Hematopoietic stem cell grafts from unrelated donors are commonly transported by aircraft. They must not be subjected to x-rays during security checks, which may cause inconvenient discussions between the courier and the airport security staff. We exposed hematopoietic stem cells from mobilized peripheral blood to a widely used x-ray hand-luggage control system. Cell viability as well as growth in vitro of mature progenitor cells (colony-forming cells), primitive progenitor cells (long-term culture-initiating cells), and lymphocytes were not altered even after 10 passages through the hand-luggage control system. Thus, repeated exposure to the low radiation dose of hand-luggage control systems (1.5 ± 0.6 μSv per exposure) seems to be harmless for hematopoietic stem cells, which should simplify the international transport of stem cell grafts.
Introduction
Hematopoietic stem cells donated from unrelated subjects are increasingly used for restoration of bone marrow function after high-dose chemoradiotherapy for hematologic malignancies. Thus, thousands of stem cell grafts are transported via aviation by a courier, and, according to existing guidelines (eg, from the European Group for Blood and Marrow Transplantation1), they must not be exposed to the radiation of x-ray hand-luggage control systems at airports. Interestingly, we found no published data about the presumed adverse effect of x-rays emitted from hand-luggage control devices on the viability and proliferative capacity of the blood cells and their progenitors.
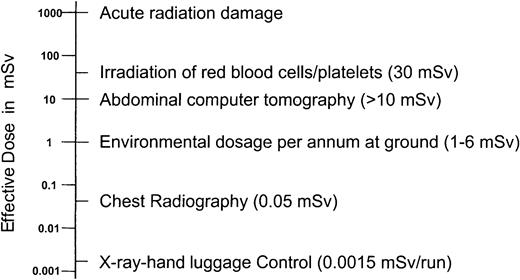
Amounts of radiation from various sources.
Figure shows radiation emitted from x-ray-hand luggage controls compared to other radiation sources in mSv (milli Sievert).
Amounts of radiation from various sources.
Figure shows radiation emitted from x-ray-hand luggage controls compared to other radiation sources in mSv (milli Sievert).
Study design
To change myths into facts, we exposed aliquots from peripheral blood progenitor cell collections (6 allogeneic, 5 autologous; mobilized by subcutaneous administration of granulocyte colony-stimulating factor) to radiation emitted from a widely used hand-luggage control device (HEIMANN HI-SCAN 6040A, Wiesbaden, Germany; radiation dosage 1.5 ± 0.6 μSv per single passage) at Innsbruck International Airport. After 0, 1, 2, 3, 5, and 10 passes through the x-ray device, the cells were checked for viability using trypan-blue dye exclusion. Mature hematopoietic progenitor cells (colony-forming cells [CFCs], including erythroid burst-forming units, granulocyte-macrophage colony-forming units, and megakaryocyte colony-forming units) and primitive progenitor cells (long-term culture-initiating cells) were enumerated in vitro as described.2 The in vitro expandability of the exposed mononuclear cells (MNCs) and CFCs was tested under serum-free conditions.3 After culturing the cells for 7 days in the presence of 1000 U/mL recombinant interleukin-2, we calculated the proliferation capacity of lymphocytes (T cells, natural killer [NK] cells) by fluorescence-activated cell-sorting analysis (CD3, CD56) and counting of the cells.
Results and discussion
After up to 10 radiographical passes, neither cell viability nor the number of hematopoietic progenitor cells was affected (Table 1). The expandability of MNCs (no x-ray: 259 ± 108-fold, 5 passages: 285 ± 132-fold), of CFCs (no x-ray: 446 ± 223-fold, 5 passages: 495 ± 251-fold), of NK cells (no x-ray: 2.4 ± 0.3-fold, 1 passage: 2.4 ± 0.4-fold, 5 passages: 2.3 ± 0.3-fold, 10 passages: 2.4 ± 0.4-fold), and of T lymphocytes (no x-ray: 1.9 ± 0.2-fold, 1 passage: 1.9 ± 0.1-fold, 5 passages: 1.9 ± 0.2-fold, 10 passages: 1.8 ± 0.2-fold) was unaltered. Also, HLA-unrestricted (NK cell) cytotoxicity against the erythroleukemia cell line K562 (effector to target ratio 5:1 and 25:1) was comparable to that of the controls (Table 1). Endothelial cells could be generated from the irradiated samples (data not shown), indicating the preservation of progenitor cells with hematoendothelial differentiation capacity.4 These data suggest that the radiation emitted from hand-luggage security-check devices exerts no harmful effect on the cellular content of hematopoietic stem cell grafts.
Cell viability, growth, expandability of hematopoietic progenitor cells, and cytotoxicity of NK cells from peripheral blood progenitor cell collections after passages through an x-ray scan device
| Parameter . | Number of passages through x-ray scan . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 5 . | 10 . | |
| Viability (n = 11) | 98 ± 1 | 97 ± 1 | 96 ± 1 | 96 ± 2 | 97 ± 1 |
| CFC (n = 11) | 98 ± 6 | 103 ± 7 | 95 ± 9 | 106 ± 13 | 109 ± 10 |
| BFU-E (n = 11) | 104 ± 10 | 109 ± 17 | 103 ± 20 | 118 ± 28 | 118 ± 23 |
| CFU-GM (n = 11) | 98 ± 7 | 112 ± 8 | 94 ± 7 | 106 ± 8 | 111 ± 8 |
| CFU-Meg (n = 7) | — | — | — | 146 ± 40 | — |
| LTC-IC (n = 11) | 102 ± 12 | 100 ± 15 | 121 ± 31 | 132 ± 23 | 108 ± 22 |
| MNC expansion (n = 3) | — | — | — | 100 ± 12 | — |
| CFC expansion (n = 3) | — | — | — | 94 ± 19 | — |
| T-cell expansion (n = 4) | 104 ± 4 | — | — | 104 ± 4 | 95 ± 2 |
| NK cell expansion (n = 4) | 101 ± 8 | — | — | 98 ± 3 | 103 ± 6 |
| NK cell cytotoxicity (n = 3) | 97 ± 7 | — | — | 101 ± 10 | 107 ± 7 |
| Parameter . | Number of passages through x-ray scan . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 5 . | 10 . | |
| Viability (n = 11) | 98 ± 1 | 97 ± 1 | 96 ± 1 | 96 ± 2 | 97 ± 1 |
| CFC (n = 11) | 98 ± 6 | 103 ± 7 | 95 ± 9 | 106 ± 13 | 109 ± 10 |
| BFU-E (n = 11) | 104 ± 10 | 109 ± 17 | 103 ± 20 | 118 ± 28 | 118 ± 23 |
| CFU-GM (n = 11) | 98 ± 7 | 112 ± 8 | 94 ± 7 | 106 ± 8 | 111 ± 8 |
| CFU-Meg (n = 7) | — | — | — | 146 ± 40 | — |
| LTC-IC (n = 11) | 102 ± 12 | 100 ± 15 | 121 ± 31 | 132 ± 23 | 108 ± 22 |
| MNC expansion (n = 3) | — | — | — | 100 ± 12 | — |
| CFC expansion (n = 3) | — | — | — | 94 ± 19 | — |
| T-cell expansion (n = 4) | 104 ± 4 | — | — | 104 ± 4 | 95 ± 2 |
| NK cell expansion (n = 4) | 101 ± 8 | — | — | 98 ± 3 | 103 ± 6 |
| NK cell cytotoxicity (n = 3) | 97 ± 7 | — | — | 101 ± 10 | 107 ± 7 |
Values are percentage of controls, which are set to 100%, ± SEM.
CFCs indicates colony-forming cells; BFU-Es, erythroid burst-forming units; CFU-GMs, granulocyte-macrophage colony-forming units; CFU-Megs, megakaryocyte colony-forming units; LTC-ICs, long-term culture-initiating cells; MNC, mononuclear cell; NK, natural killer.
The exposure of airplane personnel to radiation from extraterrestrial sources is 3 to 6 mSv/y, compared with 1 mSv at ground5 (a sievert [Sv] is a measure of the total radioactive dose, factoring in each type of radiation and its energy), and this may increase the incidence of malignant diseases. The risk of developing acute leukemia is 5.1-fold in those flying more than 5000 hours.6Pilots (and accordingly, their bone marrow) are exposed to a radiation dose of 60 μSv during a 10-hour flight, a dose that is roughly equivalent to 40 hand-luggage controls. Assuming 5 connecting flights, the radiation dose applied to hematopoietic stem cell grafts is 67.5 μSv (7.5 μSv in 5 security checks plus 60 μSv during a 10-hour overseas flight). This is approximately 1.3% of the dose acquired by pilots every year.
Only vigorously instructed personnel are authorized to obtain, handle (ie, couriers), and deliver hematopoietic stem cell transplants to human beings. Couriers are instructed by international guidelines to refuse the checking of stem cell transplants by means of x-ray–assisted devices. Thus, unsatisfying discussions are frequently entailed, ultimately resulting in the visual and tactual inspection of the graft by security staff who are unskilled to handle vital human organs. On the other hand, thick-walled containers containing human organs may be targets for bootlegging illegal goods or weapons. Our data suggest that the radiation emitted from x-ray hand-luggage control systems at airports does not harm the cellular components of peripheral blood stem cell grafts. Thus, a customary examination using x-ray devices should facilitate the delivery of stem cell grafts and minimize the risk for the graft, for the people obliged to check this, as well as for the passengers joining the flights.
We thank Ewald Dworschak and the security staff of the Innsbruck International Airport for their help.
Supported by the “Verein zur Förderung der Krebsforschung an der Universitätsklinik Innsbruck.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas L. Petzer, Abteilung für Hämatologie & Onkologie, Universitätsklinik für Innere Medizin, Anichstrasse 35, A-6020 Austria; e-mail:andreas.petzer@uibk.ac.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal