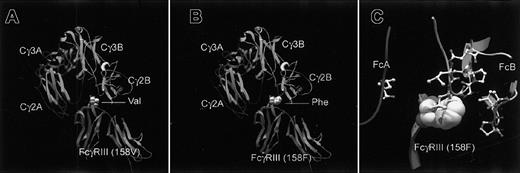
We read a recent interesting article by Cartron et al.1 The article indicated that the single nucleotide polymorphism (SNP) of IgG Fc receptor FcγRIIIa (FCGR3A) molecule affects clinical outcome of anti-CD20 monoclonal antibody therapy for follicular non-Hodgkin lymphoma.1 We created homology models of variant FCGR3A molecules based on homology between variant FCGR3A and known 3-dimensional structure of soluble CD16.2 Each model was superimposed onto a 1:1 complex of soluble CD16 and Fc fragment of human IgG1 (Figure1A-B). These models indicate that the position of variation 158Val/Phe exists at the F-G loop of the molecules that serves as binding interface and is surrounded by both chains of Fc fragments of IgG1 (Figure 1C). Since the side chain of Phe is hydrophobic and quite bigger than that of Val, the polymorphism can affect the major conformation or the hydrophobicity of the surface of the binding interface. These findings are compatible with previous observations that suggested FCGR3A binding interface3 4 and may help in understanding the way the SNP affects the binding with Fc portion of human IgG1.
Homology models of the Fc fragment of hIgG1.
The homology model has been developed using 1e4k.pdb in Brookhaven Protein Data Bank as a template.2 The modeling has been done with SwissPdbViewer and the SwissProt modeling server. The raytracing of the figure has been done using the program PovRay in SGI computer at Human Genome Center, Institute of Medical Science, the University of Tokyo. The models are superimposed onto 1e4k.pdb based on homology. Models are shown in β-strand, α-helical presentation connected with α-carbon trace. The side chains of Val158 and Phe158 are shown in space filling model. (A) Docking between FCGR3A-Val and hIgG1. (B) Docking between FCGR3A-Phe and hIgG1. (C) Details of amino acid residues close to 158F of FCGR3A-Phe molecules. The side chains of IgG1 molecule within 10Å from 158 Phe are shown in balls and sticks model.
Homology models of the Fc fragment of hIgG1.
The homology model has been developed using 1e4k.pdb in Brookhaven Protein Data Bank as a template.2 The modeling has been done with SwissPdbViewer and the SwissProt modeling server. The raytracing of the figure has been done using the program PovRay in SGI computer at Human Genome Center, Institute of Medical Science, the University of Tokyo. The models are superimposed onto 1e4k.pdb based on homology. Models are shown in β-strand, α-helical presentation connected with α-carbon trace. The side chains of Val158 and Phe158 are shown in space filling model. (A) Docking between FCGR3A-Val and hIgG1. (B) Docking between FCGR3A-Phe and hIgG1. (C) Details of amino acid residues close to 158F of FCGR3A-Phe molecules. The side chains of IgG1 molecule within 10Å from 158 Phe are shown in balls and sticks model.
Implication for how the single nucleotide polymorphism (SNP) of Fc receptor FcγRIIIa alters the interaction with anti-CD20 monoclonal antibody
The 2 available crystal structures of the extracellular part of CD16 in complex with IgG1 Fc1-1 1-2 are derived from the FcγRIIIb receptor, which is a glycosyl-phosphatidyl–anchored receptor expressed by neutrophils and eosinophils. However, due to 96% identity in the extracellular domains, it is assumed that the described structures can be used as a template to model the FcγRIIIa transmembrane receptor expressed by monocytes and natural killer (NK) cells. The FcγRIIIb receptor has a valine residue at position 158, like the FcγRIIIa-158Val allotype. We agree with Oshima and Fujimoro that a phenylalanine residue at position 158 of FcγRIIIa could alter the binding of human IgG1 Fc, notably by modifying the hydrophobic core involved in the binding to one of the CH2 domain of the antibody. The model provided by Oshima and Fujimoro supports our own results, and we thank them for this additional information.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal