BCR/ABL oncogenic tyrosine kinase activates STAT5, which plays an important role in leukemogenesis. The downstream effectors of the BCR/ABL→STAT5 pathway remain poorly defined. We show here that expression of the antiapoptotic protein A1, a member of the Bcl-2 family, and the serine/threonine kinase pim-1 are enhanced by BCR/ABL. This up-regulation requires activation of STAT5 by the signaling from SH3+SH2 domains of BCR/ABL. Enhanced expression of A1 and pim-1 played a key role in the BCR/ABL-mediated cell protection from apoptosis. In addition, pim-1 promoted proliferation of the BCR/ABL-transformed cells. Both A1 and pim-1 were required to induce interleukin 3–independent cell growth, inhibit activation of caspase 3, and stimulate cell cycle progression. Moreover, simultaneous up-regulation of both A1 and pim-1 was essential for in vitro transformation and in vivo leukemogenesis mediated by BCR/ABL. These data indicate that induction of A1 and pim-1 expression may play a critical role in the BCR/ABL-dependent transformation.
Introduction
Emergence of the Philadelphia chromosome (Ph1) results from the t(9;22) reciprocal chromosomal translocation present in most, if not all, patients with chronic myelogenous leukemia (CML)1,2 and subsets of patients with an acute myeloblastic leukemia (AML) and acute lymphoblastic leukemia (ALL).3 This translocation results in the formation ofbcr/abl hybrid genes derived from relocation of the c-abl gene from chromosome 9 to the bcr gene locus on chromosome 22. The bcr/abl hybrid genes produce BCR/ABL fusion proteins (p230, p210, and p185) that transform immature hematopoietic cells in vitro4-6 and cause a CML or acute leukemialike syndromes in mice.7-9 The process of transformation is accompanied by growth factor independence,10 reduced susceptibility to apoptosis,11 and altered motility of the BCR/ABL-expressing cells.12 The BCR/ABL oncoproteins display a constitutive tyrosine kinase activity, a feature necessary for their leukemogenic capacity.13 The BCR/ABL tyrosine kinase modulates signaling pathways activating several proteins including signal transducer and activator of transcription 5 (STAT5).14-17 Studies using STAT5 dominant-active and dominant-negative mutants showed that STAT5 is essential for the transformation of hematopoietic cells by BCR/ABL.16-19 On the other hand, experiments performed on murine bone marrow cells from STAT5 knockout mice (STAT5 KO cells) indicated that although STAT5 was not essential for BCR/ABL-dependent in vitro transformation, it was important for development of BCR/ABL-induced myeloid leukemia in vivo.20 However, the results from STAT5 KO cells should be interpreted with caution because of the high level of redundancy in the signaling pathways in hematopoietic cells. Thus, it is possible that bone marrow cells (BMCs) in surviving STAT5 KO mice21 are able to develop overlapping pathways, which overcome the absence of STAT5. In accordance with this speculation, we observed that BCR/ABL-transformed cells were able to recover partially from expression of the STAT5B dominant-negative mutant,14suggesting activation of redundant pathways. In conclusion, STAT5 seems to play an important role in the oncogenesis induced by BCR/ABL as well as other oncogenic tyrosine kinases such as TEL/JAK2 and NPM/ALK.22 The exact mechanisms regulated by STAT5 during cell transformation remain unclear.
STAT5 belongs to the family of STATs, which are latent transcription factors that become activated by phosphorylation on tyrosine and also on serine.23,24 The activated STATs dimerize, translocate to the nucleus, bind specific DNA elements, and induce transactivation of numerous genes. Activated STAT5 enhances the expression of various proteins including cyclin D1,25,26bcl-xL,25-27 CIS,28 A1,29pim-1,30 Id-1,31 OSM,32 c-fos, and c-jun.23 The role of cyclin D1,33bcl-xL,16 CIS,34 and c-Jun35 in BCR/ABL-mediated transformation has already been established. This work is focused on A1 and pim-1, the 2 proteins regulating cell apoptosis and proliferation.
A1, a member of the Bcl-2 gene family has been shown to transiently protect from apoptosis 32Dcl3 hematopoietic cells upon growth factor withdrawal.29 Bfl-1, the human homologue of A1, promotes cell survival36 and cooperates with the E1A oncogene in cell transformation.37 In addition, Bfl-1/A1 is able to suppress apoptosis induced by tumor necrosis factor α (TNF-α)38 and by ligation of an antigen to receptor.39 Mice lacking A1-a, subtype of the A1 gene, display accelerated apoptosis of neutrophils.40 A1 is localized in the mitochondria and is able to inhibit mitochondrial depolarization and release of cytochrome c.41 The exact mechanism of A1 function is not known, but probably involves binding and inactivation of the proapoptotic proteins such as Bax.42The above findings raise the possibility that A1 could be a mediator of the antiapoptotic activity and, perhaps, other functions of BCR/ABL.
Expression of pim-1 serine/threonine kinase correlated with cell mitogenesis and survival independent of growth factors.43Pim-1 synergized with c-Myc in leukemogenesis44 and enhanced transcriptional activity of c-Myb.45 Both c-Myc and c-Myb are essential for BCR/ABL leukemogenesis.46-49In addition, Cdc25A cell cycle phosphatase, a direct transcriptional target for c-Myc, is a substrate for pim-1 kinase50 and may tie pim-1–mediated mitogenic signals to the cell cycle machinery. Pim-1 may also be associated with protection of hematopoietic cells from apoptosis induced by genotoxic stress or growth factor withdrawal.51 Thus, pim-1 could potentially be involved in promotion of the cell cycle progression and inhibition of apoptosis in BCR/ABL-expressing cells.
We report here that expression of A1 and pim-1 is induced by BCR/ABL and that both are required in the BCR/ABL-mediated leukemogenesis.
Materials and methods
Plasmids
A1 and pim-1 complementary DNAs (cDNAs) in pME18S vector were obtained from Dr A. Mui (DNAX Research Institute, Palo Alto, CA). In addition, A1 cDNA in pBluescript was kindly sent by Dr M. Prystowsky (Albert Einstein College of Medicine, New York, NY). A1 sense and antisense (AS) cDNAs were cloned into pMSCV-neo retroviral construct. In addition, A1 AS was cloned into MigR1-IRES-GFP retroviral construct (gift of Dr W. Pear, University of Pennsylvania Medical School, Philadelphia). Flag-tagged pim-1 wild-type (WT) and the K67M kinase-defective mutant were from Dr S. Ness (University of New Mexico School of Medicine, Albuquerque). Both were cloned into pMX-puro and Mig-IRES-GFP retroviral constructs. BCR/ABL WT and BCR/ABLΔΔ mutant (lacking both SH3 and SH2 domains) were described before14and subcloned into the MigR1-IRES-GFP plasmid. BCR/ABL was also subcloned into the pMX-puro plasmid.
Cells
The 32Dcl3 parental cells and cells expressing various BCR/ABL proteins were described before.14 32DHN cells (expressing only hygromycin- and neomycin-resistance genes) and 32DHN-A1 cells overexpressing A1 were obtained from Dr M. Prystowsky. FD/neo cells (FDCP1 cells expressing neomycin-resistance gene) and FD/mpim44 cells (FDCP1 cells overexpressing mouse pim-1) were from Dr M. Lilly (Loma Linda University School of Medicine, Loma Linda, CA). Cell lines were maintained in Iscoves modified Dulbecco medium (IMDM) supplemented with 10% fetal bovine serum (FBS) and 15% WEHI-conditioned medium as a source of interleukin 3 (IL-3). BMCs from C57Bl/6 mice (Jackson Laboratory, Bar Harbor, ME) were obtained 6 days after the treatment with 5-fluorouracil (5-FU; 150 mg/kg body weight) as described.52,53 BMCs from patients in CML-blast crisis (CML-BC) were obtained after informed consent was obtained. CD34+ cells were isolated as described.54
Inhibition of BCR/ABL kinase
ABL kinase inhibitor STI571 (imatinib mesylate [Gleevec])55 was obtained from Novartis Pharma (Basel, Switzerland). Cells (106/mL) were incubated for 24 hours with 1 μM STI571 in the presence of IL-3, then washed and used for experiments.
Proliferation and apoptosis
Cells (105/mL) were incubated in a growth factor–free medium. Apoptotic cells were detected on cytospin slides by TACS in situ apoptosis detection kit (Trevigen, Gaithersburg, MD). Proliferation was examined by counting the cells excluding trypan blue.
Cell cycle analysis
Cells (106) were fixed in 70% ethanol for 15 minutes at 4°C, washed, and incubated in 1 mL phosphate-buffered saline (PBS) containing 0.1% NP-40 and 1 mg/mL of DNAse-free RNAse (Boehringer Mannheim, Indianapolis, IN) for 10 minutes at room temperature. DNA was stained by propidium iodide. Cells were analyzed by FACSCalibur (Becton Dickinson, San Jose, CA) using CellQuest Program.
Retroviral infections
Infections with BMCs were performed as described14,52,53 with some modifications. Briefly, BMCs from mice pretreated with 5-FU were stimulated with stem cell factor (SCF) and IL-3 for 48 hours and then cocultivated with retrovirus-producing Bosc23 packaging cells transfected with the BCR/ABL-IRES-GFP, BCR/ABLΔΔ-IRES-GFP, or IRES-GFP (control) retroviral constructs. Nonadherent green fluorescent protein-positive (GFP+) cells were obtained 72 hours later by cell sorting. These cells were expanded by 3-day stimulation with SCF plus IL-3 and subjected to the second round of infection with the viruses carrying A1 and/or pim-1 cDNA (sense, AS, or point-mutant) and puromycin- or neomycin-resistance sequences. Control cells were infected with the retroviruses containing the antibiotic resistance only. In general, GFP+ cells were cocultivated with the Bosc23 cells transfected with the retroviral constructs for 72 hours in the presence of growth factors. To increase the infection efficiency an equal volume of fresh retroviral supernatant was added to the cocultivation medium every 24 hours (the retroviral titers were usually between 1 and 3 × 106 U/mL as determined by measuring the infection efficiency on Rat-2 cells54). After infection cells were incubated for 4 days in the presence of growth factors, G418 and/or puromycin, and then spun down on Lympholyte-M (Cedarlane Laboratories, Hornby, ONT, Canada) to eliminate dead cells (about 20%-30% of cells were recovered). GFP+, G418, and/or puromycin-resistant cells were used for the experiments. In general, cell lines were infected as described.5632DHN, 32DHN-A1, FD/neo, and FD/mpim44 cells were infected with BCR/ABL-IRES-GFP, BCR/ABLΔΔ-IRES-GFP, or IRES-GFP (control) viruses. 32Dcl3 cells were infected with pMX-puro or pMX-BCR/ABL-puro retrovirus. Freshly established puromycin-resistant mixed populations were infected with pim-1(K67M)-IRES-GFP, A1(AS)-IRES-GFP, or IRES-GFP viruses. GFP+ cells were obtained by sorting and used for experiments. 32DHN-A1 cells were infected with pMX-pim-1-puro. Freshly established puromycin-resistant 32DHN-A1 clones overexpressing pim-1 (data not shown) were infected with the BCR/ABLΔΔ-IRES-GFP or IRES-GFP retrovirus. GFP+ cells were obtained by sorting and used for experiments.
Northern analysis
Cells were starved from IL-3 and serum (incubation in IMDM supplemented with 0.1% bovine serum albumin [BSA]) for 8 hours. Total RNA was isolated and probed for the presence of A1, pim-1, and GADPH using specific full-length cDNA probes end-labeled with (α32P)dCTP (NEN Life Science Products, Boston, MA).
Western analysis
Cells were solubilized in lysis buffer (10 mM Hepes, pH 7.5, 150 mM NaCl, 1% NP-40, 10% glycerol, 5 mM EDTA, 1 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, 50 mM NaF, 1 mM Na3VO4 and 10 μg/mL each aprotinin and leupeptin). The lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and examined by Western analysis using primary antibodies recognizing the following proteins: A1 (T-18 + C-19, Santa Cruz Biotechnology, Santa Cruz, CA), pim-1 (C-20 + N-16, Santa Cruz), Flag-2 (Sigma Chemical, St Louis, MO), actin (C-11, Santa Cruz), tyrosine phosphorylated proteins (P.Tyr; PY20 from Oncogene Research Products, Cambridge, MA, and 4G10 from Upstate Biotechnology, Lake Placid, NY), c-ABL (Ab-3, Oncogene), active caspase 3 (CM1, generous gift from IDUN Pharmaceuticals, La Jolla, CA), Bad (K-17, Santa Cruz), Bax (Ab-5, Oncogene), Bcl-2 (N-17, Santa Cruz), and Bcl-xL (Transduction Laboratories, Lexington, KY). Species-specific secondary antibodies linked to the horseradish peroxidase (HRP) were from Amersham Life Sciences (Arlington Heights, IL). Bands were detected with an enhanced chemiluminescence (ECL) kit (Amersham Life Sciences).
pim-1 kinase reaction
Kinase reaction was done essentially as described by Mochizuki and colleagues.50 Briefly, pim-1 was immunoprecipitated from the total cell lysates and reactions were carried out in a 30-μL volume containing 25 mM Hepes (pH 7.5), 10 mM MgCl2, 0.5 mM dithiothreitol, 10 μCi (0.37 MBq) [γ-32P] adenosine triphosphate (ATP), 10 μM ATP, and 10 μg histone H1 (Roche Molecular Biochemicals, Indianapolis, IN). The samples were incubated at 22°C for 30 minutes and then reactions were terminated by the addition of 30 μL 2 times sample buffer (100 μM Tris-HCl [pH6.8], 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, and 0.2% bromophenol blue). Samples were subjected to SDS-PAGE, followed by autoradiography.
Leukemogenesis in mice
C57Bl/6 mice (Taconic Farms, Germantown, NJ) received total body irradiation (450 rads). The next day they were injected intravenously with 105 GFP+ BMCs infected with retroviruses carrying BCR/ABL (WT or ΔΔ mutant)-IRES-GFP followed by A1 and/or pim-1 cDNAs. Terminally ill mice were killed and examined for development of leukemia as described.14 53 In brief, various organs (spleen, liver, lymph nodes, brain, lungs, kidneys, gastrointestinal tract, and skin) were harvested, fixed, embedded, sectioned, stained, and examined under the microscope. A chloroacetate esterase (Leder) staining confirmed myeloid differentiation of the leukemic cells in the selected tissue sections. Animal studies were approved by the Institutional Animal Care and Use Committee at Temple University.
Results
The expression of A1 and pim-1 is regulated by BCR/ABL SH3+SH2-STAT5 pathway
Our previous study revealed that signaling by BCR/ABL and in particular by its SH3+SH2 region activated STAT5, a signaling step essential for the BCR/ABL-mediated leukemogenesis.14To define in more detail this novel cell transforming pathway, we examined expression of the 2 downstream effectors of STAT5: A1 and pim-1.29 30
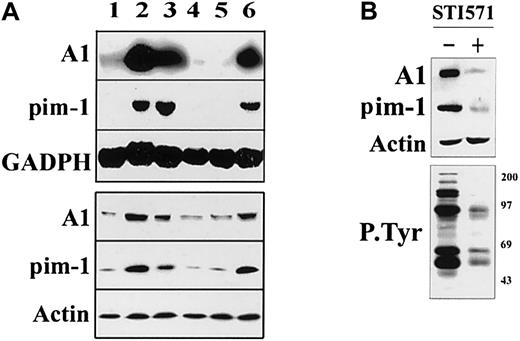
For this purpose, messenger RNA (mRNA) and protein levels of A1 and pim-1 were determined in 32Dcl3 parental cells and clones expressing either BCR/ABL WT, BCR/ABL ΔΔ mutant (SH3+SH2 deletion), which is unable to activate STAT5, STAT5 dominant-active mutant (DAM), STAT5 WT, or BCR/ABLΔΔ and STAT5-DAM. As shown in Figure1A, expression of A1 and pim-1 on both mRNA and protein levels was markedly up-regulated in cells in which STAT5 was active (BCR/ABL, STAT5-DAM, BCR/ABLΔΔ, and STAT5-DAM) in comparison to cells with an inactive STAT5 (parental, STAT5-WT, BCR/ABLΔΔ).14 To confirm that A1 and pim-1 genes represent the BCR/ABL targets also in the patient-derived leukemic cells, their expression was analyzed in CML patient cells, before and after treatment with the ABL kinase inhibitor STI571. Western analysis clearly demonstrated that the primary leukemic cells expressed both A1 and pim-1 (Figure 1B). Furthermore, this expression was dependent on the BCR/ABL kinase activity, because it was profoundly diminished after cell treatment with STI571.
Signaling from the BCR/ABL SH3+SH2 region induces expression of the A1 and pim-1 genes.
(A) 32Dcl3 parental cells (lane 1) or clones expressing WT BCR/ABL (lane 2), STAT5B dominant-active mutant (STAT5B-DAM, lane 3), STAT5B WT (lane 4), BCR/ABLΔΔ (lane 5), BCR/ABLΔΔ, and STAT5B-DAM (lane 6) were starved (8 hours) from IL-3. Expression of the indicated genes was assessed by Northern analysis (upper panel) or Western analysis (lower panel) using specific probes. Equal RNA and protein loading was confirmed by detection of GADPH and actin, respectively. Results represent 3 experiments using cell lines described previously.14 (B) A1 and pim-1 were detected by Western blot assays in total cell lysates obtained from CML-BC cells preincubated with 1 μM STI571 for 24 hours in the presence of IL-3 (upper panel). Inhibition of BCR/ABL kinase activity by STI571 was confirmed by Western blot assay with use of anti-P.Tyr antibodies (lower panel). Results are representative of 2 separate experiments.
Signaling from the BCR/ABL SH3+SH2 region induces expression of the A1 and pim-1 genes.
(A) 32Dcl3 parental cells (lane 1) or clones expressing WT BCR/ABL (lane 2), STAT5B dominant-active mutant (STAT5B-DAM, lane 3), STAT5B WT (lane 4), BCR/ABLΔΔ (lane 5), BCR/ABLΔΔ, and STAT5B-DAM (lane 6) were starved (8 hours) from IL-3. Expression of the indicated genes was assessed by Northern analysis (upper panel) or Western analysis (lower panel) using specific probes. Equal RNA and protein loading was confirmed by detection of GADPH and actin, respectively. Results represent 3 experiments using cell lines described previously.14 (B) A1 and pim-1 were detected by Western blot assays in total cell lysates obtained from CML-BC cells preincubated with 1 μM STI571 for 24 hours in the presence of IL-3 (upper panel). Inhibition of BCR/ABL kinase activity by STI571 was confirmed by Western blot assay with use of anti-P.Tyr antibodies (lower panel). Results are representative of 2 separate experiments.
Role of A1 and pim-1 in BCR/ABL-mediated growth factor independence
Prevention of cell apoptosis and induction of growth factor independence are among the major functions of BCR/ABL.57To examine the role of A1 and pim-1 in these processes, 2 types of experiments were performed. First, A1 or pim-1 proteins were up-regulated in cells expressing the BCR/ABLΔΔ mutant, and second, A1 protein and pim-1 kinase were down-modulated in the BCR/ABL+ cells by introduction of the A1 AS cDNA or kinase-deficient mutant of pim-1 (K67M).
Elevation of the expression of A1 protein in 32DHN-A1 cells to the level observed in BCR/ABL+ counterparts (Figure2A, left panel) caused only a modest protection from apoptosis in growth factor–free medium (Figure 2A, right panel). Eventually all cells died in apoptosis after 5 days of incubation without growth factor. Similar up-regulation of A1 in the BCR/ABLΔΔ cells caused more pronounced early protection from apoptosis, but eventually all cells also died on day 5. In turn, down-regulation of A1 protein expression by transfection with the A1 AS cDNA, sensitized the BCR/ABL+ cells to apoptosis in the growth factor– and serum-free conditions (Figure 2B, triangles), but not in the absence of IL-3 and presence of serum (Figure 2B, squares).
Role of A1 in the BCR/ABL-mediated leukemogenesis.
(A, left panel) 32DHN control cells and cells expressing A1 protein (32DHN-A1) were infected with the retrovirus carrying BCR/ABL WT-IRES-GFP, BCR/ABLΔΔ-IRES-GFP, or IRES-GFP. BCR/ABL and A1 proteins were detected in growth factor–starved GFP+ cells by Western analysis. Actin was detected as control for protein loading. (A, right panel) Cells were incubated in the absence of IL-3 for 5 days and apoptosis was detected by the in vitro apoptosis detection test. (B) 32Dcl3 cells expressing BCR/ABL were infected with retrovirus carrying A1 AS cDNA-IRES-GFP or with empty (E) retrovirus. GFP+ cells were analyzed for the expression of A1 protein (upper box) and actin (lower box). Apoptosis was examined in the absence of IL-3 (squares) or in the absence of IL-3 and serum (triangles). Results represent 4 experiments.
Role of A1 in the BCR/ABL-mediated leukemogenesis.
(A, left panel) 32DHN control cells and cells expressing A1 protein (32DHN-A1) were infected with the retrovirus carrying BCR/ABL WT-IRES-GFP, BCR/ABLΔΔ-IRES-GFP, or IRES-GFP. BCR/ABL and A1 proteins were detected in growth factor–starved GFP+ cells by Western analysis. Actin was detected as control for protein loading. (A, right panel) Cells were incubated in the absence of IL-3 for 5 days and apoptosis was detected by the in vitro apoptosis detection test. (B) 32Dcl3 cells expressing BCR/ABL were infected with retrovirus carrying A1 AS cDNA-IRES-GFP or with empty (E) retrovirus. GFP+ cells were analyzed for the expression of A1 protein (upper box) and actin (lower box). Apoptosis was examined in the absence of IL-3 (squares) or in the absence of IL-3 and serum (triangles). Results represent 4 experiments.
Ectopic expression of pim-1 protein in FD/mpim44 cells at the level comparable to one seen in the BCR/ABL WT-transfected FD cells (Figure 3A) protected these cells from apoptosis in the growth factor–free medium during the first 3 days of culture, but eventually almost all cells died by day 5 (Figure 3B). Increased expression of pim-1 in the BCR/ABLΔΔ cells exerted a more significant antiapoptotic effect because only less than 40% of the cells died after 5 days. Although the FD/mpim44 cells were unable to proliferate in the absence of IL-3, their growth was partially re-established after transfection with the BCR/ABLΔΔ mutant (FD/mpim44+BCR/ABLΔΔ cells; Figure 3C). The latter cells grew more slowly than the BCR/ABLWT+ cells, probably due to the fact that some of them were dying in apoptosis. To confirm the observation that pim-1 is important for growth factor independence of BCR/ABL+ cells, pim-1(K67M) kinase-deficient mutant was expressed in BCR/ABLWT+ 32Dcl3 cells. Expression of the FLAG-tagged pim-1(K67M) protein and inhibition of the pim-1 kinase activity was confirmed by Western analysis and in vitro kinase assay, respectively (Figure 3D). The mutant was able to interfere with the BCR/ABL-mediated protection from apoptosis and caused the death of about 50% of the transfected cells (BCR/ABL+pim-1[K67M] cells) in the absence of IL-3 (Figure 3E). This effect was associated with an about 2.5-fold decrease in the number of cells in the liquid culture as compared to the cells expressing BCR/ABL WT alone (Figure 3F). This observation was confirmed in the clonogenic assay performed in the methylcellulose semisolid medium: expression of the pim-1(K67M) markedly decreased the number and size of colonies formed by the BCR/ABL WT+ cells (data not shown).
Role of pim-1 in the BCR/ABL-mediated leukemogenesis.
(A) FDCP1 cells expressing neomycin-resistance gene (FD/neo) and cells overexpressing mouse pim-1 (FD/mpim44) were infected with retrovirus carrying BCR/ABLWT-IRES-GFP, BCR/ABLΔΔ-IRES-GFP, or IRES-GFP (E). BCR/ABL and pim-1 proteins were detected in growth factor–starved GFP+ cells by Western analysis. Actin was detected as control for protein loading. (B) Apoptosis was examined in IL-3–free conditions as described in the legend to Figure 2. (C) Cell proliferation in IL-3–free medium was assessed by trypan blue exclusion. (D) 32Dcl3 cells were infected with retrovirus carrying BCR/ABL or with empty virus (E). After short selection in puromycin freshly established mix populations were infected with retrovirus carrying pim-1(K67M)FLAG-IRES-GFP or IRES-GFP (E). GFP+cells were obtained by FACS and BCR/ABL and pim-1(K67M)FLAG were detected in growth factor–starved cells by Western analysis using anti-ABL and anti-FLAG antibodies, respectively. The kinase reactions were performed in anti–pim-1 immunoprecipitates containing [γ-32P]ATP and histone H1 as a substrate, which were then separated on SDS-PAGE and visualized by autoradiography (bottom box). Apoptosis (E) and proliferation potential (F) in the absence of IL-3 were assessed as described in the legend to Figure 2. Results represent at least 3 experiments.
Role of pim-1 in the BCR/ABL-mediated leukemogenesis.
(A) FDCP1 cells expressing neomycin-resistance gene (FD/neo) and cells overexpressing mouse pim-1 (FD/mpim44) were infected with retrovirus carrying BCR/ABLWT-IRES-GFP, BCR/ABLΔΔ-IRES-GFP, or IRES-GFP (E). BCR/ABL and pim-1 proteins were detected in growth factor–starved GFP+ cells by Western analysis. Actin was detected as control for protein loading. (B) Apoptosis was examined in IL-3–free conditions as described in the legend to Figure 2. (C) Cell proliferation in IL-3–free medium was assessed by trypan blue exclusion. (D) 32Dcl3 cells were infected with retrovirus carrying BCR/ABL or with empty virus (E). After short selection in puromycin freshly established mix populations were infected with retrovirus carrying pim-1(K67M)FLAG-IRES-GFP or IRES-GFP (E). GFP+cells were obtained by FACS and BCR/ABL and pim-1(K67M)FLAG were detected in growth factor–starved cells by Western analysis using anti-ABL and anti-FLAG antibodies, respectively. The kinase reactions were performed in anti–pim-1 immunoprecipitates containing [γ-32P]ATP and histone H1 as a substrate, which were then separated on SDS-PAGE and visualized by autoradiography (bottom box). Apoptosis (E) and proliferation potential (F) in the absence of IL-3 were assessed as described in the legend to Figure 2. Results represent at least 3 experiments.
Because A1 seems to be preferentially involved in protection from apoptosis29 and pim-1 appears to be engaged in both cell proliferation and apoptosis,43 we decided next to determine if the functions of A1 and pim-1 are complementary or overlapping.
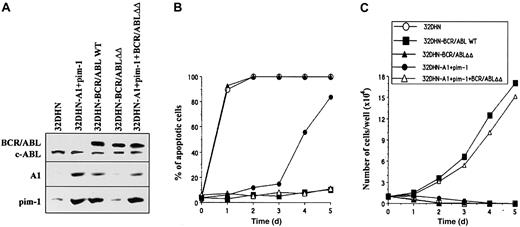
Coexpression of A1 and pim-1 in 32DHN cells (Figure4A) delayed apoptosis in the absence of IL-3, but almost all cells eventually died after 5 days of culture (Figure 4B). However, the coexpression of A1 and pim-1 rescued the factor-independent growth of the cell transformation–defective BCR/ABLΔΔ mutant. BCR/ABLΔΔ + A1 + pim-1 cells survived in the absence of IL-3 (Figure 4B) and displayed growth factor–independent proliferation comparable to that observed in the BCR/ABL WT+ cells (Figure 4C). Clonogenic assay performed in the semisolid medium confirmed this observation (data not shown). In conclusion, A1 and pim-1 seem to complement each other as downstream effectors of BCR/ABL in protection from apoptosis and promotion of growth factor independence.
Complementary functions of A1 and pim-1 in restoring the transforming capacity of the BCR/ABLΔΔ mutant.
32DHN control cells (Figure 2) and 32DHN-A1+pim-1 (overexpressing A1 and pim-1) cells were infected with BCR/ABL WT-IRES-GFP or BCR/ABLΔΔ-IRES-GFP retroviruses, respectively. GFP+cells were analyzed for (A) expression of BCR/ABL, A1 and pim-1 proteins, (B) apoptosis in IL-3–deficient medium, and (C) growth factor–independent proliferation (details are given in legends to Figures 2 and 3). Results represent 3 experiments.
Complementary functions of A1 and pim-1 in restoring the transforming capacity of the BCR/ABLΔΔ mutant.
32DHN control cells (Figure 2) and 32DHN-A1+pim-1 (overexpressing A1 and pim-1) cells were infected with BCR/ABL WT-IRES-GFP or BCR/ABLΔΔ-IRES-GFP retroviruses, respectively. GFP+cells were analyzed for (A) expression of BCR/ABL, A1 and pim-1 proteins, (B) apoptosis in IL-3–deficient medium, and (C) growth factor–independent proliferation (details are given in legends to Figures 2 and 3). Results represent 3 experiments.
Both A1 and pim-1 are required for BCR/ABL-mediated leukemogenesis
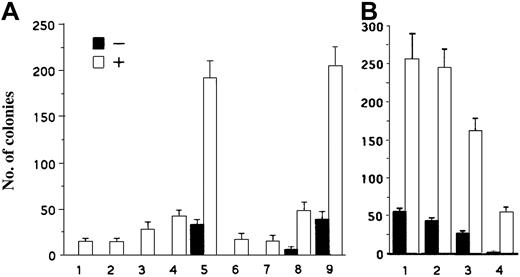
To determine if A1 or pim-1 or both play an essential role in BCR/ABL-mediated transformation of normal BMCs, we tested whether overexpression of A1 or pim-1 rescues the impaired transforming capacity of BCR/ABLΔΔ mutant, and whether down-modulation of A1 or pim-1 inhibits cell transformation mediated by BCR/ABL WT. BMCs from 5-FU–treated mice were infected with retroviral vectors carrying several different forms of BCR/ABL, A1, and pim-1, and analyzed in the in vitro colony formation assay in the growth factor–free medium. BMCs infected with BCR/ABL WT formed numerous colonies in the absence of IL-3 and many more arose in the presence of the threshold concentration of IL-3 (Figure 5A, group 5). The control, empty retrovirus-infected cells, did not form any colonies in the absence of IL-3 and only a few grew in the 0.1 U/mL IL-3 (Figure5A, group 1). BCR/ABLΔΔ mutant alone did not induce any IL-3–independent colonies and did not increase the number of colonies in IL-3 in comparison to the control cells (Figure 5A, group 6). Furthermore, no colonies were found in the absence of IL-3 and no significant colony formation enhancement was noticed in the cells carrying either A1 or pim-1 (Figure 5, group 2 and group 3, respectively) in comparison to control (Figure 5A, group 1). When BCR/ABLΔΔ+ cells were infected with either A1 or pim-1 retrovirus, their clonogenic activity was not affected by A1 or was only moderately increased by pim-1 (Figure 5A, compare group 7 with group 6, and group 8 with group 6, respectively). However, coinfection with both A1 and pim-1 fully restored the impaired transforming capacity of the BCR/ABLΔΔ mutant as compared to BCR/ABL WT (Figure5A, compare group 9 with group 5). Conversely, infection of BCR/ABL WT-expressing BMCs with either A1 AS cDNA or pim-1(K67M) mutant had no effect, or exerted only a mild effect on their clonogenicity, respectively (Figure 5B, compare group 2 with group 1, and group 3 with group 1, respectively). However, coinfection with both A1 antisense + pim-1(K67M) mutant exerted a profound negative effect on the BCR/ABL-induced colony formation (Figure 5B, compare group 4 with group 1).
Complementary effects of A1 and pim-1 in the BCR/ABL-mediated transformation of primary BMCs.
(A) A1 and pim-1 rescued transforming capacity of the BCR/ABLΔΔ mutant. Cells were infected with BCR/ABL WT-IRES-GFP (WT-GFP), BCR/ABL ΔΔ-IRES-GFP (ΔΔ-GFP) or with IRES-GFP (GFP) retroviruses. After 3 days in culture GFP+cells were isolated and coinfected with retroviruses carrying A1, pim-1, or with equivalent empty retroviruses (E). Groups are: 1, E+E+GFP; 2, A1+E+GFP; 3, E+pim-1+GFP; 4, A1+pim-1+GFP; 5, E+E+WT-GFP; 6, E+E+ΔΔ-GFP; 7, A1+E+ΔΔ-GFP; 8, E+pim-1+ΔΔ-GFP; 9, A1+pim-1+ΔΔ-GFP. (B) Simultaneous inhibition of A1 (by the AS cDNA) and pim-1 (by the dominant-negative K67M mutant) reduced transforming capacity of the BCR/ABL. Cells were infected with BCR/ABL WT-IRES-GFP retrovirus. GFP+ cells were coinfected with retroviruses containing A1 AS cDNA(AS), pim-1(K67M) dominant-negative mutant, or with equivalent empty retroviruses (E). Groups are: 1, WT-GFP+E+E; 2, WT-GFP+A1(AS)+E; 3, WT-GFP+E+pim-1(K67M); 4, WT-GFP+A1(AS)+pim-1(K67M). After infection 104 cells were plated in methylcellulose in the absence (−) or presence (+) of the threshold concentration (0.1 U/mL) of murine recombinant IL-3. Results (mean ± SD) are from 3 experiments.
Complementary effects of A1 and pim-1 in the BCR/ABL-mediated transformation of primary BMCs.
(A) A1 and pim-1 rescued transforming capacity of the BCR/ABLΔΔ mutant. Cells were infected with BCR/ABL WT-IRES-GFP (WT-GFP), BCR/ABL ΔΔ-IRES-GFP (ΔΔ-GFP) or with IRES-GFP (GFP) retroviruses. After 3 days in culture GFP+cells were isolated and coinfected with retroviruses carrying A1, pim-1, or with equivalent empty retroviruses (E). Groups are: 1, E+E+GFP; 2, A1+E+GFP; 3, E+pim-1+GFP; 4, A1+pim-1+GFP; 5, E+E+WT-GFP; 6, E+E+ΔΔ-GFP; 7, A1+E+ΔΔ-GFP; 8, E+pim-1+ΔΔ-GFP; 9, A1+pim-1+ΔΔ-GFP. (B) Simultaneous inhibition of A1 (by the AS cDNA) and pim-1 (by the dominant-negative K67M mutant) reduced transforming capacity of the BCR/ABL. Cells were infected with BCR/ABL WT-IRES-GFP retrovirus. GFP+ cells were coinfected with retroviruses containing A1 AS cDNA(AS), pim-1(K67M) dominant-negative mutant, or with equivalent empty retroviruses (E). Groups are: 1, WT-GFP+E+E; 2, WT-GFP+A1(AS)+E; 3, WT-GFP+E+pim-1(K67M); 4, WT-GFP+A1(AS)+pim-1(K67M). After infection 104 cells were plated in methylcellulose in the absence (−) or presence (+) of the threshold concentration (0.1 U/mL) of murine recombinant IL-3. Results (mean ± SD) are from 3 experiments.
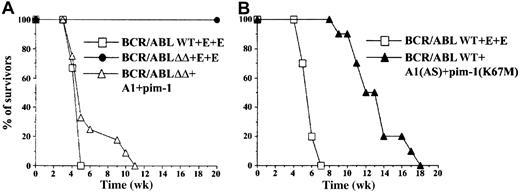
To determine whether A1 and pim-1 also play an essential role in the BCR/ABL-mediated leukemogenesis in vivo, preirradiated syngeneic mice were injected with BMCs infected with BCR/ABL, A1, or pim-1 cDNAs as described above. All mice inoculated with the cells infected with BCR/ABL WT succumbed to leukemia (median survival time [MST] of the mice ± SD = 4.2 ± 0.3 weeks; Figure6A). Histologic examination of the multiple organs (spleen, liver, lymph nodes, lung, skin, central nervous system) revealed the presence of AML in most organs. There was considerable variability in the degree of blast maturation among the mice as well as various involved sites. In one case, the histology resembled more transforming CML rather than an overt acute leukemia. All of the examined organs showed diffuse leukemic infiltrates and tissue destruction. The least differentiated cases displayed the highest mitotic and single-cell necrosis rates and areas of zonal necrosis. Myeloid origin of the blasts was confirmed in such cases by chloroacetoesterase stain. BMCs infected with the BCR/ABLΔΔ retrovirus were unable to induce leukemia in accordance with the previous report.14 Infection of the BCR/ABLΔΔ cells with either A1 or pim-1 retrovirus or coinfection of normal BMCs with A1 and pim-1 retroviruses did not rescue leukemogenic activity of the BCR/ABL mutant (data not shown). However, coinfection of BCR/ABLΔΔ cells with both A1 and pim-1 led to the development of AML in all injected mice (MST = 5.4 ± 1.7 weeks; Figure 6A). There was similar histologic diversity in the degree of blast differentiation and similar pattern of organ involvement as in the mice injected with BCR/ABL WT-carrying cells. Poorly differentiated AML with high mitotic rate and single-cell and zonal necrosis was also seen. To confirm that A1 and pim-1 are essential for BCR/ABL leukemogenesis, the BMCs expressing BCR/ABL WT were coinfected with retroviruses carrying A1 AS cDNA and pim-1(K67M) kinase-deficient mutant. The simultaneous down-modulation of A1 and pim-1 impaired the leukemogenic capacity of the BCR/ABL WT BMCs (MST = 11.4 ± 2.2 weeks,P < .001) as compared to the BMCs expressing only BCR/ABL WT (MST = 4.2 ± 0.3 weeks) without changing the phenotype of disease. The above observations indicate that A1 and pim-1 are essential downstream molecules for BCR/ABL.
A1 and pim-1 restored leukemogenic capacity of the BCR/ABLΔΔ mutant.
Syngeneic mice (10-15 mice/group) were injected intravenously with 105 cells transfected with (A) BCR/ABLΔΔ mutant and A1+pim-1 retroviruses, BCR/ABL wild-type (WT) and empty retroviruses (E+E), or BCR/ABL ΔΔ mutant and empty retroviruses (E+E); and (B) BCR/ABL WT and A1(AS) + pim-1(K67M) mutant retroviruses, or BCR/ABL WT and empty retroviruses (E+E). (Legend for Figure 5 presents detailed characterization of the transformants.). Survival time of the animals was monitored weekly.
A1 and pim-1 restored leukemogenic capacity of the BCR/ABLΔΔ mutant.
Syngeneic mice (10-15 mice/group) were injected intravenously with 105 cells transfected with (A) BCR/ABLΔΔ mutant and A1+pim-1 retroviruses, BCR/ABL wild-type (WT) and empty retroviruses (E+E), or BCR/ABL ΔΔ mutant and empty retroviruses (E+E); and (B) BCR/ABL WT and A1(AS) + pim-1(K67M) mutant retroviruses, or BCR/ABL WT and empty retroviruses (E+E). (Legend for Figure 5 presents detailed characterization of the transformants.). Survival time of the animals was monitored weekly.
A1 and pim-1 work in concert to regulate apoptosis and cell cycle
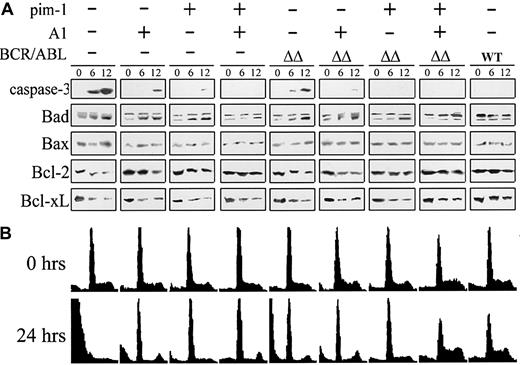
A1 and pim-1 are potent regulators of apoptosis and cell cycle progression.29 43 Because they collaborate in BCR/ABL leukemogenesis, we decided to examine their effect on expression of proapoptotic and antiapoptotic genes and on cell cycle progression in the BCR/ABL cells. After parental and BCR/ABL-, A1-, and/or pim-1–carrying cells (cell clones are described in the legend to Figure 4) were starved from IL-3, time-dependent activation of caspase 3 was detected in the parental, A1, pim-1, BCR/ABLΔΔ, and BCR/ABLΔΔ + A1 cells, whereas in the BCR/ABL WT, A1 + pim-1, BCR/ABLΔΔ + pim-1, and BCR/ABLΔΔ + A1 + pim-1 cells caspase 3 was not activated during 12 hours of starvation from IL-3 (Figure7A).
Both A1 and pim-1 are required to inhibit apoptosis and promote proliferation of the BCR/ABL-carrying cells.
(A) Apoptotic proteins. Parental cells and cell clones expressing BCR/ABL proteins, A1 protein, and/or pim-1 protein were starved from IL-3 for 6 and 12 hours and the expression of proteins regulating apoptosis was detected by Western analysis. Bad phosphorylated (upper band) and unphosphorylated (lower band) proteins are visualized. Results represent 2 experiments. (B) Cell cycle. The same cells were incubated without IL-3 for 0 or 24 hours. DNA content was determined by flow cytometry after staining with propidium iodide. Results are representative of 3 independent experiments.
Both A1 and pim-1 are required to inhibit apoptosis and promote proliferation of the BCR/ABL-carrying cells.
(A) Apoptotic proteins. Parental cells and cell clones expressing BCR/ABL proteins, A1 protein, and/or pim-1 protein were starved from IL-3 for 6 and 12 hours and the expression of proteins regulating apoptosis was detected by Western analysis. Bad phosphorylated (upper band) and unphosphorylated (lower band) proteins are visualized. Results represent 2 experiments. (B) Cell cycle. The same cells were incubated without IL-3 for 0 or 24 hours. DNA content was determined by flow cytometry after staining with propidium iodide. Results are representative of 3 independent experiments.
The high levels of unphosphorylated Bad protein correlated with casapse-3 activation, whereas Bax protein was generally not affected. Importantly, the antiapoptotic proteins Bcl-2 and Bcl-xL remained significantly up-regulated despite the IL-3 starvation in the BCR/ABL WT cells, but only the high expression of Bcl-2 was preserved in pim-1, A1+pim-1, and BCR/ABLΔΔ+A1+pim-1 cells, as compared to the parental and BCR/ABLΔΔ cells. However, after 72 hours starvation Bcl-2 was down-regulated and caspase 3 was activated in A1+pim-1–transfected cells (data not shown).
Cell cycle analysis revealed that in the absence of IL-3 parental cells were composed almost totally of cells containing subdiploid amount of DNA (a signature of apoptosis), whereas the DNA content and cell cycle progression profile of the BCR/ABL WT cells were not significantly affected by the absence of IL-3 (Figure 7B). In turn, the IL-3–starved BCR/ABLΔΔ cells accumulated in the G1 and G2/M phases or displayed a subdiploid amount of DNA. Under such conditions A1 and BCR/ABLΔΔ+A1 cells were arrested in G1 and G2/M phases with a small population already accumulated in subdiploid peak. Pim-1 and BCR/ABLΔΔ+pim-1 cells were mostly in G1 and S phase with minimal accumulation in subdiploid fraction. A1+pim-1 cells were arrested in the G1 and G2/M phases. Coexpression of both A1+pim-1 caused a dramatic change in the cell cycle distribution of the BCR/ABLΔΔ cells by fully compensating for the IL-3 withdrawal as seen in the BCR/ABL WT cells.
Discussion
Activation of STAT5 seems important for the BCR/ABL-mediated leukemogenesis, especially for the development of myeloproliferative disease.14-17,20 The mechanisms operating downstream of the BCR/ABL→STAT5 pathway remain poorly characterized. STAT5 is a powerful transcriptional factor enhancing expression of genes, whose products are involved in regulation of apoptosis and proliferation.58 Because growth factor independence, defined as protection from apoptosis and stimulation of cell cycle progression in the absence of growth factors, is one of the major effects of the BCR/ABL-mediated cell transformation, we decided to examine the role of STAT5 downstream effectors in this process. Here we report that 2 such effectors, antiapoptotic protein A1 (homologue of human Bfl-1) and proto-oncogene pim-1, seem critical for the BCR/ABL leukemogenesis by acting in a complementary fashion. Coexpression of A1 and pim-1 restored the impaired leukemogenic capacity of the BCR/ABLΔΔ mutant by rendering the target myeloid cells growth factor independent in regard to resistance to apoptosis and ability to proliferate. Conversely, down-modulation of A1 and pim-1 retarded the BCR/ABL-dependent leukemogenesis. However, the exact mechanisms of function of A1 and pim-1 in the BCR/ABL-mediated signaling and cell transformation remain to be defined. It seems that A1 and pim-1 cowork downstream of BCR/ABL SH3+SH2→STAT5 pathway.14However, it is likely that lack of SH3 and SH2 domains from BCR/ABL may abrogate activation of the additional signaling pathways. Thus, A1 and/or pim-1 may complement not only STAT5, but also other signaling molecules. This hypothesis is supported by the findings that phosphatidylinositol-3 kinase (PI-3k) and nuclear factor-κB (NF-κB) can contribute to the activation of pim-1 and overexpression of A1, respectively.38 59
We speculate that enhanced A1 expression and pim-1–induced up-regulation of Bcl-2 expression could exert synergistic/additive antiapoptotic effects, as described before.29 A1 and Bcl-2 exert their function by heterodimerizing with the proapoptotic proteins to stabilize mitochondrial membranes and prevent the release of cytochrome c.41,42 Therefore, their functions may be simply additive. However, although both A1 and Bcl-2 can heterodimerize with Bax, only Bcl-2 appears to interact with Bad.42,60 Thus, Bcl-2 seems to regulate antiapoptotic pathways, which may not be accessible to A1. Nevertheless, A1+pim-1 cells started to die after 72 hours of starvation, which was preceded by activation of caspase 3 and down-regulation of Bcl-2 (our unpublished data, May 2000). The latter phenomenon was at least partially due to the caspase 3–dependent cleavage of Bcl-2 because of the appearance of characteristic 22-kd Bcl-2 fragment,61 which can further accelerate apoptosis.62 63
In addition to its antiapoptotic effect, BCR/ABL also regulates the cell cycle.57 Pim-1 may contribute to this process through phosphorylation and activation of Cdc25A.50 Cdc25A, a phosphatase essential for G1→S transition, associates with, dephosphorylates, and activates the cell cycle kinase cyclin E-cdk2.64 Thus, pim-1–dependent activation of Cdc25A may contribute to G1→S transition,65 also in the BCR/ABL-carrying cells. Moreover, BCR/ABL→PI-3k→Akt pathway down-regulates p27kip1 and prevents inhibition of cyclin E-cdk2.66 Therefore, the BCR/ABL→STAT5→pim-1 pathway, which activates Cdc25A, and the BCR/ABL→PI-3k→Akt pathway, which down-regulates p27kip1, may exert a synergistic effect on the G1→S cell cycle progression in the leukemic cells. Furthermore, an additional proliferative signal may be delivered by the pim-1–dependent activation of c-Myb transcriptional factor,45 which is essential for the growth of Philadelphia chromosome–positive cells.47
Thus, it is probable that the combination of the strong antiapoptotic signaling (A1 and Bcl-2) with the mitogenic signal (Cdc25A and c-Myb) rescued transforming ability of the BCR/ABLΔΔ mutant. However, several mice given transplants of BCR/ABLΔΔ+A1+pim-1 cells lived longer than these inoculated with BCR/ABL cells. Therefore, it is likely that in addition to A1 and pim-1, signaling by other STAT5 downstream effectors or even activation of different signaling pathways may be required for full reconstitution of the leukemogenic activity of BCR/ABLΔΔ mutant. Although our studies showed that A1+pim-1 could rescue BCR/ABLΔΔ-mediated transformation of hematopoietic cells in vitro and leukemogenic activity in vivo without up-regulation of Bcl-xL, they should not underscore the potential role of Bcl-xL in BCR/ABL leukemogenesis.16,67 Even if the enforced overexpression of A1 and pim-1 in BCR/ABLΔΔ cells was similar to that observed in BCR/ABL WT cells, overexpression in the former cells is driven by strong retroviral promoters; conversely, overexpression in the latter cells is boosted by BCR/ABL-dependent stimulation of endogenous natural promoters. Thus, equal expression detected by Western analysis may not reflect discrete but significant differences between the expression profiles driven by retroviral promoters and BCR/ABL-mediated stimulation of endogenous promoters (eg, cell cycle–dependent changes). Another possibility is that A1, pim-1, and Bcl-xL may play cooperative and redundant functions downstream of BCR/ABL signaling. It is also worth noting that some signaling from BCR/ABLΔΔ mutant is still necessary for leukemogenesis, because A1+pim-1 induced leukemias only when coexpressed with the mutant. p21Ras is a good candidate for this function because it is activated by the BCR/ABLΔΔ mutant14 and can cooperate with STAT5 in BCR/ABL-mediated transformation.68
In conclusion, our data demonstrate that A1 and pim-1 complement each other in BCR/ABL-mediated malignant cell transformation. They may, therefore, play an important role in the pathogenesis of CML and other, BCR/ABL-related hematopoietic disorders.
Supported in part by National Institutes of Health (NIH) grants CA83700 and CA89052, by grant RPG 98-348-01 from the American Cancer Society, and by grant 501-2-1-03-97/07 from the Medical Center of Postgraduate Education (all to T.S.), and NIH grant CA89194 to M.A.W. T.S. is a Scholar of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tomasz Skorski, Center for Biotechnology, Temple University, Bio-Life Sciences Bldg, Rm 419, 1900 N 12th St, Philadelphia, PA 19122; e-mail: tskorski@astro.temple.edu


![Fig. 3. Role of pim-1 in the BCR/ABL-mediated leukemogenesis. / (A) FDCP1 cells expressing neomycin-resistance gene (FD/neo) and cells overexpressing mouse pim-1 (FD/mpim44) were infected with retrovirus carrying BCR/ABLWT-IRES-GFP, BCR/ABLΔΔ-IRES-GFP, or IRES-GFP (E). BCR/ABL and pim-1 proteins were detected in growth factor–starved GFP+ cells by Western analysis. Actin was detected as control for protein loading. (B) Apoptosis was examined in IL-3–free conditions as described in the legend to Figure 2. (C) Cell proliferation in IL-3–free medium was assessed by trypan blue exclusion. (D) 32Dcl3 cells were infected with retrovirus carrying BCR/ABL or with empty virus (E). After short selection in puromycin freshly established mix populations were infected with retrovirus carrying pim-1(K67M)FLAG-IRES-GFP or IRES-GFP (E). GFP+cells were obtained by FACS and BCR/ABL and pim-1(K67M)FLAG were detected in growth factor–starved cells by Western analysis using anti-ABL and anti-FLAG antibodies, respectively. The kinase reactions were performed in anti–pim-1 immunoprecipitates containing [γ-32P]ATP and histone H1 as a substrate, which were then separated on SDS-PAGE and visualized by autoradiography (bottom box). Apoptosis (E) and proliferation potential (F) in the absence of IL-3 were assessed as described in the legend to Figure 2. Results represent at least 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/12/10.1182_blood.v99.12.4531/4/m_h81222713003.jpeg?Expires=1765900677&Signature=autdl5J1HTgdvG2Dj2vTi50xVqGfSh5INEo8QZ9QA0telR8LhcMDxT~TnQUg8WzEO9-qmD9pJdpc269OCKK3uDfMQhpxdLXH8ucWviX4x8So3vnqwUr~FdPMVM5SBjrGDaAainKs4wa~HqV6-uek1qs1c4zOwYtZK4ofsiHuivQ3e15l-owHrkdRSla2lqNjn-MGvOX1rWyfdl6vZslbsUlOTi1lx9--v2FpqtWuHEDYZ7M-ghmAZ642JGeL3OXFSGlvNGLrK9U~poEtj9bTvXQfcJH1bFF4~l2tIUcmR~bLPV9lmIajg91VzfhwNLkB-ySgk346kbWfQ7wKt6uUYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal