Abstract
Interleukin-10 (IL-10) is constitutively produced by peritoneal B1a lymphocytes, and stromal cell–derived factor-1 (SDF-1) by mesothelial cells. Independent studies have shown that both IL-10 and SDF-1 are involved in the persistence of the peritoneal B-lymphocyte compartment. This study shows that IL-10 and SDF-1 act in synergy on peritoneal B lymphocytes. Indeed, autocrine production of IL-10 was absolutely required for all effects of SDF-1 on these cells, including increased proliferation, survival, and chemotaxis. Moreover, adding IL-10 to peritoneal B lymphocytes increased the effects of SDF-1. Neither IL-5, IL-6, nor IL-9 affected the response of peritoneal B lymphocytes to SDF-1. IL-10 was chemokinetic for peritoneal B lymphocytes, increasing their random mobility. It also potentiated the SDF-1–induced reorganization of the cytoskeleton without affecting CXCR4 gene expression by peritoneal B lymphocytes. Despite its chemokinetic properties, IL-10 abolished the migration of peritoneal B lymphocytes in response to B-lymphocyte chemoattractant (BLC), a chemokine targeting B lymphocytes to lymphoid organ follicles. The ability of B1a lymphocytes to produce IL-10 constitutively, combined with the opposite effects of this cytokine on the responses to SDF-1 and BLC, may account for the selective accumulation of B1 lymphocytes in body cavities.
Introduction
Peritoneal B lymphocytes are divided into 3 different subpopulations identified on the basis of the expression of the Mac-1 and CD5 antigens.1-3 B lymphocytes expressing neither of these antigens are referred to as B2 lymphocytes. They are thought to correspond to conventional B lymphocytes, the main B-cell population outside body cavities. The second peritoneal B-lymphocyte subpopulation, the B1b cells, express Mac-1 but not CD5. Like peritoneal B2 lymphocytes, they are derived from a bone marrow precursor. However, they are rare outside body cavities. The third subpopulation, the B1a cells, express Mac-1 and CD5 and have the unique property of self-renewal in the peritoneal cavity, independent of bone marrow–derived progenitors. B1a lymphocytes seem to be directly derived from fetal liver precursors and to persist from birth in body cavities as an autonomous cell population; the repopulation of immune-deficient mice with peritoneal B lymphocytes restores only the B1a subpopulation.4-6 B1a lymphocytes play a critical role in innate immunity.3,4,7,8 They are also involved in the production of autoantibodies.9-12
The mechanism by which B-lymphocyte populations are retained, survive, and expand in body cavities is not completely understood, but the production of a limited set of cytokines appears to be required. Mice transgenic for interleukin-9 (IL-9) display considerable expansion of the peritoneal B-lymphocyte compartment because of the accumulation of B1b lymphocytes and, to a lesser extent, of B1a cells.13Mice lacking the IL-5 or IL-5R gene14-17 display transient and partial depletion of peritoneal B1a lymphocytes. IL-5 induces the expansion of B1a lymphocytes in vitro and in vivo,18,19and mice transgenic for IL-5 produce autoantibodies.20 It is unknown whether IL-5 and IL-9 are produced in body cavities, and their potential sources at these sites have not been identified. IL-10 is another cytokine that may be involved in B1a self-renewal. The neutralization of IL-10 by anti–IL-10 monoclonal antibody (mAb) administration depletes B1a lymphocytes in normal mice21and prevents autoimmunity in NZB/W F1 mice, a model of systemic lupus erythematosus in which the B1a-lymphocyte compartment is abnormally expanded.22 In vivo administration of IL-10 activates peritoneal B1a lymphocytes and stimulates autoantibody production in predisposed mice.19 Because B1a lymphocytes produce IL-10,23 this cytokine may act as an autocrine growth factor for these cells.
Chemokines may also affect peritoneal B lymphocytes. B-lymphocyte chemoattractant (BLC, CXCL13)24 plays a critical role in the homing of B lymphocytes to follicles in the Peyer patches and spleen.25 Experiments in vitro have shown that BLC attracts peritoneal B lymphocytes.26 Stromal cell–derived factor-1 (SDF-1, CXCL12)24 also acts on peritoneal B lymphocytes. Mice with a disrupted SDF-1 gene have impaired B-lymphocyte development.27,28 SDF-1 stimulates the proliferation29 and chemotaxis30-32 of conventional B lymphocytes and of their precursors, and it protects lymphocytic leukemia B cells from spontaneous apoptosis.33In vivo, SDF-1 is required for retention of the B lineage in the bone marrow.34,35 We recently showed that SDF-1 is involved in the persistence of peritoneal B lymphocytes.6 In vitro, SDF-1 stimulates the migration and proliferation of peritoneal B lymphocytes, and it prevents their apoptosis. The in vivo neutralization of SDF-1 largely depletes the peritoneal B-lymphocyte compartment without significantly affecting lymphoid tissues. Mesothelial cells constitutively produce SDF-1 in embryos and in adults.6 36 This chemokine therefore presumably acts as a nursing paracrine factor for the retention, survival, and expansion of B lymphocytes in body cavities.
These reports indicate that the persistence of B lymphocytes in the body-cavity compartment, and particularly the self-renewal of B1a lymphocytes, may involve interactions between various cytokines and chemokines. Recently, IL-10 has been shown to prevent the signal transduction induced by a large set of chemokines in dendritic cells (DCs), indicating that for these cells, IL-10 acts as a decoy cytokine for chemokine receptor function.37 We show here that, as for DCs, IL-10 prevents the effects of the chemokine BLC on peritoneal B lymphocytes. In contrast, IL-10 has the opposite effect on SDF-1 function: There is a strong synergy between IL-10 and SDF-1 for the proliferation, migration, and survival of peritoneal B lymphocytes, and autocrine production of IL-10 by peritoneal cells is essential for all SDF-1 effects on these cells. Therefore, the constitutive production of IL-10 by peritoneal B1a lymphocytes, combined with the opposite effects of this cytokine on the responses to BLC and SDF-1, may favor the action of SDF-1 on these cells, accounting for the scarcity of B1 lymphocytes in lymphoid organs and their selective accumulation in body cavities.
Materials and methods
Mice and cell preparation
Mice were purchased from Iffa Credo (L'Abresle, France). All mice were 7- to 10-week-old Balb/c females. B-cell subpopulations were characterized by flow cytometry, as reported previously.6Peritoneal cells were recovered by washing the peritoneal cavity with 30 mL phosphate-buffered saline (PBS). Approximately 3 million peritoneal cells were recovered per mouse, comprising 7% to 10% B1a cells, 3% to 5% B1b cells, and 10% to 15% B2 cells. Unless specified, cells from 5 to 10 mice were pooled. Spleen B lymphocytes routinely contained less than 2% B1 cells.
IL-10 production studies
Peritoneal and spleen cells were plated (96-well flat-bottom plates; Costar, Brumath, France) at a density of 2 × 106cells/mL in 100 μL RPMI medium supplemented with 10% fetal calf serum (FCS; Life Technologies, Cergy Pontoise, France) with or without various concentrations of human SDF-1. Cells were incubated for 24 hours at 37°C in an atmosphere containing 5% CO2. Supernatants of triplicate wells were pooled and tested by enzyme-linked immunosorbent assay for murine IL-10 (Quantikine M; R&D Systems, Minneapolis, MN). The effects on IL-10 production of the murine secondary lymphoid-tissue chemokine (SLC/6Ckine/CCL21),24 murine BLC/BCA-1/CXCL13, and murine fractalkine/neurotactin/CX3CL124 (R&D Systems) were also studied, with concentrations of chemokines from 0.5 to 500 ng/mL.
For the detection of IL-10–containing cells, peritoneal cells were cultured at 37°C in RPMI medium (2 × 106 cells/mL) with or without SDF-1 (1 μg/mL). Eighteen hours later, cells were recovered, washed, and labeled with fluorescent mAbs (CD19, CD5, and Mac-1) at 4°C for 30 minutes. Cells were washed with 2% FCS in PBS, fixed with 2% formaldehyde in PBS for 30 minutes at room temperature, washed again, and labeled for 1 hour by incubation in a permeabilizing saponin buffer (0.5%; Sigma, St Louis, MO) with the fluorescein isothiocyanate (FITC)–conjugated rat antimouse IL-10 Ab (clone JES5-16E3) or the FITC-conjugated rat IgG2b isotype control Ab (clone A95-1). Cells were washed twice in the saponin buffer and analyzed by 4-color flow cytometry (FACScan; Becton Dickinson, Rungis, France). All mAbs were from Becton Dickinson. Whether or not SDF-1 was present, less than 1% IL-10–containing cells were detected if the saponin buffer was omitted.
Functional evaluation of chemokine receptors
Proliferation and survival experiments, using the 5(6) carboxyfluorescein diacetate, succinimidyl ester (CFDA, SE) and the 3,3′-dihexylocarbocyanine iodide (DIOC6 (3)) dye assay, respectively, were performed as described previously.6Murine IL-10 (R&D Systems) was used at a concentration of 100 ng/mL. A goat antimouse IL-10R Ab and a control Ab (R&D Systems) were used at a concentration of 10 μg/mL. SDF-1 and BLC were each used at a concentration of 1 μg/mL.
To investigate the effect of IL-10R neutralization on cytoskeleton rearrangement, we stained freshly isolated cells with fluorescent mAbs at 4°C for 30 minutes. Cells were washed in 2% FCS in PBS and incubated at 37°C for 2 hours in RPMI medium containing 20 mM HEPES (5 × 106 cells/mL), with or without the anti–IL-10R or the control Ab (10 μg/mL). Cells were washed and incubated at 37°C in RPMI medium (5 × 106cells/mL) before assessing cytoskeleton rearrangement. The actin polymerization induced by SDF-1 (250 ng/mL) was assessed as described previously.6 38
The chemotaxis of cell subpopulations was evaluated using the Transwell system (Costar) as described previously,6 except that one million cells were added to the upper chamber. The chemokines SDF-1, BLC, and SLC were used at a concentration of 1 μg/mL, and the cytokines IL-5, IL-6, IL-9, and IL-10 (all from R&D Systems) were used at 100 ng/mL. The anti–IL-10R and control Abs were used at a concentration of 10 μg/mL. Unless specified, the chemokines were added to the lower chamber only, whereas IL-5, IL-6, IL-9, IL-10, and the Abs were added to both the upper and lower chambers.
Purification of CD19+ peritoneal cells and reverse transcriptase–polymerase chain reaction studies
Peritoneal cells from 20 Balb/c mice were obtained by intraperitoneal washing and were purified using streptavidin-coated magnetic beads (CELLection Dynabeads; Dynal, Oslo, Norway) and a biotinylated CD19 mAb (Becton Dickinson). The final preparation contained more than 95% CD19+ cells, less than 1% CD3+ cells, and less than 4% CD4+CD3− cells. Cells were cultured at 37°C at a density of 2 × 106 cells/mL in RPMI medium supplemented with 10% FCS, with or without the anti–IL-10R Ab (10 μg/mL) or the control Ab for 4 hours, or with or without IL-10 (100 ng/mL) for 2 hours.
Finally, cells were recovered and stored in RNA-B extraction solution (Quantum; Appligene, Illkirch, France). Expression of the murine CXCR4 and CXCR5 genes was analyzed by RT-PCR from 100 ng total RNA. Thirty-one (for CXCR4) or 40 (for CXCR5) cycles of PCR (each cycle consisting of 60 seconds at 94°C, 60 seconds at 57°C, and 90 seconds at 72°C) were performed, using the 5′-TCATCACACTCCCCTTCTGG-3′ sense primer and the 5′-GGGTAAAGGCGGTCACAGAT-3′ antisense primer for CXCR4, and the 5′-CTAGAGCCTGGTTCACCTCC-3′ sense primer and the 5′-GCTGCTATTCACAGCCTTCAGCC-3′ antisense primer for CXCR5. Amplified products were subjected to 1.2% agarose gel electrophoresis, detected by ethidium bromide staining, and quantified by computed-assisted densitometry using the ScanAnalysis software (Biosoft, Cambridge, United Kingdom).
Statistical analysis
Results were compared using the Mann-Whitney Utest.
Results
IL-10 increases the effect of SDF-1 on the proliferation and survival of peritoneal B lymphocytes
The effects of SDF-1 and IL-10 on the proliferation of peritoneal B lymphocytes were analyzed at the single-cell level using the 5(6) CFDA, SE staining technique. As reported previously,6 SDF-1 stimulated the proliferation of a fraction of peritoneal B lymphocytes. The number of cells that divided during culture, characterized by a low 5(6) CFDA, SE content, was 4 to 5 times higher in the presence of SDF-1 than in control cultures. IL-10 also stimulated the proliferation of peritoneal B lymphocytes, although to a lesser extent than SDF-1. The highest level of proliferation was observed if SDF-1 and IL-10 were added together, with approximately one quarter of the cells resulting from cell division (Table1) and with more cycles of division for proliferating cells (Figure 1). BLC alone had little, if any, effect on the proliferation of peritoneal B lymphocytes. The addition of IL-10 did not increase the number of cells proliferating in the presence of BLC (Figure 1).
Induction of proliferation by SDF-1 and IL-10
| SDF-1 . | IL-10 . | Peritoneal cell subpopulations . | ||
|---|---|---|---|---|
| B1a . | B1b . | B2 . | ||
| − | − | 2.1 ± 0.4 | 3.1 ± 0.5 | 2.2 ± 0.2 |
| + | − | 10.7 ± 1.9* | 13.6 ± 2.1* | 9.2 ± 1.5* |
| − | + | 5.5 ± 0.5* | 7.6 ± 1.5* | 4.8 ± 0.5* |
| + | + | 25.8 ± 4.2*,† | 30.1 ± 4.5*,† | 21.2 ± 3.5*,† |
| SDF-1 . | IL-10 . | Peritoneal cell subpopulations . | ||
|---|---|---|---|---|
| B1a . | B1b . | B2 . | ||
| − | − | 2.1 ± 0.4 | 3.1 ± 0.5 | 2.2 ± 0.2 |
| + | − | 10.7 ± 1.9* | 13.6 ± 2.1* | 9.2 ± 1.5* |
| − | + | 5.5 ± 0.5* | 7.6 ± 1.5* | 4.8 ± 0.5* |
| + | + | 25.8 ± 4.2*,† | 30.1 ± 4.5*,† | 21.2 ± 3.5*,† |
Results are from 4 independent 5(6) CFDA, SE assays. They are expressed as the percentage (mean ± SEM) of cells resulting from cell division at the end of culture.
SDF-1 indicates stromal cell-derived factor-1; IL-10, interleukin 10.
P < .05 versus controls.
P < .05 versus SDF-1-induced proliferation.
SDF-1 and BLC effects on B1a-lymphocyte proliferation.
The effects of SDF-1, BLC, and IL-10 on the proliferation of B1a lymphocytes were evaluated using the 5(6) CFDA, SE assay. Results are from one representative out of 2 experiments. Similar results were obtained for B1b and B2 lymphocytes (data not shown). The percentage of B1a cells with a low concentration of 5(6) CFDA, SE is shown in the upper left quadrant.
SDF-1 and BLC effects on B1a-lymphocyte proliferation.
The effects of SDF-1, BLC, and IL-10 on the proliferation of B1a lymphocytes were evaluated using the 5(6) CFDA, SE assay. Results are from one representative out of 2 experiments. Similar results were obtained for B1b and B2 lymphocytes (data not shown). The percentage of B1a cells with a low concentration of 5(6) CFDA, SE is shown in the upper left quadrant.
The effect of SDF-1 and IL-10 on the spontaneous apoptosis of peritoneal B lymphocytes was evaluated by DIOC6 (3) labeling and flow cytometry. The addition of SDF-1 resulted in 26% to 47% fewer apoptotic cells at the end of a 2-day culture period. IL-10 also prevented cell death, although to a lesser extent than SDF-1. The simultaneous addition of SDF-1 and IL-10 resulted in a lower proportion of apoptotic cells than for cells cultured alone or in the presence of a single cytokine (Table 2). BLC had no effect on the apoptosis of peritoneal B lymphocytes, even if IL-10 was added to the cultures (data not shown).
Effects of SDF-1 and IL-10 on the survival of peritoneal B cells
| SDF-1 . | IL-10 . | Peritoneal cell subpopulations . | ||
|---|---|---|---|---|
| B1a . | B1b . | B2 . | ||
| − | − | 24.0 ± 0.7 | 32.3 ± 1.8 | 32.3 ± 2.3 |
| + | − | 12.8 ± 0.6* | 22.3 ± 1.5* | 23.9 ± 2.8* |
| − | + | 15.9 ± 1.5* | 26.1 ± 2.1 | 25.7 ± 2.5 |
| + | + | 9.3 ± 0.7*,† | 19.0 ± 1.5* | 17.6 ± 1.3* |
| SDF-1 . | IL-10 . | Peritoneal cell subpopulations . | ||
|---|---|---|---|---|
| B1a . | B1b . | B2 . | ||
| − | − | 24.0 ± 0.7 | 32.3 ± 1.8 | 32.3 ± 2.3 |
| + | − | 12.8 ± 0.6* | 22.3 ± 1.5* | 23.9 ± 2.8* |
| − | + | 15.9 ± 1.5* | 26.1 ± 2.1 | 25.7 ± 2.5 |
| + | + | 9.3 ± 0.7*,† | 19.0 ± 1.5* | 17.6 ± 1.3* |
Results are from 4 independent experiments and are expressed as the percentage (mean ± SEM) of apoptotic cells in the DIOC6 (3) labeling assay.
Abbreviations are explained in Table 1.
P < .05 versus controls.
P < .05 versus cultures in the presence of SDF-1 alone.
The 3 B-lymphocyte subpopulations, B1a, B1b, and B2 cells, were all sensitive to the combined effects of SDF-1 and IL-10 on proliferation and apoptosis, with the effects most pronounced for B1a cells in both assays.
IL-10 potentiates the chemotactic effect of SDF-1
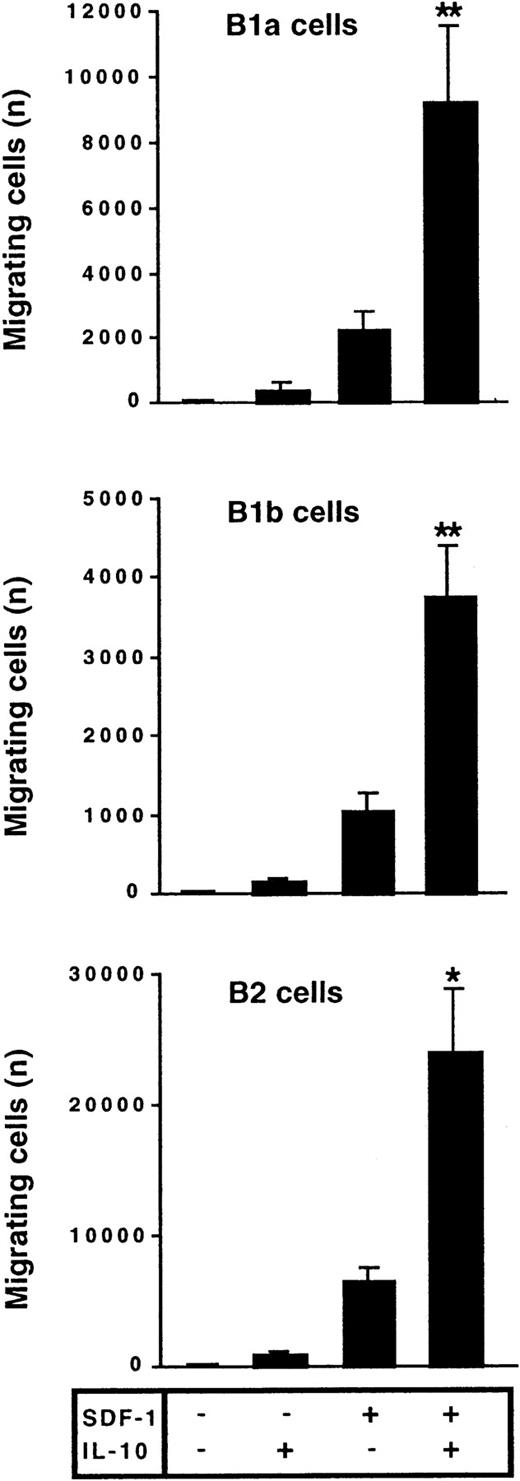
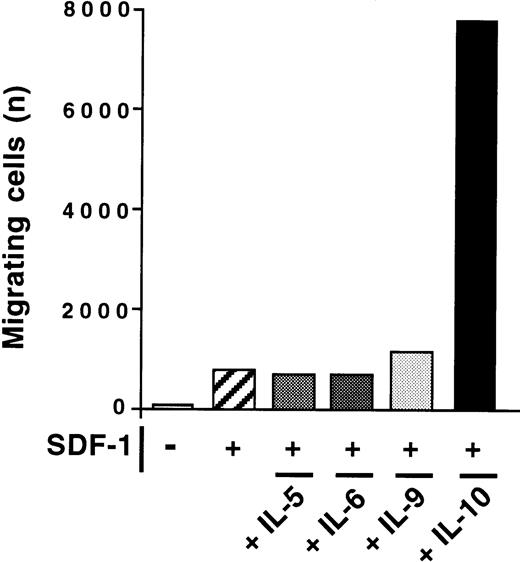
The effect of IL-10 and SDF-1 on the migration of peritoneal B lymphocytes was evaluated in Transwell chemotaxis experiments with freshly isolated peritoneal cells. The number and phenotype of the B lymphocytes migrating to the lower chamber were determined by flow cytometry. SDF-1 stimulated the chemotaxis of peritoneal B lymphocytes. IL-10 slightly increased migration if added alone, but it strongly increased the effect of SDF-1. B1a, B1b, and B2 lymphocytes were all sensitive to this synergistic induction of migration by IL-10 and SDF-1 (Figure 2). In contrast to IL-10, neither IL-5, IL-6, nor IL-9 potentiated the SDF-1–induced migration of peritoneal B1a cells (Figure 3) or of B1b or B2 cells (data not shown).
IL-10 potentiates the chemotactic activity of SDF-1 on peritoneal B lymphocytes.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and added to the upper chamber of a Transwell system. The effect of SDF-1 and/or IL-10 on cell migration was evaluated. Cells in the lower chamber at the end of the assay were characterized and counted by flow cytometry. Results (mean ± SEM) are from 9 experiments. *P < .05 and **P < .005 versus cultures in the presence of SDF-1 alone.
IL-10 potentiates the chemotactic activity of SDF-1 on peritoneal B lymphocytes.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and added to the upper chamber of a Transwell system. The effect of SDF-1 and/or IL-10 on cell migration was evaluated. Cells in the lower chamber at the end of the assay were characterized and counted by flow cytometry. Results (mean ± SEM) are from 9 experiments. *P < .05 and **P < .005 versus cultures in the presence of SDF-1 alone.
Absence of effect of IL-5, IL-6, and IL-9 on SDF-1–induced chemotaxis.
The chemotactic response of peritoneal B cells to SDF-1 was tested with or without IL-5, IL-6, IL-9, or IL-10. Results shown are for B1a cells in 1 of 2 experiments. Similar findings were observed for B1b and B2 lymphocytes.
Absence of effect of IL-5, IL-6, and IL-9 on SDF-1–induced chemotaxis.
The chemotactic response of peritoneal B cells to SDF-1 was tested with or without IL-5, IL-6, IL-9, or IL-10. Results shown are for B1a cells in 1 of 2 experiments. Similar findings were observed for B1b and B2 lymphocytes.
SDF-1 stimulates IL-10 production by peritoneal B cells
We investigated the effect of SDF-1 on the production of IL-10 by peritoneal cells. Peritoneal cells were cultured for 24 hours with various concentrations of SDF-1. IL-10 production by spleen cells was studied in parallel. In the absence of stimulation, peritoneal cells produced larger amounts of IL-10 than did spleen cells. SDF-1 induced a dose-dependent increase in IL-10 production by peritoneal cells. It had no effect on spleen cells (Figure4A). In the presence of 500 ng/mL SDF-1, approximately 8 times more IL-10 was produced by peritoneal cells than by spleen cells. Neither BLC, SLC, nor fractalkine stimulated IL-10 production by peritoneal cells (data not shown).
SDF-1 stimulates IL-10 production by peritoneal B cells.
(A) Peritoneal cells (black columns) or spleen cells (gray columns) were cultured either alone or in the presence of various SDF-1 concentrations. IL-10 concentration was determined in the supernatant. Results (mean ± SEM) are from 3 experiments. (B-D) Peritoneal cells were incubated with or without SDF-1. They were then labeled with CD19, CD5, Mac-1 mAb, and with an anti–IL-10 mAb. (B) B1a cells; (C) B1b cells; (D) B2 cells. Results (mean ± SEM from 6 experiments) are the proportion of cells containing IL-10. No labeling of IL-10 was detected if an FITC-coupled control mAb was used instead of an anti–IL-10 mAb or if the cell permeabilization step was omitted (data not shown). *P < .05 and **P < .005 compared with cells cultured in the absence of SDF-1.
SDF-1 stimulates IL-10 production by peritoneal B cells.
(A) Peritoneal cells (black columns) or spleen cells (gray columns) were cultured either alone or in the presence of various SDF-1 concentrations. IL-10 concentration was determined in the supernatant. Results (mean ± SEM) are from 3 experiments. (B-D) Peritoneal cells were incubated with or without SDF-1. They were then labeled with CD19, CD5, Mac-1 mAb, and with an anti–IL-10 mAb. (B) B1a cells; (C) B1b cells; (D) B2 cells. Results (mean ± SEM from 6 experiments) are the proportion of cells containing IL-10. No labeling of IL-10 was detected if an FITC-coupled control mAb was used instead of an anti–IL-10 mAb or if the cell permeabilization step was omitted (data not shown). *P < .05 and **P < .005 compared with cells cultured in the absence of SDF-1.
To determine whether B lymphocytes contributed to IL-10 production by peritoneal cells and which B-cell subpopulation produced IL-10, we performed flow cytometry experiments using an anti–IL-10 mAb in combination with B-lymphocyte subpopulation markers. In the absence of SDF-1, only a few cells contained IL-10. The incubation of peritoneal cells with SDF-1 increased the proportion of IL-10–containing cells in the 3 B-lymphocyte subpopulations, with the highest proportion of positive cells in the B1a subpopulation (Figure 4B-D).
Endogenous IL-10 is required for all SDF-1 effects on peritoneal B lymphocytes
The ability of SDF-1 to increase IL-10 production by peritoneal B lymphocytes raised the question of the role of the autocrine production of IL-10 on the effects of SDF-1. To address this issue, we investigated the effect of a blocking anti–IL-10R Ab on SDF-1–induced peritoneal B-lymphocyte proliferation and survival. The stimulating effect of SDF-1 on the proliferation of B1a lymphocytes was totally abolished in the presence of the anti–IL-10R Ab (Figure5A). The antiapoptotic effect of SDF-1 also required the endogenous production of IL-10 because it was totally abolished in the presence of the anti–IL-10R Ab (Figure 5B). The influence of the anti–IL-10R Ab on the SDF-1–induced proliferation and apoptosis of B1b and B2 peritoneal lymphocytes was similar (data not shown).
An anti–IL-10R Ab abolishes the effects of SDF-1 on the proliferation and survival of peritoneal B1a lymphocytes.
Proliferation (A) and survival (B) of peritoneal B1a lymphocytes were evaluated using the 5(6) CFDA, SE and DIOC6 (3) labeling assays, respectively. An anti–IL-10R or a control Ab was tested in combination with SDF-1. Results (mean ± SEM) are from 6 experiments (A) and 3 experiments (B). *P < .05 and **P < .005 as compared with cultures with SDF-1 and the control antibody, respectively.
An anti–IL-10R Ab abolishes the effects of SDF-1 on the proliferation and survival of peritoneal B1a lymphocytes.
Proliferation (A) and survival (B) of peritoneal B1a lymphocytes were evaluated using the 5(6) CFDA, SE and DIOC6 (3) labeling assays, respectively. An anti–IL-10R or a control Ab was tested in combination with SDF-1. Results (mean ± SEM) are from 6 experiments (A) and 3 experiments (B). *P < .05 and **P < .005 as compared with cultures with SDF-1 and the control antibody, respectively.
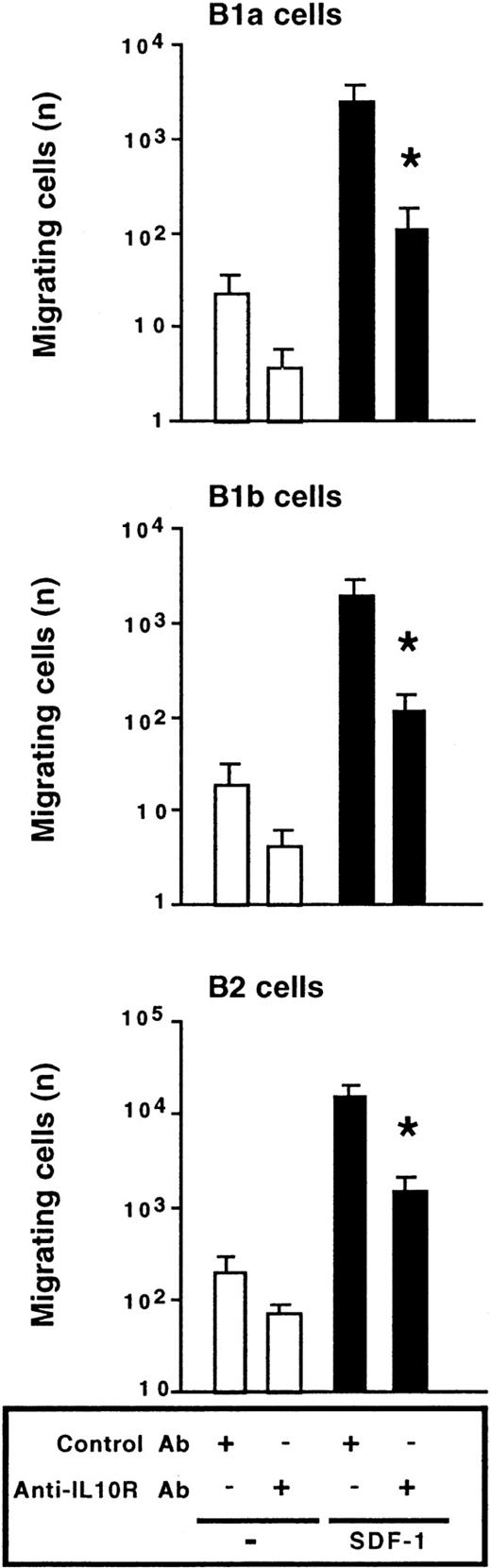
We also investigated the effect of the anti–IL-10R Ab on the migration of peritoneal B lymphocytes. The chemotactic effect of SDF-1 was inhibited by more than 90% in the presence of the anti–IL-10R Ab. Migration of the 3 peritoneal B-lymphocyte subpopulations was affected by blocking IL-10R (Figure 6). A rat antimouse IL-10R mAb (1B1.3A; 10 μg/mL) and a rat IgG1 control mAb (GL113; kindly provided by R. Coffman, DNAX, Palo Alto, CA) were also tested in migration assays and gave similar results to the goat polyclonal Abs (data not shown).
Endogenous IL-10 is required for SDF-1–induced chemotaxis.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and tested in Transwell experiments for SDF-1–induced chemotaxis in the presence of an anti–IL-10R or a control Ab. Results (mean ± SEM from 4 experiments) are the numbers of cells migrating to the lower chamber. *P < .05 compared with cells migrating in the presence of SDF-1 and the control Ab.
Endogenous IL-10 is required for SDF-1–induced chemotaxis.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and tested in Transwell experiments for SDF-1–induced chemotaxis in the presence of an anti–IL-10R or a control Ab. Results (mean ± SEM from 4 experiments) are the numbers of cells migrating to the lower chamber. *P < .05 compared with cells migrating in the presence of SDF-1 and the control Ab.
Therefore, experiments analyzing proliferation, prevention of apoptosis, and migration all led to the same conclusion: The effects of SDF-1 on peritoneal B lymphocytes absolutely require production of IL-10 by peritoneal cells.
Lack of synergy between IL-10 and SDF-1 for spleen B2 lymphocytes
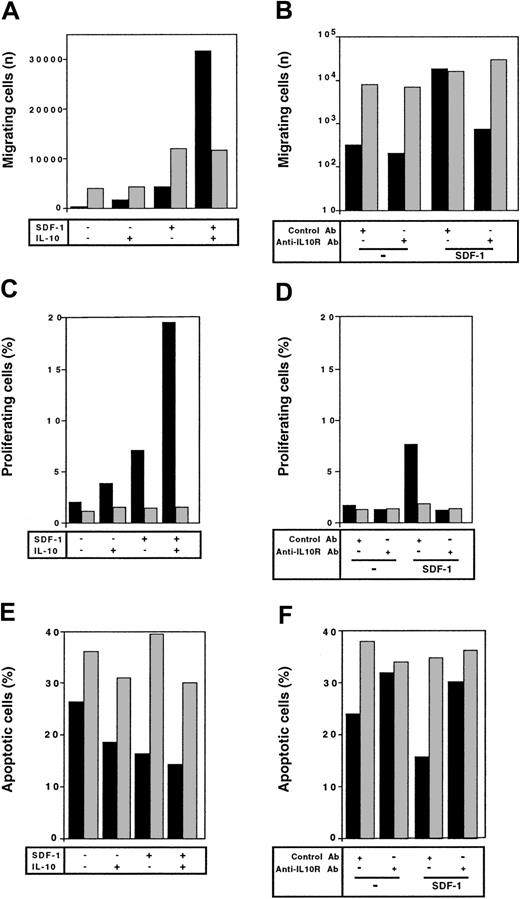
We evaluated whether the synergistic effects between IL-10 and SDF-1 evidenced with peritoneal B lymphocytes also occurred with spleen B lymphocytes. The frequency of spleen B1 lymphocytes was too low to address this question for this cell subpopulation. On spleen B2 lymphocytes, SDF-1 did not stimulate proliferation (Figure7C), did not prevent apoptosis (Figure7E), and did not trigger actin polymerization (data not shown). In contrast, it stimulated chemotaxis of these cells (Figure 7A). Neither the addition of IL-10 nor the addition of an anti–IL-10R Ab changed the response of spleen B2 cells to SDF-1 (Figure 7). Therefore, the synergistic effect between IL-10 and SDF-1 is specific for peritoneal B lymphocytes.
Different responses to SDF-1 of peritoneal versus spleen B2 lymphocytes.
The response to SDF-1 of peritoneal (black columns) and spleen (gray columns) B2 lymphocytes was evaluated for chemotaxis (A,B), proliferation (C,D), and survival (E,F). In each case, the influence of IL-10 (left panels) or anti–IL-10R Ab (right panels) on this response was tested. Results shown are from 1 of 2 (C-F) or 3 (A,B) experiments.
Different responses to SDF-1 of peritoneal versus spleen B2 lymphocytes.
The response to SDF-1 of peritoneal (black columns) and spleen (gray columns) B2 lymphocytes was evaluated for chemotaxis (A,B), proliferation (C,D), and survival (E,F). In each case, the influence of IL-10 (left panels) or anti–IL-10R Ab (right panels) on this response was tested. Results shown are from 1 of 2 (C-F) or 3 (A,B) experiments.
Mechanism of synergy between IL-10 and SDF-1 in peritoneal B-cell migration
To gain insight into the mechanism by which IL-10 affects SDF-1–induced migration, we investigated whether IL-10 acted as a chemokinetic agent, potentiating the chemotactic effect of SDF-1. In Transwell experiments, IL-10 or SDF-1 was added to the lower chamber only or to both the lower and upper chambers. As expected, the main effect of SDF-1 was chemotactic; the number of cells migrating to the lower chamber was largest if SDF-1 was added to the lower chamber only. Adding SDF-1 to both the upper and lower chambers decreased migration by approximately 90%. IL-10 weakly stimulated cell migration. However, unlike SDF-1, it acted as a chemokinetic agent; similar numbers of cells migrated to the lower chamber regardless of whether IL-10 was added to the lower chamber only or to both chambers (Figure 8). In contrast to IL-10, IL-5, IL-6, and IL-9 were not chemokinetic for peritoneal B lymphocytes (data not shown).
Chemokinetic effect of IL-10 and chemotactic effect of SDF-1 on peritoneal B1a lymphocytes.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and tested for migration in Transwell experiments. SDF-1 or IL-10 was added to either the lower chamber or to both the lower and upper chambers. Results shown (mean ± SEM from 5 experiments) are the numbers of B1a cells migrating to the lower chamber. *P < .05 compared with cells migrating in response to SDF-1 added alone to the lower chamber. Similar findings were observed for peritoneal B1b and B2 cells.
Chemokinetic effect of IL-10 and chemotactic effect of SDF-1 on peritoneal B1a lymphocytes.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and tested for migration in Transwell experiments. SDF-1 or IL-10 was added to either the lower chamber or to both the lower and upper chambers. Results shown (mean ± SEM from 5 experiments) are the numbers of B1a cells migrating to the lower chamber. *P < .05 compared with cells migrating in response to SDF-1 added alone to the lower chamber. Similar findings were observed for peritoneal B1b and B2 cells.
We investigated the synergy between IL-10 and SDF-1 further by studying the effect of the anti–IL-10R Ab on SDF-1–induced cytoskeleton rearrangement. Freshly isolated peritoneal cells were incubated at 37°C with or without the blocking anti–IL-10R Ab or a control Ab. Two hours later, cells were stimulated with SDF-1, and actin polymerization was monitored for 60 seconds by flow cytometry. The control Ab had no effect on SDF-1–induced cytoskeleton rearrangement. In contrast, the anti–IL-10R Ab abolished SDF-1–induced actin polymerization, regardless of the B-lymphocyte subpopulation considered (Figure 9). Similar findings were obtained when using a rat anti–IL-10R mAb (data not shown). This indicates that the continuous production of IL-10 by peritoneal cells is required for SDF-1–induced cytoskeleton rearrangement.
Endogenous IL-10 is required for SDF-1–induced cytoskeleton rearrangement.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and tested by flow cytometry for SDF-1–induced cytoskeleton rearrangement. This effect was tested with an anti–IL-10R Ab (black squares) or a control Ab (gray triangles), or without Ab addition (empty diamonds). Results (mean ± SEM from 3 experiments) show the kinetics of actin polymerization following SDF-1 addition. 100% corresponds to the baseline level.
Endogenous IL-10 is required for SDF-1–induced cytoskeleton rearrangement.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and tested by flow cytometry for SDF-1–induced cytoskeleton rearrangement. This effect was tested with an anti–IL-10R Ab (black squares) or a control Ab (gray triangles), or without Ab addition (empty diamonds). Results (mean ± SEM from 3 experiments) show the kinetics of actin polymerization following SDF-1 addition. 100% corresponds to the baseline level.
IL-10 does not affect CXCR4 gene expression in peritoneal B lymphocytes
The modulation of the effects of SDF-1 by IL-10 or IL-10R neutralization may reflect a role of IL-10 on CXCR4 expression. We determined whether IL-10 or the anti–IL-10R Ab affected the level of CXCR4 gene expression by peritoneal cells. Addition of IL-10 for 2 hours to freshly isolated CD19+ peritoneal B lymphocytes did not modify the level of CXCR4 gene expression (Figure10B). The amount of CXCR4 mRNA was higher in cultured CD19+ peritoneal B lymphocytes than in freshly isolated cells. Addition of the anti–IL-10R Ab did not prevent this increase in expression of the CXCR4 gene (Figure 10A). Therefore, the effects of IL-10 and of the anti–IL-10R Ab on SDF-1 responsiveness were observed independently of an effect of these agents on CXCR4 gene expression.
Regulation of CXCR4 and CXCR5 gene expression by IL-10.
(A) The level of CXCR4 and CXCR5 expression by peritoneal B lymphocytes was evaluated by RT-PCR in freshly isolated cells (lane 1) or in cells cultured for 4 hours with a control antibody (lane 2) or an anti–IL-10R Ab (lane 3). Results are from one representative out of 2 experiments. Densitometry analysis of amplified products showed that the CXCR4/β-actin ratio (mean ± SEM) was 0.43 ± 0.09, 1.02 ± 0.07, and 1.01 ± 0.02 for lanes 1 to 3, respectively. Similar analysis for CXCR5 gene expression showed a CXCR5/β-actin ratio (mean ± SEM) of 0.78 ± 0.06, 0.91 ± 0.07, and 1.01 ± 0.03 for lanes 1 to 3, respectively. (B) The level of CXCR4 and CXCR5 expression by peritoneal B lymphocytes was evaluated by RT-PCR in freshly isolated cells (lane 4) or in cells cultured for 2 hours without (lane 5) or with IL-10 (lane 6). Results are from a single experiment. Densitometry analysis of amplified products showed that the CXCR4/β-actin ratio was 0.35, 0.88, and 0.85 for lanes 4 to 6, respectively. Similar analysis for CXCR5 gene expression showed a CXCR5/β-actin ratio of 0.44, 0.63, and 0.45 for lanes 4 to 6, respectively.
Regulation of CXCR4 and CXCR5 gene expression by IL-10.
(A) The level of CXCR4 and CXCR5 expression by peritoneal B lymphocytes was evaluated by RT-PCR in freshly isolated cells (lane 1) or in cells cultured for 4 hours with a control antibody (lane 2) or an anti–IL-10R Ab (lane 3). Results are from one representative out of 2 experiments. Densitometry analysis of amplified products showed that the CXCR4/β-actin ratio (mean ± SEM) was 0.43 ± 0.09, 1.02 ± 0.07, and 1.01 ± 0.02 for lanes 1 to 3, respectively. Similar analysis for CXCR5 gene expression showed a CXCR5/β-actin ratio (mean ± SEM) of 0.78 ± 0.06, 0.91 ± 0.07, and 1.01 ± 0.03 for lanes 1 to 3, respectively. (B) The level of CXCR4 and CXCR5 expression by peritoneal B lymphocytes was evaluated by RT-PCR in freshly isolated cells (lane 4) or in cells cultured for 2 hours without (lane 5) or with IL-10 (lane 6). Results are from a single experiment. Densitometry analysis of amplified products showed that the CXCR4/β-actin ratio was 0.35, 0.88, and 0.85 for lanes 4 to 6, respectively. Similar analysis for CXCR5 gene expression showed a CXCR5/β-actin ratio of 0.44, 0.63, and 0.45 for lanes 4 to 6, respectively.
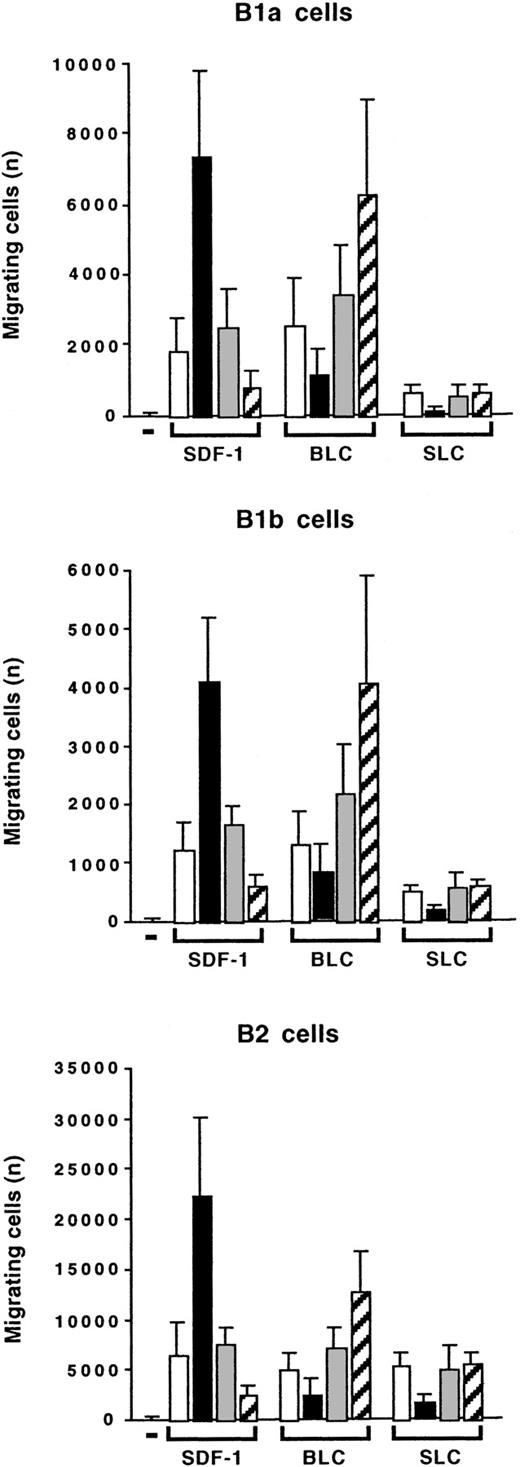
IL-10 has opposite effects on SDF-1–induced and BLC-induced chemotaxis
We compared the effects of IL-10 on the chemotaxis of peritoneal B lymphocytes induced by SDF-1, BLC, and SLC. BLC was as efficient as SDF-1 at stimulating the migration of B1a, B1b, and B2 lymphocytes. SLC was as efficient as the other 2 chemokines at stimulating the migration of B2 lymphocytes. However, it had only a weak effect on B1a and B1b lymphocytes (Figure11). Interestingly, IL-10 had opposite effects on the migration induced by BLC and on that induced by SDF-1: Addition of IL-10 inhibited the BLC-induced migration of B1a, B1b, and B2 lymphocytes, whereas the anti–IL-10R Ab increased the response to BLC (Figure 11). The effects of IL-10 and the anti–IL-10R Ab correlated with their effects on CXCR5 gene expression. The anti–IL-10R Ab weakly increased CXCR5 gene expression, whereas IL-10 decreased this expression (Figure 10). Therefore, the concentration of IL-10 in the microenvironment determines which chemokine induces a response in peritoneal B lymphocytes, with starvation of IL-10 selecting for a response to BLC and excess of IL-10 selecting for a response to SDF-1.
Opposite effects of IL-10 on SDF-1–induced and BLC-induced chemotaxis.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and tested in Transwell experiments for SDF-1–induced or BLC-induced chemotaxis. Migration was evaluated without (empty columns) or with IL-10 (black columns), with a control Ab (gray columns), or with an anti–IL-10R Ab (hatched columns). Results (mean ± SEM from 3 experiments) are the numbers of cells migrating to the lower chamber.
Opposite effects of IL-10 on SDF-1–induced and BLC-induced chemotaxis.
Peritoneal cells were labeled with CD19, CD5, and Mac-1 mAbs and tested in Transwell experiments for SDF-1–induced or BLC-induced chemotaxis. Migration was evaluated without (empty columns) or with IL-10 (black columns), with a control Ab (gray columns), or with an anti–IL-10R Ab (hatched columns). Results (mean ± SEM from 3 experiments) are the numbers of cells migrating to the lower chamber.
Discussion
SDF-1 and IL-10 are cytokines produced in the body-cavity microenvironment. SDF-1 is constitutively synthesized by mesothelial cells,6,36 and IL-10 by peritoneal B lymphocytes.23 Independent studies have shown that SDF-1 and IL-10 each contribute to the persistence of the peritoneal B-lymphocyte compartment, and particularly of the B1a-lymphocyte subpopulation.6,19,21,22 The results of this study confirm and extend those of previous studies by showing that SDF-1 and IL-10 do not act independently on peritoneal B cells, but instead act in synergy. All the effects of SDF-1 on these cells require the presence of IL-10, including proliferation, prevention of apoptosis, and chemotaxis. IL-10 produced by peritoneal cells is needed for SDF-1 effects because these effects are no longer observed in the presence of an anti–IL-10R Ab. These findings demonstrate the critical role of autocrine IL-10 production by peritoneal B lymphocytes, sensitizing these cells to SDF-1. Moreover, we found that the addition of IL-10 increased the effects of SDF-1 on peritoneal B lymphocytes, indicating that the concentration of IL-10 in the microenvironment of peritoneal B lymphocytes modulates the intensity of their response to SDF-1. Because SDF-1 increases the production of IL-10 by peritoneal B lymphocytes, it may sensitize them to its own action in a self-amplifying loop. It is possible that other peritoneal cells in addition to B lymphocytes contributed to the local IL-10 production. Thus, overall, our results suggest that the role of autocrine IL-10 production in the self-renewal of B1a peritoneal lymphocytes23 is to potentiate the effects of SDF-1. By facilitating migration toward the local source of SDF-1 (mesothelial cells), IL-10 may favor the retention of B lymphocytes in the peritoneal cavity. By potentiating the effects of SDF-1 on proliferation and on the prevention of apoptosis, IL-10 may favor the local survival and expansion of these cells. This may be the reason why the treatment of mice with either an anti–IL-1021 or an anti–SDF-16 mAb leads to the depletion of peritoneal B lymphocytes.
B1 lymphocytes accumulate not only in body cavities, but also in the gut-associated lymphoid tissue and in mucosa.3,17,18,39,40SDF-1 is produced in large amounts by digestive epithelial cells,41 and IL-10 is produced in the gut.42It is thus possible that SDF-1 and IL-10 also have a combined effect on B1 lymphocytes in the intestinal mucosa, leading to the accumulation and survival of B1 lymphocytes at this site. A continuous exchange of B1 lymphocytes between the intestinal wall, the peritoneal cavity, and the omentum has been suggested,3,18,43 and this would imply that changes in the peritoneal B1-lymphocyte compartment may also indirectly affect intramucosal B1 cells. B1 lymphocytes are an important source of mucosal IgA.39 They also play a critical role in innate immunity.3,4,7 8 The combined effects of IL-10 and SDF-1 on B1-lymphocyte expansion and retention in the body cavities, and possibly in the intestinal wall, may thus be important in mucosal and innate immunity.
In contrast to the situation in anti–IL-10 mAb-treated mice, no abnormalities of the peritoneal B-lymphocyte compartment were noted in mice with a disrupted IL-10 or IL-10R gene, based on the analysis of the CD5 and B220 markers of B-lymphocyte subpopulations.1,44 Using CD5 and Mac-1 as markers of B-lymphocyte subpopulations, we observed a significant decrease in the numbers of peritoneal B1a and B1b lymphocytes (more than 70% and more than 50%, respectively) in IL-10−/− mice as compared with either Balb/c or C57BL/6 control mice (data not shown). In contrast, the numbers of peritoneal B2 lymphocytes were in the same range between IL-10−/− and control mice. This indicates that whereas IL-10 plays no role in the development of peritoneal B2 lymphocytes, it stimulates that of peritoneal B1a and B1b lymphocytes. Our findings suggest that the role of IL-10 in this process is to sensitize peritoneal B1a and B1b lymphocytes to the action of locally produced SDF-1. Mice transgenic for the IL-9 gene accumulate increased numbers of B1b lymphocytes in the peritoneum, whereas the numbers of peritoneal B1a and B2 lymphocytes are within the normal range.13 We confirmed this finding and observed that peritoneal B lymphocytes in such mice respond to SDF-1 with the same magnitude as in control mice (data not shown). This indicates that the role of IL-9 in expanding peritoneal B1b lymphocytes differs from that of IL-10, a hypothesis consistent with the absence of synergy between IL-9 and SDF-1 in our in vitro experiments.
The mechanism of synergy between IL-10 and SDF-1 on cell migration involves a chemokinetic effect of IL-10 and a chemotactic effect of SDF-1: IL-10 increases the random mobility of peritoneal B lymphocytes, which then migrate more efficiently along a gradient of SDF-1. Such synergy between a chemokinetic and a chemotactic agent has already been reported for eosinophils. IL-5 and eotaxin act synergistically to recruit these cells, with IL-5 being chemokinetic and eotaxin being a chemoattractant.45 The mechanism of cooperation between IL-10 and SDF-1 involves an early step in SDF-1 action because the presence of IL-10 is essential for cytoskeleton rearrangement during the few seconds following SDF-1 addition. The effect of IL-10 is also transient; SDF-1 loses its ability to act on peritoneal B lymphocytes after only 2 hours of incubation of the cells with an anti–IL-10R Ab. Thus, the constitutive production of IL-10 by peritoneal cells continuously sensitizes them to SDF-1, and variations in IL-10 concentration rapidly affect sensitivity to SDF-1. This modulation occurs without changes in the expression level of the CXCR4 gene. This suggests that IL-10 does not influence the expression of the receptor on the cell surface, but this point could not be tested directly in view of the lack of reagent allowing CXCR4 detection in the mice. Several examples are known in which CXCR4 is expressed but does not function.46-49
D'Amico et al37 showed that IL-10 abolishes the function on DCs of many chemokine receptors. Our results with BLC are consistent with such a decoy effect of IL-10 on the function of chemokine receptors. However, our results extend these findings by showing that the effect of IL-10 on the response to a chemokine is not always inhibitory. For peritoneal B lymphocytes, IL-10 is absolutely required for the appropriate action of SDF-1 on its receptor. Thus, depending on the concentration of IL-10, peritoneal B lymphocytes are highly sensitive to SDF-1 but almost unresponsive to BLC if IL-10 concentration is high; the opposite is true in the absence of IL-10.
If this phenomenon extends to other conditions, then, in a tissue with several superimposed chemokine gradients, a single cytokine in the microenvironment may determine which chemokine gradient a cell ignores and which one it follows. Conversely, acute changes in the cytokine content of the microenvironment rapidly redirect cell migration by changing the chemokine gradient selected by the cell. In the case of peritoneal B lymphocytes, such a dual and opposite effect of IL-10 on BLC and SLC effects may explain the scarcity of B1 lymphocytes in lymphoid organs and their accumulation in body cavities. BLC is involved in the homing of B lymphocytes to the B-cell zones of lymphoid organs.25,50 51 The constitutive production of IL-10 by B1 lymphocytes may prevent their accumulation in lymphoid organs, an effect amplified by their poor response to another chemokine targeting lymphocytes to lymphoid tissues, SLC. In contrast, this constitutive production of IL-10 probably sensitizes B1 cells to mesothelial-derived SDF-1, leading to their retention in body cavities.
SDF-1 is a chemokine with pleiotropic effects. However, most, if not all, of the effects of SDF-1 on its various target cells require the simultaneous presence of specific cytokines, which differ according to the target considered: macrophage-derived chemokine for platelets; thrombopoietin for megakaryocyte precursors; stem cell factor/kit ligand, IL-6, and steel factor for hematopoietic progenitors; and vascular endothelial growth factor for endothelial cells.52-58 Our findings provide another example of this phenomenon, identifying IL-10 as the key cytokine required for SDF-1 effects on peritoneal B lymphocytes. Therefore, despite the multiplicity of effects of SDF-1, the cytokine composition of the microenvironment determines the local effects of SDF-1, thereby limiting the influence of this chemokine in each body compartment to a particular set of cells.
In NZB and NZB/W mice, the B1-lymphocyte compartment is expanded, a feature that is dependent on IL-10 and leads to B-lymphoid malignancies and to autoimmune disorders.22,59-61 Elimination of peritoneal B lymphocytes or IL-10 neutralization in a transgenic mouse model of autoimmunity prevents the emergence of autoimmune manifestations.12,62 A similar mechanism may occur in human systemic lupus erythematosus, in which there is an expansion of IL-10–producing B lymphocytes, IL-10–dependent autoantibody production, and an improvement in clinical symptoms in patients treated with an anti–IL-10 mAb.63-65 B1 lymphocytes of NZB/W mice are extremely sensitive to SDF-1 stimulation, and this hypersensitivity is totally dependent on the endogenous production of IL-10 by B1 cells (unpublished results, December 2000). This suggests that SDF-1 plays a role in conjunction with IL-10 in this model of B1-lymphocyte–mediated autoimmunity, a hypothesis currently being tested in vivo.
Our findings show that the retention of B lymphocytes as a self-renewing compartment within body cavities requires the combined effects of SDF-1, produced by mesothelial cells, and IL-10, produced by peritoneal cells themselves. This synergistic effect results in greater migration, proliferation, and survival of B lymphocytes. Moreover, the amount of IL-10 acting on peritoneal B lymphocytes modulates their sensitivity to SDF-1 and to BLC in opposite ways, demonstrating the contribution of the cytokine content of the microenvironment to driving the response of immune cells to a complex network of chemokines.
Yolande Richard is acknowledged for critical review of the manuscript. Robert Coffman is acknowledged for the gift of the 1B1.3A anti–IL-10R and the GL113 control mAbs. Jean-Christophe Renauld (Ludwig Institute for Cancer Research, Brussels, Belgium) is acknowledged for kindly providing IL-9 transgenic (Tg5) and control (FVB) mouse strains.
Supported by the Association pour la Recherche sur le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dominique Emilie, INSERM U131, Institut Paris-Sud sur les Cytokines, 32 rue des Carnets, 92140 Clamart, France; e-mail:emilie@ipsc.u-psud.fr.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal