Abstract
Thrombotic microangiopathy (TM) is associated with abnormalities of von Willebrand factor–cleaving protease (VWCP) and other hemostatic factors. This study hypothesized that TM patients might have genetically determined thrombotic risk factors that predispose them to aberrant microvascular thrombosis. DNA samples from 30 white and 12 African American adult TM patients were analyzed for genetic alleles associated with vascular thrombosis, and plasma samples were analyzed for levels of VWCP activity. DNA was analyzed by using allele-specific polymerase chain reaction for factor V 1691A (Leiden), factor II 20 210A, methylenetetrahydrofolate reductase 667T, type 1 plasminogen activator inhibitor 4G/5G, and platelet GPIa 807T. Patients were segregated by race (white or African American) and plasma level of VWCP activity (normal or deficient). The prevalence of factor V Leiden was significantly increased among the white TM patients that had normal VWCP activity: 4 (36%) of 11 patients compared with 6 (3%) of 186 white control subjects possessed the factor V Leiden allele (P < .001; odds ratio, 17.1; 95% confidence interval, 5.4-54.0). No factor V Leiden alleles were detected in 19 white TM patients with intermediate or deficient levels of VWCP activity or in any of 12 African American patients. The prevalence of other thrombosis-associated alleles did not differ between TM patients and control subjects. These findings suggest that factor V Leiden may be a pathogenic risk factor in TM patients that have normal VWCP activity.
Introduction
Thrombotic microangiopathy (TM) disorders, including thrombotic thrombocytopenic purpura (TTP) and the hemolytic uremic syndrome (HUS), are characterized by widespread microvascular thrombosis leading to end-organ injury. The etiologies and pathogenic mechanisms of TM syndromes remain poorly understood. However, evidence implicates a deficiency of von Willebrand factor (VWF)–cleaving protease (VWCP) activity in the pathogenesis of TM in patients with the clinical diagnosis of TTP.1,2 Deficiency of VWCP activity has been proposed to impair proteolytic processing of ultralarge endothelial cell–derived VWF multimers, resulting in a predisposition to platelet thrombosis.2 3
Deficient VWCP activity provides support for a platelet-VWF–dependent mechanism of microvascular thrombosis in a subset of TM patients. However, the mechanism of microvascular thrombosis in TM patients with normal levels of VWCP activity remains obscure. Patients who have TM associated with bone marrow transplantation4 and enteropathic Escherichia coli infection,5 as well as some patients with idiopathic TM,6 have been reported to have normal VWCP activity. Microvascular thrombosis in these TM patients might involve a wide range of hemostatic abnormalities, and the syndromic nature of TM suggests the possibility of multiple pathogenic factors.
An increasing number of genetic mutations and polymorphisms have been found to be associated with various manifestations of vascular thrombosis. The implicated mutant or polymorphic alleles affect hemostatic mechanisms by encoding variant proteins or altered regulatory sequences that have the potential to enhance thrombus formation or decrease fibrinolysis. We hypothesized that thrombosis-associated mutations or polymorphisms may contribute to the pathogenic mechanisms of TM disorders. Moreover, because certain hemostatic abnormalities, such as deficient VWCP activity, are found in some TM patients but not in others, we hypothesized that the prevalence of genetic thrombotic risk factors may differ among subsets of TM patients.
We studied 42 patients treated with plasma exchange for TM syndromes. Patients were segregated by plasma levels of VWCP activity and, because of significant differences in the prevalence of thrombosis-associated polymorphisms between races, by racial background. Each subject was analyzed for 5 alleles associated with pathologic thrombosis: (1) factor V 1691A (Leiden), associated with resistance to activated protein C7-9; (2) factor II 20 210A, associated with elevated plasma prothrombin levels10-12; (3) methylenetetrahydrofolate reductase (MTHFR) 667T, associated in the homozygous state with elevated plasma homocysteine levels and premature vascular disease13,14; (4) type 1 plasminogen activator inhibitor (PAI-1) 4G/5, in which the homozygous 4G allele is associated with increased plasma PAI-1 concentration15-17; and (5) platelet GPIa 807T, associated with increased concentration of the collagen receptor α2 β1 and increased platelet adhesion to collagen.18 19
Patients, materials, and methods
Patients and control subjects
Blood samples were obtained from 42 patients referred to The Blood Center of Southeastern Wisconsin for plasma exchange treatment for TM. Of the 42 patients, 30 were white and 12 were African American. Diagnostic criteria for TM included thrombocytopenia, microangiopathic hemolytic anemia, elevated lactate dehydrogenase, minimal or no abnormalities of prothrombin time or partial thromboplastin time, and a negative direct antiglobulin test for immune hemolysis. Clinical data were obtained from medical records. In cases with recurrent TM episodes, defined as occurrence of diagnostic criteria after 2 weeks or more of sustained remission, data reported are from the earliest TM episode from which data were available. Follow-up for recurrence is passive for all patients. Informed consent was obtained in all cases in accordance with a protocol approved by the Human Research Review Committee of The Blood Center of Southeastern Wisconsin. We found no evidence of kinship between any TM patients in this study.
Peripheral blood collected from a total of 232 African American and 281 white control subjects was used for genetic studies. The number of control samples reported for each marker reflects the number of samples available at the time assays were performed. The ethnic origin of subjects was determined by self-identification. African American control subjects resided in the Midwest, Southwest, and Pacific Northwest regions of the United States. White control subjects resided in the metropolitan Milwaukee area.
VWCP activity assay
VWCP activity was measured in patient plasma samples obtained from sodium citrate–anticoagulated whole blood collected before initial plasmapheresis or from the first 300 mL waste plasma bag (anticoagulated with acid citrate dextrose) obtained during the initial plasmapheresis procedure. Acid citrate dextrose and sodium citrate–anticoagulated plasma demonstrated comparable VWCP activity (data not shown). Samples were stored at −80°C until use.
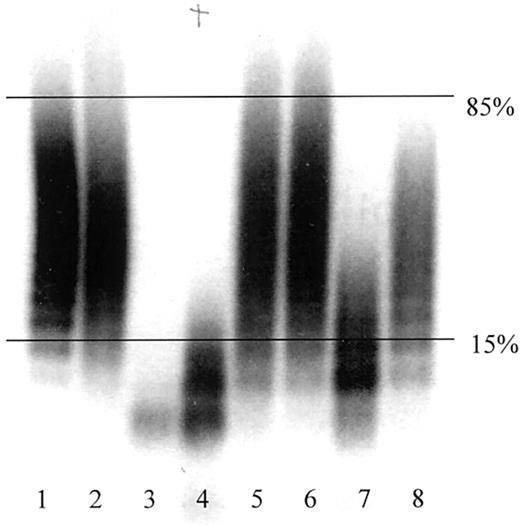
VWCP activity was determined in a modified version of the assay described by Furlan et al.20 To activate the protease, 98 μL undiluted plasma was incubated in 3 μL 10 mM Pefabloc SC (Boehringer, Mannheim, Germany) and 8 μL 10 mM BaCl2 for 5 minutes at 37°C. Purified VWF (50 μL; gift of Dr Robert Montgomery) was added to a final concentration of 3 U/mL. The incubation mixture was dialyzed (1.5 M urea, 0.005 M Tris, pH 8.0) on a hydrophilic filter membrane (VSWP, 47-mm diameter; Millipore, Bedford, MA) for 15 hours at 37°C. The reaction was quenched with 10 μL 0.2 M EDTA, pH 7.4. Undigested VWF or VWCP-proteolyzed VWF was electrophoresed into 0.65% agarose in the presence of sodium dodecyl sulfate. The gel was fixed, washed, blocked, and incubated overnight with a mixture of 125I-labeled monoclonal anti-VWF antibodies as previously described.21 The gel was then washed, dried, and exposed to x-ray film. VWCP activity was determined by analysis of electrophoretic pattern and categorized as follows: (1) normal activity, the largest remaining VWF multimer band was smaller than the 15th percentile of the undigested VWF multimer range; (2) intermediate activity, the largest remaining VWF multimer band was between the 15th and 85th percentiles of the undigested VWF multimer range; and (3) deficient activity, the largest remaining VWF multimer band was larger than the 85th percentile of the undigested VWF multimer range (Figure 1). Undigested VWF multimer ranges were defined on each gel by measurement of the difference in electrophoretic mobility between the highest and lowest molecular weight bands.
Representative autoradiograph of VWCP activity assay.
Undigested VWF and VWCP-proteolyzed VWF were detected by antibody labeling and radiography as described in “Patients, materials, and methods.” Horizontal lines indicate the 15th and 85th percentiles of the undigested VWF multimer range. Lanes 1 and 2, undigested VWF; lane 3, normal VWCP activity; lanes 4, 7, and 8, intermediate VWCP activity; lanes 5 and 6, deficient VWCP activity.
Representative autoradiograph of VWCP activity assay.
Undigested VWF and VWCP-proteolyzed VWF were detected by antibody labeling and radiography as described in “Patients, materials, and methods.” Horizontal lines indicate the 15th and 85th percentiles of the undigested VWF multimer range. Lanes 1 and 2, undigested VWF; lane 3, normal VWCP activity; lanes 4, 7, and 8, intermediate VWCP activity; lanes 5 and 6, deficient VWCP activity.
Isolation of DNA
Genomic DNA was isolated from 200 to 250 μL buffy coat by using the QIAmp Blood Kit (QIAGEN, Valencia, CA), and plasmid DNA was isolated by using the QIAprep (QIAGEN) Miniprep kit according to the manufacturer's instructions. DNA was quantified spectrophotometrically; the A260/A280 ratios of template DNA samples ranged from 1.6 to 1.8.
Genotyping by allele-specific polymerase chain reaction
Genotyping by allele-specific polymerase chain reaction (PCR) was performed for prothrombin G20 210A, factor V Leiden, MTHFR C677T, and Gp1a C807T as previously described.22,23 The primers reported by Catto et al24 were used for PAI-1 4G/5G genotyping. Briefly, analysis was accomplished by subjecting samples to 2 separate PCR amplifications. One amplification detected normal alleles; the second amplification detected abnormal alleles. Each reaction was performed with 125 ng genomic DNA, 10 mM Tris · HCl, pH 8.3, 50 mM KCl, 1.5 to 2.0 mM MgCl2, 0.2 μM each dNTP (Pharmacia, Pitscataway, NJ), 80 to 250 ng appropriate forward and reverse primer, and 1 U AmpliTaq DNA Polymerase (Perkin-Elmer, Boston, MA). Reactions were conducted in a total volume of 25 to 50 μL. PAI-1 4G/5G reactions were amplified with the following thermal profile: 35 cycles consisting of 94°C for 30 seconds, 71°C for 30 seconds, and 72°C for 30 seconds in a Perkin Elmer 9600 thermal cycler. Each amplified PCR product (15-20 μL) was analyzed by electrophoresis through 2% agarose gels stained with ethidium bromide.
Control templates and primer specificity
A control template for the prothrombin mutant allele was produced by PCR-mediated mutagenesis, using a primer having the sequence of the mutant allele. This product was then ligated into the pCR 1.1 vector (Invitrogen, Carlsbad, CA), transformed into TOP10F′ competent E coli cells (Invitrogen), and screened according to the manufacturer's instructions. Clones possessing the prothrombin 20 210A insert were confirmed by direct sequence analysis. The factor V 1691 G/A primer specificity was confirmed by testing selected primers and reaction conditions against known homozygotes; such samples were identified as previously described.25 The MTHFR 677 C/T, GP1a 807 C/T, and PAI-1 4G/5G primer specificity was confirmed by using DNA isolated from known homozygotes, which were identified through nucleotide sequencing. Nucleotide sequencing was conducted with either an ABI model 373A Sequencing System or a Micro Gene Blaster (Visible Genetics, Toronto, ON, Canada) using dye-primer chemistry.
Statistical analysis
Patients with TM were stratified into groups based on race (white or African American) and VWCP activity (normal or deficient). This grouping resulted in 3 analyzable strata: white with normal VWCP activity, white with deficient VWCP activity, and African American with deficient VWCP activity. Descriptive statistics were calculated to summarize the clinical features of the TM patients. TM patients were compared with control subjects of the same racial group. The analysis was stratified by racial background because previous studies have demonstrated significant differences between racial groups in the prevalence of thrombosis-associated mutations or polymorphisms.26 A subject had an allele abnormality if one or both alleles were abnormal in Factor V G1691A, Factor II G20 210A, or GP1a C807T, or if both alleles were abnormal in MTHFR C677T or PAI-1 4G/5G. The prevalence of allele abnormalities was compared for each of the 5 alleles between control subjects and TM patients for each stratum by using the chi-square test or Fisher exact test (if sample size assumptions were not met). SAS software, version 6.12 (SAS Institute, Cary, NC), was used to perform all analyses. AP value of < .05 was considered statistically significant.
Results
VWCP activity in TM patients
VWCP activity was measured in all 42 TM patients. VWCP activity was classified as normal, intermediate, or deficient as shown in Figure1. Among 12 African American TM patients, VWCP activity was deficient in 10, intermediate in 1, and normal in 1. Among 30 white TM patients, VWCP activity was deficient in 16, intermediate in 3, and normal in 11. Because of population size limitations the patient groups with 3 or fewer subjects were not analyzed for prevalence of allele abnormalities. Control subjects were not evaluated for VWCP activity.
Clinical data in TM patients
Selected clinical data from the 3 analyzed patient groups are shown in Table 1. The 11 white patients with normal VWCP activity have had no known recurrences of TM. In contrast, whites and African Americans with deficient VWCP activity have had 19% and 60% recurrence rates, respectively. There were trends toward higher platelet counts and creatinine values in the white patients with normal VWCP activity compared with the other 2 groups.
Clinical data in patients with thrombotic microangiopathy
| . | White with normal VWCP . | White with deficient VWCP . | African American with deficient VWCP . |
|---|---|---|---|
| n | 11 | 16 | 10 |
| No. of episodes | |||
| One | 11 | 13 | 4 |
| Two or more | 0 | 3 | 6 |
| Platelet count (μL−1)* | 60 000 ± 32 000 | 22 000 ± 19 000 | 15 000 ± 13 000 |
| Creatinine (mg/dL)* | 4.6 ± 2.7 (n = 10) | 1.4 ± 0.6 (n = 8) | 0.9 ± 0.2 (n = 3) |
| LDH (mg/dL)* | 3 808 ± 2 661 (n = 9) | 2 356 ± 1 956 (n = 14) | 1 972 ± 1 762 (n = 10) |
| . | White with normal VWCP . | White with deficient VWCP . | African American with deficient VWCP . |
|---|---|---|---|
| n | 11 | 16 | 10 |
| No. of episodes | |||
| One | 11 | 13 | 4 |
| Two or more | 0 | 3 | 6 |
| Platelet count (μL−1)* | 60 000 ± 32 000 | 22 000 ± 19 000 | 15 000 ± 13 000 |
| Creatinine (mg/dL)* | 4.6 ± 2.7 (n = 10) | 1.4 ± 0.6 (n = 8) | 0.9 ± 0.2 (n = 3) |
| LDH (mg/dL)* | 3 808 ± 2 661 (n = 9) | 2 356 ± 1 956 (n = 14) | 1 972 ± 1 762 (n = 10) |
LDH indicates low-density lipoprotein.
Numbers are mean ± SD.
Prevalence of factor V Leiden allele abnormality in TM patients and control subjects
Among all white TM patients, 4 (13%) of 30 possessed the factor V Leiden allele abnormality. All 4 white patients who possessed the factor V Leiden allele abnormality were among the 11 that demonstrated normal VWCP activity (Table 2). Three of these patients were heterozygous, and one was homozygous for the factor V Leiden allele. Six (3.2%) of 186 white control subjects possessed the factor V Leiden allele abnormality, and all were heterozygous (P < .001; odds ratio, 17.1; 95% confidence interval, 5.4-54.0 for white TM patients with normal VWCP versus white control subjects) (Table 2). The factor V Leiden allele abnormality was not detected in any of the 16 white TM patients with deficient VWCP activity or in any of the 10 African American patients with deficient protease activity (Table 3).
Genetic analysis of white patients with thrombotic microangiopathy and white control subjects
| . | Control subjects . | TM patients with normal VWCP . | TM patients with deficient VWCP . | |||||
|---|---|---|---|---|---|---|---|---|
| n . | % abnormal (heterozygous/ homozygous) . | n . | % abnormal (heterozygous/ homozygous) . | P* . | n . | % abnormal (heterozygous/ homozygous) . | P† . | |
| Factor V G1691A | 186 | 3 (6/0) | 11 | 36 (3/1) | < .001 | 16 | 0 (0/0) | > .99 |
| Factor II G20210A | 186 | 3 (5/0) | 11 | 0 (0/0) | > .99 | 16 | 6 (1/0) | .394 |
| MTHFR C677T | 186 | 11‡ (20) | 11 | 0‡ (0) | .607 | 16 | 0‡ (0) | .377 |
| GP1a C807T | 280 | 60 (133/34) | 11 | 73 (7/1) | .535 | 16 | 50 (7/1) | .4452-153 |
| PAI-1 4G/5G | 173 | 29‡ (51) | 11 | 27‡ (3) | > .99 | 15 | 27‡ (4) | > .99 |
| . | Control subjects . | TM patients with normal VWCP . | TM patients with deficient VWCP . | |||||
|---|---|---|---|---|---|---|---|---|
| n . | % abnormal (heterozygous/ homozygous) . | n . | % abnormal (heterozygous/ homozygous) . | P* . | n . | % abnormal (heterozygous/ homozygous) . | P† . | |
| Factor V G1691A | 186 | 3 (6/0) | 11 | 36 (3/1) | < .001 | 16 | 0 (0/0) | > .99 |
| Factor II G20210A | 186 | 3 (5/0) | 11 | 0 (0/0) | > .99 | 16 | 6 (1/0) | .394 |
| MTHFR C677T | 186 | 11‡ (20) | 11 | 0‡ (0) | .607 | 16 | 0‡ (0) | .377 |
| GP1a C807T | 280 | 60 (133/34) | 11 | 73 (7/1) | .535 | 16 | 50 (7/1) | .4452-153 |
| PAI-1 4G/5G | 173 | 29‡ (51) | 11 | 27‡ (3) | > .99 | 15 | 27‡ (4) | > .99 |
TM indicates thrombotic microangiopathy; VWCP, von Willebrand factor–cleaving protease; MTHFR, methylenetetrahydrofolate reductase; PAI-1, type 1 plasminogen activator inhibitor.
Comparison between control subjects and TM patients with normal VWCP.
Comparison between control subjects and TM patients with deficient VWCP.
For MTHFR C677T and PAI-1 4G/5G, the abnormal is homozygous only, heterozygous individuals are classified as normal.
P value is from χ2; all otherP values are from Fisher exact test.
Genetic analysis of African American patients with thrombotic microangiopathy and African American control subjects
| . | Control subjects . | TM patients with deficient VWCP . | P . | ||
|---|---|---|---|---|---|
| n . | % abnormal (heterozygous/ homozygous) . | n . | % abnormal (heterozygous/ homozygous) . | ||
| Factor V G1691A | 190 | 2 (3/0) | 10 | 0 (0/0) | > .99 |
| Factor II G20210A | 191 | 1 (1/0) | 10 | 0 (0/0) | > .99 |
| MTHFR C677T | 192 | 23-150 (3) | 10 | 03-150 (0) | > .99 |
| GP1a C807T | 231 | 56 (102/28) | 10 | 30 (3/0) | .117 |
| PAI-1 4G/5G | 202 | 83-150 (17) | 10 | 203-150 (2) | .222 |
| . | Control subjects . | TM patients with deficient VWCP . | P . | ||
|---|---|---|---|---|---|
| n . | % abnormal (heterozygous/ homozygous) . | n . | % abnormal (heterozygous/ homozygous) . | ||
| Factor V G1691A | 190 | 2 (3/0) | 10 | 0 (0/0) | > .99 |
| Factor II G20210A | 191 | 1 (1/0) | 10 | 0 (0/0) | > .99 |
| MTHFR C677T | 192 | 23-150 (3) | 10 | 03-150 (0) | > .99 |
| GP1a C807T | 231 | 56 (102/28) | 10 | 30 (3/0) | .117 |
| PAI-1 4G/5G | 202 | 83-150 (17) | 10 | 203-150 (2) | .222 |
All P values are from Fisher exact test.
For MTHFR C677T and PAI-1 4G/5G, the abnormal is homozygous only; heterozygous individuals are classified as normal.
Prevalence of the factor II 20 210A allele abnormality in TM patients and control subjects
Prevalence of the factor II 20 210A allele abnormality did not differ between white TM patients with normal VWCP activity (0%) and control subjects (3%) or between white patients with deficient VWCP activity (6%) and control subjects (Table 2). The factor II 20 210A allele abnormality was not detected in any African American TM patients or African American control subjects (Table 3). No individuals homozygous for the factor II 20 210A allele were detected in any group.
Prevalence of a MTHFR 667T allele abnormality in TM patients and control subjects
Prevalence of a GP1a 807T allele abnormality in TM patients and control subjects
Prevalence of PAI-1 4G/5G allele abnormality in TM patients and control subjects
Discussion
In this study we found a significantly increased prevalence of the factor V Leiden allele abnormality in a subset of white TM patients who had normal VWCP activity. The 12-fold increase in factor V Leiden allele abnormality compared with race-matched control subjects implicates this abnormality of the thrombomodulin/protein C anticoagulant pathway as a genetic risk factor for TM in a subset of patients. A major advantage of genetic analyses of potential pathogenic factors, in contrast to analyses of protein antigen or activity levels, is that statistical differences in genetic factors suggests a causative pathogenic role, whereas differences in protein levels may reflect a causative pathogenic role or a consequence of a disease process. Statistical differences in a particular genetic factor can, however, be interpreted erroneously as causative if the population possessing the trait is a kindred that could share other potentially pathogenic genetic or acquired disease risk factors. The family medical histories of our population of white patients with normal VWCP did not reveal genetic relatedness or exposure to common risk factors. We therefore conclude that the highly statistically significant increased prevalence of factor V Leiden in the white patient group with normal VWCP levels represents a genetic risk factor for this subtype of TM syndrome.
Pathogenic mechanisms of TM syndromes have been proposed to involve a wide range of quantitative and qualitative abnormalities of hemostatic factors. Among the abnormalities of platelet function reported in TM are abnormal in vitro aggregation attributed to platelet aggregating factors,27-29 abnormal VWF multimers,30 and platelet autoantibodies.31Studies have shown that a very high proportion of TM patients with the clinical diagnosis of TTP have a deficiency of VWCP.1,2Reported abnormalities of coagulation proteins in TM include increased PAI-1 activity and deficiencies of protein C, protein S, tissue plasminogen activator, and urokinase-type plasminogen activator.32-35 Studies in kindreds with familial HUS indicate that genetic alterations of complement factor H, a plasma protein that down-regulates complement activity, are prevalent in this subset of TM patients.36-39 Complement activation is known to induce expression of tissue factor and adhesion molecules on endothelial cells and procoagulant alterations on platelet surfaces.40-42
Inactivation of factor Va by activated protein C is impaired in persons with the factor V Leiden mutation.9 This alteration confers increased risk of venous thrombosis.9,43,44 Our observation that the prevalence of the factor V Leiden allele in TM patients is increased in a subset of TM patients is consistent with previous studies that have implicated the thrombomodulin/protein C anticoagulant pathway in TM. Takahashi et al34 reported low functional levels of protein S, and Glas-Greenwalt et al35observed low levels of protein C in some TM patients. Decreased protein S function has been reported to sensitize animals to the development of microvascular thrombosis in a model of E coliendotoxemia.45
TM syndromes are associated with systemic lupus erythematosus and antiphospholipid antibodies,46 and the inactivation of factor Va by activated protein C is inhibited by antiphospholipid antibodies, suggesting a pathogenic defect similar to factor V Leiden.47,48 Abnormalities of fibrinolysis have been implicated in the pathogenesis of TM syndromes,32,33,35,49and activated protein C has been shown to enhance fibrinolysis indirectly by down-regulating production of thrombin and thrombin-activatable fibrinolysis inhibitor.50 In vitro studies have shown that acceleration of fibrinolysis by activated protein C is markedly attenuated by factor V Leiden,51which might promote microvascular thrombosis.
The participation of multiple genetic factors in the development of TM is suggested by experimental murine models in which occurrence of a thrombotic phenotype resulting from introduction of factor V Leiden was dependent on the genetic background of the mice.52 When introduced into C57BL/J mice, factor V Leiden had no effect on fetal development or survival, whereas, in a mixed 129Sv-C57BL/6J genetic background, homozygous factor V Leiden resulted in perinatal disseminated intravascular thrombosis and increased mortality. Similar variability is observed in humans with mutations of complement factor H. Some mutations have been associated with development of HUS, others with systemic lupus erythematosus, type II membranoproliferative glomerulonephritis, and other disorders.36-38 53 Factor V Leiden may be among a constellation of genetic risk modifiers that when present in certain combinations are permissive of the development of TM and in other combinations result in deep-vein thrombosis or other thrombotic manifestations. Acquired risk factors, such as infection with Shiga toxin–producing bacteria, are undoubtedly important components of the risk profile for TM. Although no patients in this study were diagnosed with diarrhea-associated TM, reliable methods for detection of Shiga toxin are not widely available or used. Studies of patients with Shiga toxin–associated TM could reveal genetic risk factors.
Genetic studies, together with the wide clinical variability and multitude of observed hemostatic abnormalities in TM, suggest that critical pathogenic factors likely differ among individuals or groups of TM patients. Our results suggest that factor V Leiden may be an important pathophysiologic risk factor in subsets of TM patients, particularly those with normal VWCP activity. Additional studies of genetic risk modifiers may identify other relevant pathogenic factors in TM patients. Identification of relevant genetic and acquired risk factors, in combination with prospective clinical correlation studies, may lead to more rational and individualized treatments for TM disorders.
We thank Robert Luhm and David Dinauer of the Product Development Laboratory and Loni Kagen and Sharon Graminske of the Transfusion Medicine Laboratory of The Blood Center of Southeastern Wisconsin for their assistance in this study.
Supported by grants HL55556 and DK58949 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas J. Raife, Department of Pathology, University of Iowa College of Medicine, 200 Hawkins Dr, C250 GH, Iowa City, IA 52242; e-mail: thomas-raife@uiowa.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal