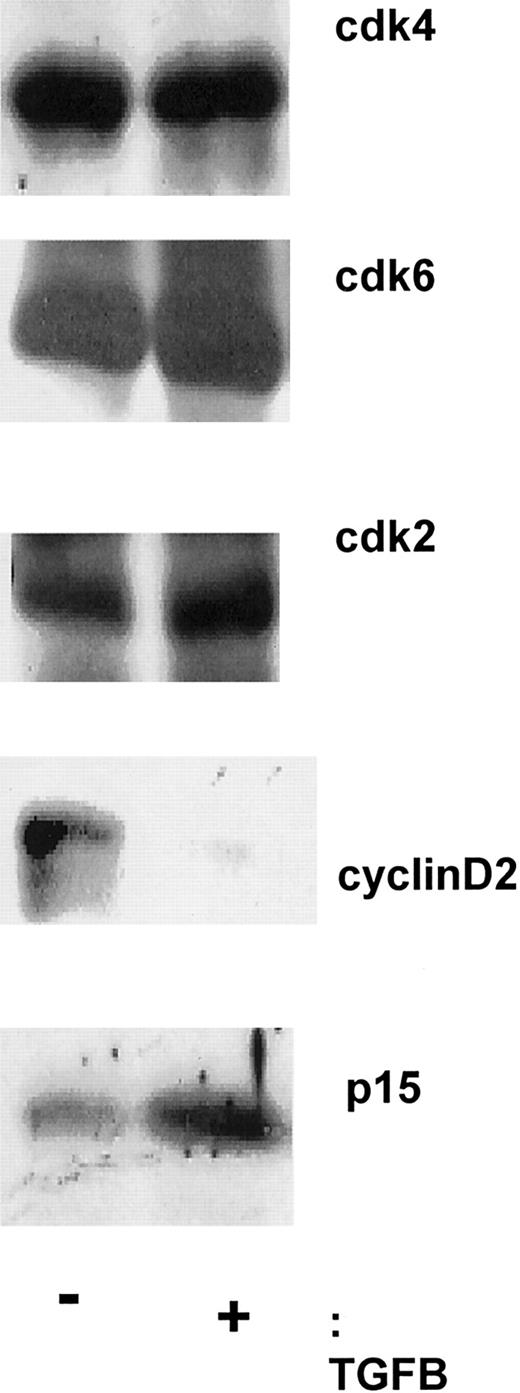
Effects of TGF-β addition versus neutralization on cyclin and cdk levels and association
To examine the molecular mechanisms by which TGF-β decreases cell proliferation, protein analyses for cell-cycle–related proteins were performed on CD34+ cells. D-type cyclins (cyclin D1, D2, and D3) are cell-cycle–modulated proteins, and adequate levels of at least one of the isotypes are required for progression through the early G1 phase. Hematopoietic cells express cyclin D2 and D3, but not cyclin D1.24 No alterations in the levels of cyclin D3 were observed after addition of TGF-β. Immunoblot analyses showed a specific decrease in cyclin D2 levels and an increase in levels of the CDKI p15 in human CD34+ cells after 18 hours of culture with TGF-β (Figure 3). The levels of the cdk2, cdk4, and cdk6 proteins were not modulated in CD34+ cells cultured in medium with cytokines alone compared with the addition of TGF-β (Figure 3).
Addition of soluble TGF-β1 alters the levels of cyclin D2, not cyclin D3, cdk2, cdk4, or cdk6.
After incubating with and without soluble TGF-β1 for 18 hours, CD34+ progenitors were lysed in 1% NP-40, loaded onto a 10% or 15% SDS-PAGE gel, and transferred to Immunobilon PSQ membrane. Immunoblotting with antibodies to cdk4 (SC-749), cdk6 (SC-7961), cyclin D2 (SC-754), cyclin D3 (D-7), and p15 (SC-613) was then performed.
Addition of soluble TGF-β1 alters the levels of cyclin D2, not cyclin D3, cdk2, cdk4, or cdk6.
After incubating with and without soluble TGF-β1 for 18 hours, CD34+ progenitors were lysed in 1% NP-40, loaded onto a 10% or 15% SDS-PAGE gel, and transferred to Immunobilon PSQ membrane. Immunoblotting with antibodies to cdk4 (SC-749), cdk6 (SC-7961), cyclin D2 (SC-754), cyclin D3 (D-7), and p15 (SC-613) was then performed.
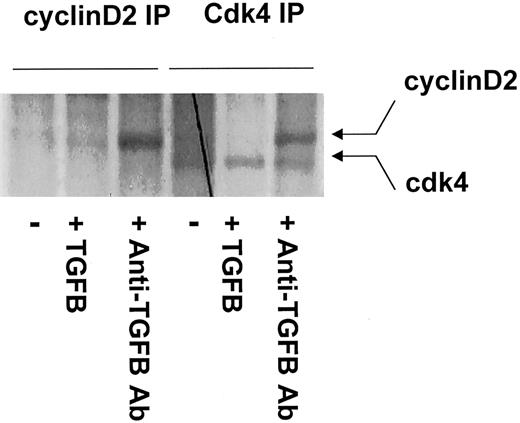
D-type cyclins associate with cdk4 to form active kinase complexes
To measure the amount of cyclin D/cdk4 complexes formed in vitro, CD34+ cells were metabolically labeled with35S-methionine for 18 hours. Serial immunoprecipitations were performed to quantitate the levels of associated cyclin D2/cdk4 complexes. There was no detectable association of cyclin D2/cdk4 complex in CD34+ cells treated with TGF-β (Figure4). Compared to the control, treatment with anti–TGF-β antibody significantly increased the levels of cyclin D2/cdk4 complexes (Figure 4). In summary, our data show that addition of TGF-β to cultures of primary human CD34+cells caused a decrease in the levels of cyclin D2, resulting in a decrease in cyclin D2/cdk4 complexes, whereas neutralization of TGF-β caused an increase in the levels of cyclin D2 and in its association with cdk4. Association of cyclin D with cdk4 allows cells to progress from quiescence into the initial stages of cell-cycle progression.
Immunocomplexes between cdk4 and cyclin D2 were detected only in the presence of anti–TGF-β antibody.
After starvation in methionine-free DMEM for 4 hours, CD34+progenitors were metabolically labeled for 18 hours with35S-methionine/cysteine in the presence or absence of soluble TGF-β1 or anti–TGF-β antibody. Cell lysates were first immunoprecipitated with agarose-coated polyclonal CDK4 antibody. Subsequent serial immunoprecipitation was then carried out further using agarose-coated polyclonal cyclin D2 antibody. Samples were run on a 10% SDS-PAGE gel, dried, and exposed to autoradiogram film.
Immunocomplexes between cdk4 and cyclin D2 were detected only in the presence of anti–TGF-β antibody.
After starvation in methionine-free DMEM for 4 hours, CD34+progenitors were metabolically labeled for 18 hours with35S-methionine/cysteine in the presence or absence of soluble TGF-β1 or anti–TGF-β antibody. Cell lysates were first immunoprecipitated with agarose-coated polyclonal CDK4 antibody. Subsequent serial immunoprecipitation was then carried out further using agarose-coated polyclonal cyclin D2 antibody. Samples were run on a 10% SDS-PAGE gel, dried, and exposed to autoradiogram film.
Effects of TGF-β addition versus neutralization on phosphorylation of pRb
To further examine the consequences of TGF-β–mediated alterations in cyclin-dependent kinase activity in primary human hematopoietic cells, we analyzed phosphorylation of the downstream target protein, pRb. pRb, a substrate of the cyclin D/cdk4 and cyclin E/cdk2 kinase complexes, can be detected as 3 isoforms: underphosphorylated, hypophosphorylated, and hyperphosphorylated. Phosphorylation of pRb by cyclin D/cdk4 causes release of E2F, which allows cell-cycle progression through the G1phase.
To assess the effect of TGF-β on the phosphorylation of pRb, CD34+ cells were treated with cytokines, cytokines with TGF-β, or cytokines with anti–TGF-β antibody. After an 18-hour incubation, cells were directly lysed for total protein analysis. Immunoblotting with a monoclonal antibody that recognizes all isoforms of pRb was performed to assess the effect of TGF-β on the phosphorylation of pRb. TGF-β treatment significantly reduced the phosphorylation of pRb at the 48-hour time point, as shown by a decrease in the high molecular weight pRb and an increased detection of the lower molecular weight pRb (Figure5A). The broad appearance of the band is due to the fact that there are at least 16 potential phosphorylation sites on the pRb protein. However, a key residue that is a target for phosphorylation, to allow conformational change on pRb, is Ser811.25 Using an antibody specific to phosphorylated Ser811 for immunoblotting, we observed an increase in intensity of pRb phosphorylation at Ser811 in CD34+ cells that had been cultured in the presence of anti–TGF-β antibody (Figure 5B). Equal protein loading was measured by incubating the blot in amido black staining as a final step, to stain all proteins. We have shown that treatment with anti–TGF-β antibody led to an increased level of the cdk4/cyclin D complex (Figure 4). Neutralizing TGF-β with anti–TGF-β antibody increased the levels of association of this complex, which resulted in increased phosphorylation of pRb at Ser811 (Figure 5B). The phosphorylated form of pRb allows progression from the G0 to the G1 phase of the cell cycle.
Soluble TGF-β1 specifically decreased the level of pRb phosphorylation.
CD34+ progenitors were incubated in the presence or absence of soluble TGF-β1 or neutralizing antibody to TGF-β1 (a-TGFB) for 48 hours. Total lysates were run on 7.5% SDS-PAGE gel and transferred onto Immunobilon membrane. (A) Immunoblotting with an antibody that recognizes underphosphorylated, hypophosphorylated, and hyperphosphorylated forms of pRb. (B) Immunoblotting with an antibody specific to Ser811 pRb. Membranes were also stained in amido black staining to ensure equal protein loading in all lanes.
Soluble TGF-β1 specifically decreased the level of pRb phosphorylation.
CD34+ progenitors were incubated in the presence or absence of soluble TGF-β1 or neutralizing antibody to TGF-β1 (a-TGFB) for 48 hours. Total lysates were run on 7.5% SDS-PAGE gel and transferred onto Immunobilon membrane. (A) Immunoblotting with an antibody that recognizes underphosphorylated, hypophosphorylated, and hyperphosphorylated forms of pRb. (B) Immunoblotting with an antibody specific to Ser811 pRb. Membranes were also stained in amido black staining to ensure equal protein loading in all lanes.
Effects of TGF-β neutralization on human hematopoietic stem cell engraftment and differentiation in a long-term xenograft model
Recently, several reports have suggested that cycling human and murine hematopoietic stem cells display reduced levels of homing and engraftment, as compared to their quiescent counterparts.13-15 We sought to determine whether neutralization of TGF-β, which we have demonstrated in the current studies to enhance cell-cycle progression at the molecular level, had an effect on human hematopoietic stem/progenitor cell engraftment and subsequent lineage development in an immune-deficient mouse xenograft system. For the in vivo assays, we used bnx mice, which have a longer life span than the commonly used nonobese diabetic/severe combined immunodeficiency (NOD/SCID) strain, which can succumb to thymoma at 4 to 6 months of age.26 The bnx strain has a 2-year life span and thus allows relatively long-term analysis of engrafted human cells (6-12 months), as we have described.18,19,22,27 28
In the initial series of studies, primary CD34+ progenitor cells from human BM were cultured on the fibronectin fragment CH-296 (Retronectin) with cytokines for 24 to 48 hours, with and without neutralizing antibody to TGF-β. The Retronectin molecule was used because we have previously demonstrated that it maintains the regenerative capacity of long-term engrafting human hematopoietic cells through an in vitro culture period.29Following the culture period, a small portion of the cells from each sample was plated in a CFU assay and the remainder was transplanted into immunodeficient mice as described.18,19,22,27 28 CFU assays were enumerated on days 14 to 21 after plating. The average number of colonies obtained after 48 hours of culture in 5% serum-containing medium with cytokines was 526 ± 44 CFU/1 × 105 CD34+ cells plated versus 770 ± 54 after the same duration of culture in identical conditions, but with neutralizing antibody to TGF-β added to a final concentration of 5 μg/mL (7 separate experiments). Addition of neutralizing antibody to TGF-β significantly increased the levels of the colonies that developed (P = .004). However, because the colony-forming assay measures effects on relatively mature, committed progenitors, the effects of neutralization of TGF-β on the more primitive, engrafting cells in an in vivo transplantation system were also examined.
Both CD34+ cells and CD34+/CD38−cells, which are a rare and quiescent subset of the CD34+population,16,17 were used in the in vivo studies. Each cell sample, cultured with and without addition of neutralizing antibody to TGF-β, was transplanted into immune-deficient bnx mice with IL-3–secreting mesenchymal stem cells to provide species-specific cytokine support, as we have previously described in detail.19 28 Mice were harvested 6 to 12 months after transplantation and the human hematopoietic cell content in the murine BM was assessed by labeling with human-specific antibodies and flow cytometry. The total human leukocyte levels in the BM of bnx mice receiving transplants of human CD34+ and CD34+/CD38− cells cultured for 24 to 48 hours in medium containing cytokines alone versus the percentage of human leukocytes in the BM of mice receiving transplants with the same cell populations, cultured in identical conditions but with addition of neutralizing antibody to TGF-β, were not significantly different (Table 1). The total percentage of human CD45+ cells in the BM of each group of mice, as detected by FACS, was 15.8 ± 2.4 for the cytokines alone group (n = 11) versus 17.7 ± 2.1 for the cytokines plus anti–TGF-β group (n = 21, Table 1).
Human CFUs and hematopoietic lineages recovered from BM of bnx mice 6 to 12 months after transplantation
| Mouse no. . | Culture conditions . | No. human CFUs . | Percentage human cells of each lineage in bnx BM . | ||||
|---|---|---|---|---|---|---|---|
| CD45 . | CD4 . | CD8 . | CD33 . | CD19 . | |||
| 1 | Cytokines only | 18 | 14.3 | 2.6 | 3.4 | 6.5 | 1.2 |
| 2 | 30 | 12.0 | 1.8 | 4.4 | 2.8 | 2.5 | |
| 3 | 51 | 24.2 | 5.0 | 6.4 | 10.1 | 2.1 | |
| 4 | 22 | 6.2 | 1.2 | 2.0 | 3.2 | 0.9 | |
| 5 | 75 | 7.1 | 1.9 | 2.7 | 2.1 | 1.0 | |
| 6 | 63 | 30.0 | 3.4 | 5.7 | 17.9 | 2.9 | |
| 7 | 33 | 15.7 | 3.1 | 3.5 | 7.8 | 0.3 | |
| 8 | 55 | 22.2 | 4.4 | 5.1 | 8.6 | 0.4 | |
| 9 | 38 | 16.3 | 1.5 | 3.2 | 9.5 | 1.1 | |
| 10 | 40 | 5.5 | 0.9 | 1.4 | 2.4 | 0.6 | |
| 11 | 29 | 20.4 | 2.4 | 4.2 | 10.3 | 1.4 | |
| Avg ± SEM− | 41.3 ± 5.4 | 15.8 ± 2.4 | 2.6 ± 0.4 | 3.5 ± 0.5 | 7.4 ± 1.4 | 1.3 ± 0.3 | |
| 12 | Cytokines + Anti–TGF-β antibody | 46 | 21.6 | 3.6 | 5.1 | 10.4 | 2.9 |
| 13 | 25 | 7.7 | 1.5 | 2.4 | 3.0 | 0.4 | |
| 14 | 32 | 26.3 | 5.8 | 7.2 | 11.9 | 1.3 | |
| 15 | 21 | 11.5 | 2.9 | 3.6 | 3.1 | 1.9 | |
| 16 | 66 | 12.4 | 2.3 | 3.8 | 4.8 | 1.4 | |
| 17 | 39 | 12.9 | 3.4 | 4.4 | 3.2 | 0.9 | |
| 18 | 48 | 33.8 | 2.1 | 5.4 | 20.1 | 3.9 | |
| 19 | ND | 14.4 | 2.2 | 3.2 | 6.3 | 1.6 | |
| 20 | 44 | 18.3 | 4.6 | 4.5 | 6.7 | 2.3 | |
| 21 | 74 | 6.2 | 1.5 | 2.0 | 2.3 | 0.3 | |
| 22 | 26 | 27.9 | 4.1 | 8.5 | 11.6 | 2.0 | |
| 23 | 31 | 10.1 | 2.2 | 3.2 | 2.8 | 0.9 | |
| 24 | 28 | 31.5 | 9.7 | 6.6 | 13.0 | 2.2 | |
| 25 | ND | 17.5 | 3.1 | 4.3 | 8.9 | 1.1 | |
| 26 | 15 | 5.0 | 0.8 | 1.6 | 2.1 | 0.4 | |
| 27 | 40 | 21.8 | 3.6 | 4.7 | 9.4 | 1.4 | |
| 28 | 37 | 42.3 | 6.2 | 12.8 | 16.4 | 3.3 | |
| 29 | 93 | 14.3 | 1.9 | 3.5 | 6.1 | 1.5 | |
| 30 | 81 | 10.5 | 1.1 | 2.5 | 5.0 | 1.0 | |
| 31 | 46 | 14.3 | 2.9 | 3.7 | 5.4 | 0.8 | |
| 32 | 42 | 10.8 | 1.4 | 2.6 | 5.1 | 1.0 | |
| Avg ± SEM− | 43.9 ± 4.8 | 17.7 ± 2.1 | 3.7 ± 0.5 | 4.6 ± 0.6 | 7.5 ± 1.1 | 1.5 ± 0.2 | |
| Mouse no. . | Culture conditions . | No. human CFUs . | Percentage human cells of each lineage in bnx BM . | ||||
|---|---|---|---|---|---|---|---|
| CD45 . | CD4 . | CD8 . | CD33 . | CD19 . | |||
| 1 | Cytokines only | 18 | 14.3 | 2.6 | 3.4 | 6.5 | 1.2 |
| 2 | 30 | 12.0 | 1.8 | 4.4 | 2.8 | 2.5 | |
| 3 | 51 | 24.2 | 5.0 | 6.4 | 10.1 | 2.1 | |
| 4 | 22 | 6.2 | 1.2 | 2.0 | 3.2 | 0.9 | |
| 5 | 75 | 7.1 | 1.9 | 2.7 | 2.1 | 1.0 | |
| 6 | 63 | 30.0 | 3.4 | 5.7 | 17.9 | 2.9 | |
| 7 | 33 | 15.7 | 3.1 | 3.5 | 7.8 | 0.3 | |
| 8 | 55 | 22.2 | 4.4 | 5.1 | 8.6 | 0.4 | |
| 9 | 38 | 16.3 | 1.5 | 3.2 | 9.5 | 1.1 | |
| 10 | 40 | 5.5 | 0.9 | 1.4 | 2.4 | 0.6 | |
| 11 | 29 | 20.4 | 2.4 | 4.2 | 10.3 | 1.4 | |
| Avg ± SEM− | 41.3 ± 5.4 | 15.8 ± 2.4 | 2.6 ± 0.4 | 3.5 ± 0.5 | 7.4 ± 1.4 | 1.3 ± 0.3 | |
| 12 | Cytokines + Anti–TGF-β antibody | 46 | 21.6 | 3.6 | 5.1 | 10.4 | 2.9 |
| 13 | 25 | 7.7 | 1.5 | 2.4 | 3.0 | 0.4 | |
| 14 | 32 | 26.3 | 5.8 | 7.2 | 11.9 | 1.3 | |
| 15 | 21 | 11.5 | 2.9 | 3.6 | 3.1 | 1.9 | |
| 16 | 66 | 12.4 | 2.3 | 3.8 | 4.8 | 1.4 | |
| 17 | 39 | 12.9 | 3.4 | 4.4 | 3.2 | 0.9 | |
| 18 | 48 | 33.8 | 2.1 | 5.4 | 20.1 | 3.9 | |
| 19 | ND | 14.4 | 2.2 | 3.2 | 6.3 | 1.6 | |
| 20 | 44 | 18.3 | 4.6 | 4.5 | 6.7 | 2.3 | |
| 21 | 74 | 6.2 | 1.5 | 2.0 | 2.3 | 0.3 | |
| 22 | 26 | 27.9 | 4.1 | 8.5 | 11.6 | 2.0 | |
| 23 | 31 | 10.1 | 2.2 | 3.2 | 2.8 | 0.9 | |
| 24 | 28 | 31.5 | 9.7 | 6.6 | 13.0 | 2.2 | |
| 25 | ND | 17.5 | 3.1 | 4.3 | 8.9 | 1.1 | |
| 26 | 15 | 5.0 | 0.8 | 1.6 | 2.1 | 0.4 | |
| 27 | 40 | 21.8 | 3.6 | 4.7 | 9.4 | 1.4 | |
| 28 | 37 | 42.3 | 6.2 | 12.8 | 16.4 | 3.3 | |
| 29 | 93 | 14.3 | 1.9 | 3.5 | 6.1 | 1.5 | |
| 30 | 81 | 10.5 | 1.1 | 2.5 | 5.0 | 1.0 | |
| 31 | 46 | 14.3 | 2.9 | 3.7 | 5.4 | 0.8 | |
| 32 | 42 | 10.8 | 1.4 | 2.6 | 5.1 | 1.0 | |
| Avg ± SEM− | 43.9 ± 4.8 | 17.7 ± 2.1 | 3.7 ± 0.5 | 4.6 ± 0.6 | 7.5 ± 1.1 | 1.5 ± 0.2 | |
The BM cells were recovered from bnx mice 6 to 12 months after cotransplantation of human hematopoietic cells and IL-3–producing stromal cells. Human-specific colony-forming assays were plated from each bnx/human marrow sample. Colonies were enumerated on day 21. The CFU values shown were obtained from 3 × 105 bnx/human BM cells plated. FACS analyses were done to determine the percentage of human cells of each lineage in the bnx BM. The results shown were the percentages of human cells of each lineage within 10 000 total, ungated bnx/human BM samples analyzed by FACS.
In addition to the total human white blood cell numbers in the BM of the mice after long-term transplantation, as determined by FACS for human-specific CD45, we also determined levels of human myeloid (CD33+), T-lymphoid (CD3+/CD4+ and CD3+/CD8+), and B-lymphoid (CD19+) lineages. The levels of human myeloid cells, as determined by labeling with an anti-CD33 antibody, were 2.6 ± 0.4 for mice given transplants with cells cultured in cytokines alone versus 3.2 ± 0.5 for mice receiving transplants with cells cultured in the same medium but with addition of neutralizing antibody to TGF-β (Table 1). Again, culture of the stem/progenitor cells with anti–TGF-β antibody in vitro, prior to transplantation, did not significantly affect human myeloid cell development. Similar results were obtained for T- and B- lymphocyte development, as is shown in Table 1. No significant differences in development of any human hematopoietic lineage by culture of the human stem/progenitor cells with neutralization of TGF-β prior to transplantation were detected.
As a final measure to ensure that there was no significant effect on long-term human hematopoiesis from culturing stem and progenitor cells for 24 to 48 hours with neutralization of TGF-β prior to transplantation, in comparison to culture with cytokines alone, we measured the human-specific colony-forming progenitors that could be recovered from both groups of long-term engrafted mice. In addition to the lack of effect on lineage development by neutralization of TGF-β during the in vitro culture period prior to transplantation, there were also no significant differences in the numbers of total (erythroid, myeloid, or mixed lineage) CFUs recovered from the BM of mice given transplants with cells cultured in the absence or presence of anti–TGF-β antibody, as shown in Table 1. An average of 41.3 ± 5.4 versus 43.9 ± 4.8 total colonies were grown from 3 × 105 bnx BM cells, plated from each mouse in the respective groups (Table 1). Again, there was no significant difference in these values. Together, the lineage development and colony replating data demonstrate that there was no differentiation effect imposed on the reconstituting hematopoietic cells by 24 to 48 hours in culture with neutralization of TGF-β prior to transplantation, in comparison to cells cultured for the same period with cytokines alone. In summary, we have determined that neutralization of TGF-β during 1 to 2 days of culture induced cell-cycle progression from quiescence into the G1 phase of the cycle, but did not have an impact on the long-term hematopoietic capacity of primitive hematopoietic progenitors.
Discussion
Multipotential hematopoietic progenitors are highly responsive to TGF-β1, and less responsive to TGF-βΙΙ.30Secretion of TGF-β has been shown to occur via autocrine as well as paracrine pathways. Treatment with soluble TGF-β1 has been shown to reduce the proliferation of CD34+ cells as measured by CFU assay, whereas addition of anti–TGF-β antibody increased in vitro CFU counts.9 10 We also saw this effect in the current studies; however, addition of anti–TGF-β antibody did not alter the number of human CFUs that could later be grown from the marrow of long-term engrafted immunodeficient mice. These data suggest that neutralization of TGF-β alone may not significantly alter the most primitive human hematopoietic stem cell compartment.
Cashman et al8 made the seminal observation that the levels of TGF-β accumulate with time in the medium of long-term cultures, inhibiting cell cycle entry of progenitors within the stromal monolayer. When the conditioned medium was exchanged for fresh medium, the levels of TGF-β dropped, and a portion of the progenitors was released from inhibition and entered the cell cycle.8 Hatzfeld and colleagues have done further elegant studies to examine the negative regulatory effects of TGF-β on cultured cells.9 10 These studies show the importance of TGF-β as an inhibitor of cell-cycle entry, in primary human hematopoietic progenitors.
In the current studies we determined the molecularmechanisms by which TGF-β inhibits proliferation of primary human CD34+ progenitors. Using Ki67/7-AAD staining to delineate between G0, G1, and S/G2M populations, we initially showed that TGF-β1–treated human CD34+ progenitors accumulate in the G0 phase of the cell cycle, whereas cells treated with an anti–TGF-β neutralizing antibody progressed from the G0 into the G1 phase of the cell cycle. Interestingly, we showed that the anti–TGF-β antibody-treated cells accumulate in G1and did not progress further into S phase. A possible explanation might be that the anti–TGF-β antibody treatment recruits quiescent progenitors out of G0 into G1 due to availability of cyclin D2/ckd4 kinase activity for initial phosphorylation of pRb. However, the subsequent phosphorylation of pRb to recruit cells from G1 into S/G2M depends on another complex, cyclin E/cdk2. In our experiments, TGF-β did not affect the levels of cdk2 protein or the kinase activity of cyclin E/cdk2 (data not shown). We had previously reported that an additional CDKI, p27Kip1, which functions primarily by blocking cyclin E/CDK2 activity, impedes the cycling of deeply quiescent progenitors.22,23 Thus, the CDKI p15, which is regulated by TGF-β levels, and p27 work together to maintain quiescence in primitive human hematopoietic cells.22 23
Our current data thus further confirm that addition of anti–TGF-β antibody alone is not sufficient for recruitment of the population of quiescent human hematopoietic stem cells into the S phase of the cell cycle, but rather recruits them out of G0 and into G1. These data suggest that anti–TGF-β antibody treatment of quiescent human stem/progenitor cells might be useful to augment transduction by lentiviral vectors, which require the cells to be in the G1, rather than G0 phase, for successful transduction. Sutton et al31 have hypothesized that this requirement is due to the lack of sufficient dNTP pools for efficient completion of viral reverse transcription when the cells are in the G0 phase.31
When the cells block in G1 phase after TGF-β treatment, the CKDI p27Kip1 might then act as the primary cell-cycle “gatekeeper,” exerting its effect on cyclin E/cdk2. It is also particularly interesting that neutralization of TGF-β with cytokines resulted in an equivalent percentage of cells in S/G2M when compared with cells cultured in cytokines alone. Lardon et al32 had reported that within the CD34+population, there is a subset of cells that is responsive to IL-3 but insensitive to TGF-β. The report also stated that in the presence of IL-3, the inhibitory effect of TGF-β on cell cycle occurs at the second round of the cell cycle. Because our current studies focused on time points between 18 and 48 hours, the first cell cycle, it is possible that during that time, there was an expansion of an IL-3–responsive TGF-β–insensitive cell population, which masked the effect of TGF-β neutralization on the TGF-β–sensitive population. Future studies will address the effect of TGF-β neutralization on the second round of the cell cycle in the presence of IL-3, SCF, and IL-6.
The molecular mechanism by which TGF-β alters cell-cycle progression in primary CD34+ hematopoietic progenitors had not been elucidated prior to the current study, although some reports had been published using hematopoietic cell lines such as 32D3.33In our studies using primary human CD34+ progenitors, there was a significant increase in p15Ink4b with a decrease in cyclin D2 after addition of TGF-β to cells in culture. The level of cdk4 remained unchanged. These findings are contrary to results observed in 32D3 cells whereby TGF-β induced a decrease in cdk4 and no changes in cyclin D2 and D3 levels.33 The inconsistencies between our current results and the previous reported findings may be partially due to differences in hematopoietic cell lines versus primary hematopoietic progenitors. The murine IL-3–dependent cell line, 32D, proliferates in response to the IL-3 signaling pathway alone, and cell-cycle inhibition by factors other than IL-3 withdrawal is dysregulated. As reported,33 TGF-β interferes with the IL-3–mediated induction of cdk4 in 32D cells, resulting in reduced cdk4 kinase activity. Overexpression of cdk4 renders these cells resistant to TGF-β, restoring the kinase activity of both cdk4 and cdk2. In our studies, CD34+ progenitors were cultured in the presence of a 3-cytokine combination (IL-3, IL-6, and SCF), which has been shown to enhance retroviral transduction of human hematopoietic progenitors34,35 and is often used in clinical gene therapy protocols.36-39 Because all 3 cytokines have been shown to have independent as well as synergistic mitogenic effects on CD34+ cells, it is plausible that the presence of IL-6 or SCF during culture may counteract the effect of TGF-β on cdk4 expression in these cells. For instance, CD34+ progenitors transduced with a retrovirus encoding the complementary DNA for c-kit, the receptor for the Steel factor, showed reduced sensitivity to TGF-β, indicating the ability of SCF to at least partially decrease its effects.40 Alternately, our observation that CD34+ cells have decreased cyclin D2 protein levels and decreased association of cyclin D2 with cdk4 following TGF-β treatment suggests the possibility that TGF-β–mediated regulation of cell-cycle proteins in primary cells operates through a different route than in immortalized cell lines. Whether the decrease is in cdk4 as seen in 32D cells or in cyclin D2 as shown in CD34+progenitors in the current studies, the end result of both studies was a decrease in phosphorylation of the downstream target of the active cyclin D/cdk4 kinase complex, the retinoblastoma gene protein product (pRb).
pRb plays a major role in maintenance of cells in G0 and its initial phosphorylation depends on cyclin D/cdk4. Formation of the cyclin D/cdk4 complex is inhibited by the CDKI, p15, which is up-regulated by TGF-β signaling. pRb is a tumor suppressor protein that binds and inactivates the DNA-binding protein, E2F, which is required for activating the transcription of S phase–related genes such as thymidine kinase and DNA polymerase-δ. Phosphorylation of pRb in mid G1 is a prerequisite for the release of free E2F for DNA binding. Initial hypophosphorylation of pRb is performed by the cdk4/cyclin D complex, allowing cell-cycle progression. Hypophosphorylation is then followed by pRb hyperphosphorylation and inactivation by cdk2/cyclin E at the G1/S transition phase. In our current studies, we observed an increase in the degree of phosphorylation of pRb in CD34+ progenitors cultured in the presence of anti–TGF-β antibody. Because this would cause enhanced cell-cycle entry, the data are consistent with the increased levels of cell-cycle progression in murine and human hematopoietic progenitors treated with anti-TGFβ antibody that has been observed by our group and others.41-44
In vitro neutralization of TGF-β using a panspecific anti–TGF-β antibody enhanced colony formation in cells plated immediately after the culture period. However, there was no significant increase in the number of “secondary human colonies” that could be recovered from the BM of immunodeficient bnx mice receiving transplants of human cells treated with anti–TGF-β antibodies. In accordance with these data, there were no significant differences in the total human hematopoietic cell engraftment or in the levels of any of the human blood cell lineages, T, B, or myeloid, that developed in the bnx mice over a period of 6 to 12 months after transplantation of stem cell populations treated with or without neutralization of TGF-β.
Glimm et al45 have reported that after a 5-day in vitro culture with cytokines, cells from the G1 fraction engrafted NOD/SCID mice better than the cells in the G0phase. In contrast, Gothot's group reported that only cells that remained in the G0 phase of the cell cycle after cytokine stimulation would engraft NOD/SCID mice.46 These disparities may be explained by the fact that different cytokines and culture conditions were used by the 2 groups, as was nicely discussed in the manuscript from Dr Eaves' group.45 We used bnx mice as the recipients of the cultured cells, in the current studies, and they may engraft with different kinetics than the NOD/SCID strain. Dr Eaves' group has recently reported some very interesting disparities in the engraftment of different human hematopoietic progenitor populations in NOD/SCID/B2M knockout mice, in comparison to NOD/SCID mice.47 Therefore, it is quite feasible that the different strains of xenograft recipients may allow human cells with different properties, such as phenotype, adhesion molecule density, or cell-cycle status, to engraft with different efficiencies.
Recently, TGF-β has been described as a factor that maintains the “stem cell state” of CD34+ cells.9 In our current study, we measured stem cell state based on the pluripotentiality of the cell population tested, using multilineage development of human blood cell lineages and retention of secondary colony-forming capacity following transplantation in a relatively long-term xenograft system. In this xenograft system, the primitive hematopoietic stem cells reconstituted the sublethally irradiated recipients, giving rise to all human blood lineages. After treatment with or without anti–TGF-β antibody, cultures of human CD34+ and CD34+CD38− cells successfully engrafted immunodeficient bnx mice for 6 to 12 months. BM from the chimeric mice contained human myeloid, T-, and B-lymphoid cells and retained secondary human-specific colony-forming capacity, confirming that after culture the human stem cells had not been induced to differentiate by neutralization of TGF-β. Therefore, addition of anti–TGF-β neutralizing antibody to 24- to 48-hour in vitro cultures did not result in loss of the engrafting human stem cell pool, in our system.
In summary, our data show that neutralization of TGF-β during 1 to 2 days of culture induced a portion of the primary human CD34+ progenitors to progress from quiescence (G0) into the G1 phase of the cell cycle, but not further into S/G2/M phase. The transition from G0 into the G1 phase did not have an impact on the long-term hematopoietic capacity of the primitive hematopoietic progenitors, in the long-term bnx/human xenograft system.
Thank you to Naomi Taylor for useful discussion and insight. We thank Craig Jordan for advice and help with the cell-cycle analyses. We very much appreciate Kaiser Permanente, Sunset Boulevard, Los Angeles, for the donation of umbilical cord blood samples. Thank you to Sally Worttman, who heads our animal facility, and to Renee Traub-Workman and Miriam Figueroa, who maintain the bnx mouse colony.
Supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (RO1 DK53041) and the National Heart, Lung and Blood Institute (SCOR no. 1-P50-HL54850). J.H. is a summer intern from Troy High School, Fullerton, CA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jan A. Nolta, University of Southern California School of Medicine, 4650 Sunset Blvd, Mailstop #62, Los Angeles, CA 90027; e-mail: jnolta@chla.usc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal