Thalidomide (Thal) achieves responses even in the setting of refractory multiple myeloma (MM). Although increased angiogenesis in MM bone marrow and the antiangiogenic effect of Thal formed the empiric basis for its use in MM, we have shown that Thal and its immunomodulatory analogs (IMiDs) directly induce apoptosis or growth arrest of MM cells, alter adhesion of MM cells to bone marrow stromal cells, inhibit the production of cytokines (interleukin-6 and vascular endothelial growth factor) in bone marrow, and stimulate natural killer cell anti-MM immunity. In the present study, we demonstrate that the IMiDs trigger activation of caspase-8, enhance MM cell sensitivity to Fas-induced apoptosis, and down-regulate nuclear factor (NF)-κB activity as well as expression of cellular inhibitor of apoptosis protein–2 and FLICE inhibitory protein. IMiDs also block the stimulatory effect of insulinlike growth factor–1 on NF-κB activity and potentiate the activity of TNF-related apoptosis-inducing ligand (TRAIL/Apo2L), dexamethasone, and proteasome inhibitor (PS-341) therapy. These studies both delineate the mechanism of action of IMiDs against MM cells in vitro and form the basis for clinical trials of these agents, alone and coupled with conventional and other novel therapies, to improve outcome in MM.
Introduction
Despite the use of high-dose chemotherapy with autologous hematopoietic stem cell transplantation, few, if any, patients with multiple myeloma (MM) are cured. Based upon its antiangiogenic effects and increased angiogenesis observed in MM bone marrow, thalidomide (Thal) was used empirically to treat MM refractory to conventional and high-dose therapy; remarkably, 30% responses were observed.1 When Thal was used together with dexamethasone (Dex) early after diagnosis as initial therapy for MM, responses could be achieved in the majority (80%) of cases.2 Although the in vivo anticancer efficacy of Thal has been attributed to its potent antiangiogenic activity,3 the precise mechanism of its action is unknown. Our studies have demonstrated multiple anti-MM activities of Thal other than antiangiogenesis: direct induction of apoptosis or growth arrest in MM cells,4 inhibition of interleukin-6 and vascular endothelial growth factor (VEGF) secretion triggered by MM cell adhesion to bone marrow stromal cells,5 inhibition of VEGF-mediated MM cell growth and migration,6 inhibition of tumor necrosis factor α (TNF-α) signaling,4 and stimulation of patients' natural killer cell anti-MM immunity.7 Moreover, our studies have confirmed anti-MM, as well as antiangiogenic, effects of Thal in a murine MM model.6 More potent immunomodulatory analogs of Thal (IMiDs) have been developed,8 which are now showing promise in early clinical testing in MM.9
The exact mechanism of the teratogenic effects of Thal is not fully elucidated. One hypothesis suggests that Thal or its breakdown product(s) inhibits the stimulatory effects of insulinlike growth factor 1 (IGF-1) and fibroblast growth factor 2 (FGF-2) on angiogenesis in the developing limb bud,10,11 resulting in limb malformation. This hypothesis is supported by the fact that Thal is an inhibitor of angiogenesis induced by bFGF in a rabbit cornea micropocket assay and that its antiangiogenic activity correlates with teratogenicity, but not with the sedation or the immunosuppression.3,12 Moreover, high pretreatment plasma bFGF levels in patients with progressive MM are associated with subsequent response to Thal therapy.13 Because there is increasing evidence that IGF-1 is also an important growth and survival factor in MM,14,15 inhibition of IGF-1 signaling by Thal may also contribute to its anti-MM activity. Specifically, IGF-1 triggers phosphatidylinositol-3′-kinase (PI-3K) signaling, with downstream mitogen-activated protein kinase (MAPK) activation and proliferation, as well as activation of Akt,15,16 with downstream phosphorylation and inactivation of the proapoptotic Bcl-2 family member Bad, thereby inhibiting caspase activity.16IGF-1 also confers protection against Dex-induced apoptosis in MM cells in vitro.17,18 Finally, IGF-1 enhances the growth of the MM cell line OPM-6 in severe combined immunodeficiency mice,16 further supporting its role in MM pathophysiology and raising the possibility that inhibition of IGF-1 may account, at least in part, for the anti-MM effects of Thal or IMiDs.
In the present study, we demonstrate that IMiD-induced apoptosis in MM cells is associated with several outcomes: activation of caspase-8; enhanced sensitivity to Fas-mediated apoptosis; down-regulation of nuclear factor (NF)-κB activity as well as cellular inhibitor of apoptosis protein 2 (cIAP-2) and FLICE inhibitory protein (FLIP) expression; inhibition of the prosurvival effects of IGF-1; and potentiation of the anti-MM activity of TNF-related apoptosis-inducing ligand (TRAIL/Apo2L), Dex, and the proteasome inhibitor PS-341. These studies both delineate the mechanism of action of IMiDs and provide the framework for clinical application of these agents, alone and in combination, to improve outcome in MM.
Materials and methods
Materials
Thalidomide, IMiD1, and IMiD3 (Celgene, Warren, NJ), and PS-341 (Millennium, Cambridge, MA) were dissolved in dimethylsulfoxide (DMSO). The final concentration of DMSO in all experiments was less than 0.01%, and all treatment conditions were compared with vehicle controls. Apo2L/TRAIL was obtained from Genentech (South San Francisco, CA). The mouse anti–Bcl-2 monoclonal antibody and rabbit anti–intercellular adhesion molecule 1 (ICAM-1) antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); polyclonal antibody against cIAP-2 was from R&D Systems (Minneapolis, MN); and rabbit polyclonal antibody against FLIP was from Upstate Biotechnologies (Lake Placid, NY). IGF-1 and TNF-α were purchased from R&D Systems. Dex was purchased from Sigma (St Louis, MO). The caspase-8 inhibitor (IETD-FMK) and caspase-9 inhibitor (LEHD-FMK) were purchased from Calbiochem (La Jolla, CA) and used at a concentration of 20 μM.
Tissue culture
MM.1S cells were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL); OCI-My-5 cells were obtained from Dr H. A. Messner (Ontario Cancer Institute, Toronto, Ontario, Canada); S6B45 cells were provided by Dr T. Kishimoto (Osaka University, Osaka, Japan). MM patients' bone marrow mononuclear cells were processed by flow cytometric cell sorting in an EPICS cell sorter (Beckman Coulter, Hialeah, FL) to obtain CD38+CD138+ tumor cells of greater than 95% purity. All cells were cultured in RPMI 1640 medium (GIBCO Laboratories, Grand Island, NY) supplemented with 10% charcoal dextran–treated fetal bovine serum (Hyclone, Logan, UT), as well asl-glutamine, penicillin, and streptomycin (GIBCO).
Caspase activity assay
To perform caspase-8 and caspase-9 activity assays, we treated MM.1S cells with IMiD1 (1 μM for 72 hours) or vehicle in medium containing 1% serum and then assayed them using respective ApoAlert Caspase Colorimetric Assay Kits (Clontech, Palo Alto, CA), according to the instructions of the manufacturer.
Immunoblotting analysis
Immunoblotting analysis was performed as previously described.19 Briefly, cells were lysed for 30 minutes on ice in lysis buffer (50 mM Tris-HCl, pH 8, with 120 mM NaCl and 1% NP-40) supplemented with the Complete-TM mixture (Gibco) of proteinase inhibitors. The samples were cleared by microcentrifugation (14 000 rpm for 30 minutes at 4°C) and assessed for protein concentration. Thirty micrograms of protein/sample was subjected to electrophoresis in a 12% sodium dodecyl sulfate–polyacrylamide gel and electroblotted onto nitrocellulose membranes. After 1 hour of incubation in blocking solution (20% IgG-free normal horse serum in phosphate-buffered saline [PBS]), the membranes were exposed overnight at 4°C to the primary antibody. Following washing in PBS, the respective secondary peroxidase–labeled antibody was applied at 1:10 000 dilution for 1 hour at room temperature. Proteins were visualized using enhanced chemiluminescence.
Evaluation of NF-κB activity
The DNA binding activity of NF-κB in MM.1S cells was quantified by enzyme-linked immunosorbent assay using the Trans-AM NF-κB p65 Transcription Factor Assay Kit (Active Motif North America, Carlsbad, CA), according to the instructions of the manufacturer. Briefly, nuclear extracts were prepared as previously described20 and incubated in 96-well plates coated with immobilized oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) containing a consensus (5′-GGGACTTTCC-3′) binding site for the p65 subunit of NF-κB. NF-κB binding to the target oligonucleotide was detected by incubation with primary antibody specific for the activated form of p65 (Active Motif North America), visualized by anti-IgG horseradish peroxidase conjugate and Developing Solution, and quantified at 450 nm with a reference wavelength of 655 nm. Background binding, obtained by incubation with a 2-nucleotide mutant oligonucleotide (5′-AGTTGAGGCCACTTTCCCAGGC-3′), was subtracted from the value obtained for binding to the consensus DNA sequence.
MTT colorimetric survival assay
The survival of MM cells was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay, as previously described.19 Cells were plated in 48-well plates at 70% to 80% confluence and then treated as indicated. At the end of each treatment, cells were incubated with 1 mg/mL MTT for 4 hours at 37°C; a mixture of isopropanol and 1 N HCl (23:2, vol/vol) was then added under vigorous pipetting to dissolve the formazan crystals. Dye absorbance (A) in viable cells was measured at 570 nm, with 630 nm as a reference wavelength. Cell survival was estimated as a percentage of the value of untreated controls. Percentage cell death, quantified as 100% minus percentage survival, includes both apoptotic and necrotic cell death. All experiments were repeated at least 3 times, and each experimental condition was repeated at least in quadruplicate wells in each experiment.
Statistical analysis
Statistical significance was examined by a 2-way analysis of variance, followed by Duncan post hoc test. A value ofP < .05 was considered significant in all analyses.
Results
IMiD1 induces caspase-8, but not caspase-9, activity in MM cells
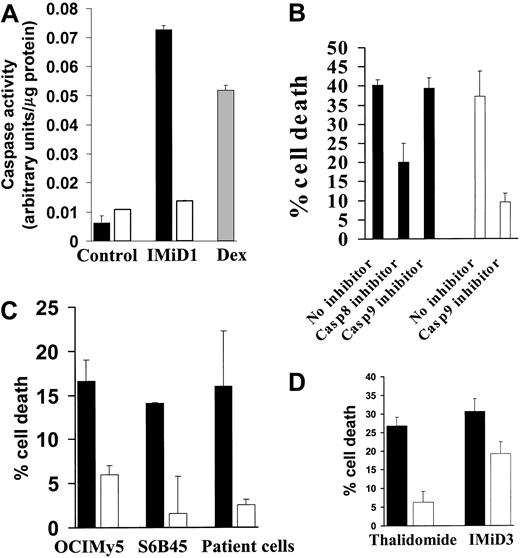
Our prior studies have shown that IMiD1 (CC4047, CDC394, Actimid)21 is severalfold more potent than Thal in inhibiting the growth of MM cells and directly induces apoptosis in the MM cell line MM.1S.4 IMiD1 was therefore the primary focus of our study. We first investigated the mechanism of its proapoptotic activity, in particular the role of caspases. Using a colorimetric activity assay, we demonstrated that IMiD1 induced caspase-8, but not caspase-9, activity in MM.1S cells (Figure1A). In contrast, Dex (1 μM for 48 hours) activated caspase-9, as in our prior study,22 and served as a positive control.
Caspase-8 mediates IMiD-induced apoptosis.
(A) Quantification of caspase-8 (black bars) and caspase-9 (white bars) activity was performed using the ApoAlert caspase colorimetric assay kit in MM.1S cells treated with IMiD1 (1 μM for 72 hours in 1% serum) after normalization for cellular protein. Treatment with Dex (1 μM for 48 hours) activated caspase-9 and served as a positive control (gray bar). Data shown (mean ± SD) are representative of 3 experiments. (B) The caspase-8–specific inhibitor IETD-FMK, but not the caspase-9 inhibitor LEHD-FMK (both used at 20 μM), protected MM.1S cells from cell death induced by IMiD1 (1 μM for 72 hours in 1% serum), as quantified by the MTT assay (black bars). LEHD-FMK attenuated apoptosis induced by Dex (1 μM for 48 hours; white bars) and served as a positive control. Data shown (mean ± SD) are representative of 3 experiments. (C) The caspase-8–specific inhibitor IETD-FMK (20 μM) protected OCI-My-5, S6B45, and primary MM patient cells from cell death induced by IMiD1 (1 μM for 72 hours in 1% serum), as quantified by the MTT assay (black bars: no inhibitor; white bars: caspase-8 inhibitor). Data shown (mean ± SD) are representative of 3 experiments. (D) The caspase-8–specific inhibitor IETD-FMK (20 μM) protected MM.1S cells from cell death induced by Thal (100 μM for 72 hours in 1% serum) or IMiD3 (1 μM for 72 hours in 1% serum), as quantified by the MTT assay (black bars: no inhibitor; white bars: caspase-8 inhibitor). Data shown (mean ± SD) are representative of 3 experiments.
Caspase-8 mediates IMiD-induced apoptosis.
(A) Quantification of caspase-8 (black bars) and caspase-9 (white bars) activity was performed using the ApoAlert caspase colorimetric assay kit in MM.1S cells treated with IMiD1 (1 μM for 72 hours in 1% serum) after normalization for cellular protein. Treatment with Dex (1 μM for 48 hours) activated caspase-9 and served as a positive control (gray bar). Data shown (mean ± SD) are representative of 3 experiments. (B) The caspase-8–specific inhibitor IETD-FMK, but not the caspase-9 inhibitor LEHD-FMK (both used at 20 μM), protected MM.1S cells from cell death induced by IMiD1 (1 μM for 72 hours in 1% serum), as quantified by the MTT assay (black bars). LEHD-FMK attenuated apoptosis induced by Dex (1 μM for 48 hours; white bars) and served as a positive control. Data shown (mean ± SD) are representative of 3 experiments. (C) The caspase-8–specific inhibitor IETD-FMK (20 μM) protected OCI-My-5, S6B45, and primary MM patient cells from cell death induced by IMiD1 (1 μM for 72 hours in 1% serum), as quantified by the MTT assay (black bars: no inhibitor; white bars: caspase-8 inhibitor). Data shown (mean ± SD) are representative of 3 experiments. (D) The caspase-8–specific inhibitor IETD-FMK (20 μM) protected MM.1S cells from cell death induced by Thal (100 μM for 72 hours in 1% serum) or IMiD3 (1 μM for 72 hours in 1% serum), as quantified by the MTT assay (black bars: no inhibitor; white bars: caspase-8 inhibitor). Data shown (mean ± SD) are representative of 3 experiments.
IMiD-induced MM cell death is caspase-8–dependent
To further support the functional role for caspase-8 in mediating IMiD1-induced apoptosis in MM cells, we used specific caspase-8 and caspase-9 inhibitors. As can be seen in Figure 1B, the caspase-8–specific inhibitor IETD-FMK, but not the caspase-9 inhibitor LEHD-FMK, protected MM.1S cells from IMiD1-induced cell death. As a positive control, the caspase-9 inhibitor LEHD-FMK protected against Dex-induced apoptosis, as in our prior studies.22
We then expanded our investigation to additional cell lines and MM patients' cells. As seen in Figure 1C, the caspase-8 inhibitor attenuated IMiD1-induced apoptosis in OCI-My-5 and S6B45 cells, as well as in patients' MM cells, confirming the involvement of caspase-8 in IMiD1-induced apoptosis in MM cells.
IMiD3 (CC5013, CDC501, Revimid)21 is the Thal analog used in phase 1 clinical trials.21 Therefore, we extended our studies to include IMiD3, as well as the parent compound Thal. As seen in Figure 1D, the caspase-8 inhibitor attenuated apoptosis induced by IMiD3 and Thal in MM.1S cells. Collectively, these data suggest that caspase-8 is an obligate mediator of apoptosis induced by Thal and its analogs in MM cells.
IMiD1 sensitizes MM cells to Fas-mediated apoptosis
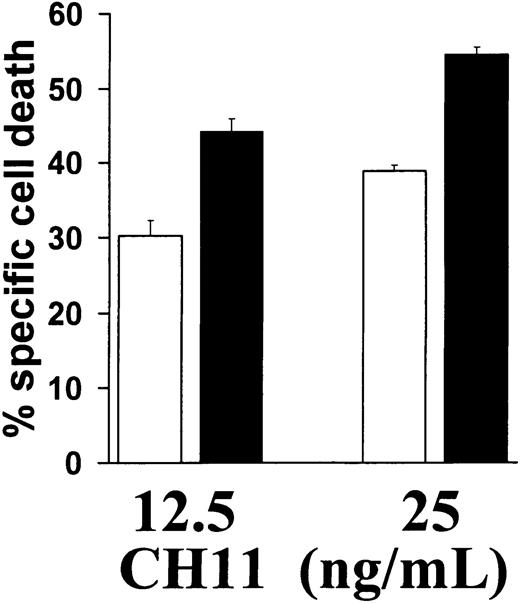
Our finding that IMiD induced caspase-8 activation suggested that IMiDs may synergize with other activators of the caspase-8–dependent apoptotic pathway. Because the death receptor Fas triggers apoptosis via caspase-8,23 24 we next determined whether IMiD1 sensitized MM cells to Fas-mediated apoptosis. As can be seen in Figure2, IMiD1 increased the sensitivity of MM.1S cells to low concentrations (12.5 or 25 ng/mL) of the Fas cross-linking antibody CH11. This finding further supports the involvement of caspase-8 in IMiD-triggered MM cell apoptosis.
IMiD1 enhances Fas-mediated apoptosis.
Pretreatment of MM.1S cells with (black bars) or without (white bars) 1 μM IMiD1 for 24 hours increased their sensitivity to apoptosis induced by Fas cross-linking antibody CH11 (12.5-25 ng/mL for 18 hours). Percentage specific cell death was calculated with the formula: % specific cell death = 100 − (absorbance in cells treated with IMiD1 and CH11)/(absorbance in cells treated with IMiD1 alone). Data shown (mean ± SD) are representative of 3 experiments.
IMiD1 enhances Fas-mediated apoptosis.
Pretreatment of MM.1S cells with (black bars) or without (white bars) 1 μM IMiD1 for 24 hours increased their sensitivity to apoptosis induced by Fas cross-linking antibody CH11 (12.5-25 ng/mL for 18 hours). Percentage specific cell death was calculated with the formula: % specific cell death = 100 − (absorbance in cells treated with IMiD1 and CH11)/(absorbance in cells treated with IMiD1 alone). Data shown (mean ± SD) are representative of 3 experiments.
IMiD1 sensitizes MM cells to TRAIL/Apo2L–induced apoptosis
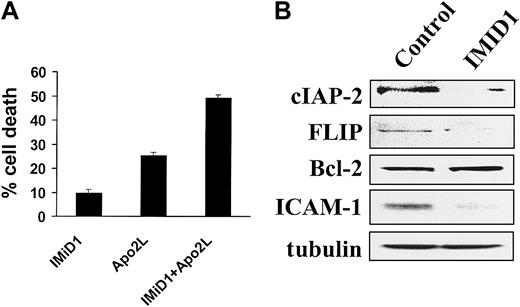
The clinical relevance of the interaction of IMiD with Fas signaling is limited because the enhanced apoptosis is modest; however, FasL is unlikely to be used clinically because of its toxicity. Another important member of this family of death ligands, TRAIL/Apo2L, exhibits selective anticancer activity and is undergoing early clinical evaluation.24 We therefore evaluated the effect of a pretreatment (4 hours) with IMiD1 on TRAIL/Apo2L–induced apoptosis. We found a synergistic effect (Figure 3A), suggesting the potential therapeutic utility of combining these agents.
IMiD1 enhances TRAIL/Apo2L–mediated apoptosis and down-regulates the expression of the antiapoptotic proteins cIAP-2 and FLIP.
(A) Combined proapoptotic effect of IMiD1 with TRAIL/Apo2L in MM.1S cells. Cells were pretreated with or without 1 μM IMiD1 for 4 hours, and a low concentration of TRAIL/Apo2L (50 ng/mL) was then added for an additional 24 hours. Data shown (mean ± SD) are representative of 3 experiments. IMiD1 potentiated the apoptotic effect of TRAIL/Apo2L. (B) Immunoblotting analysis for the antiapoptotic molecules cIAP-2, FLIP, Bcl-2, ICAM-1, and tubulin in MM.1S cells. Treatment with IMiD1 (1 μM for 72 hours) decreased the protein levels of caspase-8 inhibitors cIAP-2, FLIP, and ICAM-1, but not Bcl-2.
IMiD1 enhances TRAIL/Apo2L–mediated apoptosis and down-regulates the expression of the antiapoptotic proteins cIAP-2 and FLIP.
(A) Combined proapoptotic effect of IMiD1 with TRAIL/Apo2L in MM.1S cells. Cells were pretreated with or without 1 μM IMiD1 for 4 hours, and a low concentration of TRAIL/Apo2L (50 ng/mL) was then added for an additional 24 hours. Data shown (mean ± SD) are representative of 3 experiments. IMiD1 potentiated the apoptotic effect of TRAIL/Apo2L. (B) Immunoblotting analysis for the antiapoptotic molecules cIAP-2, FLIP, Bcl-2, ICAM-1, and tubulin in MM.1S cells. Treatment with IMiD1 (1 μM for 72 hours) decreased the protein levels of caspase-8 inhibitors cIAP-2, FLIP, and ICAM-1, but not Bcl-2.
IMiD1 down-regulates the expression of the caspase-8 inhibitors cIAP-2 and FLIP
We have recently demonstrated that the antiapoptotic proteins cIAP-2 and FLIP inhibit caspase-8 activation triggered by TRAIL/Apo2L in MM cells.25 In view of the sensitizing effect of IMiD1 on TRAIL/Apo2L–induced apoptosis, we next determined whether IMiD1 alters the expression of these antiapoptotic proteins. As can be seen in Figure 3B, IMiD1-induced apoptosis in MM.1S cells was associated with down-regulation of cIAP-2 and FLIP, but not Bcl-2, protein expression. Because cIAP-2 expression may be regulated by the transcription factor NF-κB,25 26 this observation suggested that IMiD1 inhibits NF-κB activity. Moreover, IMiD1 also down-regulated the expression of another NF-κB target gene, the adhesion molecule ICAM-1 (Figure 3B), suggesting that IMiD1 may also modulate MM cell adhesion.
IMiD1 down-regulates constitutive NF-κB activity in MM.1S cells
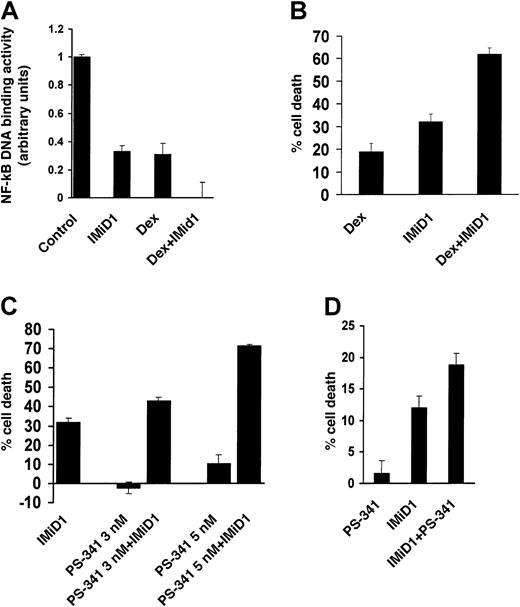
We next examined directly the effect of IMiD1 on NF-κB activity in MM.1S cells. As seen in Figure 4A, IMiD1 treatment down-regulated constitutive NF-κB activity in MM.1S cells, consistent with a recent report that Thal down-regulated TNF-α–induced NF-κB activation in endothelial and Jurkat cells.27 Because NF-κB activity mediates survival and Dex resistance in MM cells,28 down-regulation of its activity by IMiD1, as recently observed with proteasome inhibitors,29 could also contribute to its anti-MM activity.
IMiD1 inhibits NF-κB activity and enhances the effectiveness of Dex and PS-341 in MM.
(A) Quantification of the DNA binding activity of the transcriptional factor NF-κB in MM.1S cells treated with or without 1 μM IMiD1 for 48 hours, 1 μM Dex for 24 hours, or both, after normalization for cellular protein. Data shown (mean ± SD) are representative of 3 experiments. (B) Combined proapoptotic effect of IMiD1 with Dex in MM.1S cells. Cells were pretreated with or without 1 μM IMiD1 for 24 hours, and then Dex (0.25 μM) was added for an additional 48 hours. IMiD1 potentiated the apoptotic effect of Dex. Data shown (mean ± SD) are representative of 3 experiments. (C) Combined proapoptotic effect of IMiD1 with PS-341 in MM.1S cells. Cells were pretreated with or without 1 μM IMiD1 for 48 hours, and subtoxic concentrations of PS-341 (3 or 5 nM) were added for an additional 24 hours. IMiD1 potentiated the apoptotic effect of PS-341. Data shown (mean ± SD) are representative of 3 experiments. (D) Combined proapoptotic effect of IMiD1 with PS-341 in primary MM cells. Cells were pretreated with or without 1 μM IMiD1 for 48 hours, and a subtoxic concentration of PS-341 (3 nM) was added for an additional 24 hours. IMiD1 potentiated the apoptotic effect of PS-341. Data shown (mean ± SD) are representative of 3 experiments.
IMiD1 inhibits NF-κB activity and enhances the effectiveness of Dex and PS-341 in MM.
(A) Quantification of the DNA binding activity of the transcriptional factor NF-κB in MM.1S cells treated with or without 1 μM IMiD1 for 48 hours, 1 μM Dex for 24 hours, or both, after normalization for cellular protein. Data shown (mean ± SD) are representative of 3 experiments. (B) Combined proapoptotic effect of IMiD1 with Dex in MM.1S cells. Cells were pretreated with or without 1 μM IMiD1 for 24 hours, and then Dex (0.25 μM) was added for an additional 48 hours. IMiD1 potentiated the apoptotic effect of Dex. Data shown (mean ± SD) are representative of 3 experiments. (C) Combined proapoptotic effect of IMiD1 with PS-341 in MM.1S cells. Cells were pretreated with or without 1 μM IMiD1 for 48 hours, and subtoxic concentrations of PS-341 (3 or 5 nM) were added for an additional 24 hours. IMiD1 potentiated the apoptotic effect of PS-341. Data shown (mean ± SD) are representative of 3 experiments. (D) Combined proapoptotic effect of IMiD1 with PS-341 in primary MM cells. Cells were pretreated with or without 1 μM IMiD1 for 48 hours, and a subtoxic concentration of PS-341 (3 nM) was added for an additional 24 hours. IMiD1 potentiated the apoptotic effect of PS-341. Data shown (mean ± SD) are representative of 3 experiments.
Our finding that IMiD1 down-regulates the constitutive activity of NF-κB in MM cells further suggests that it may have combined anti-MM activity with conventional or novel therapies that also target NF-κB. Dex is a mainstay of MM therapy and down-regulates NF-κB activity, as shown in Figure 4A and in previous reports.28 The combination of Dex with IMiD1 resulted in complete abrogation of NF-κB activity in MM.1S cells (Figure 4A), suggesting the clinical utility of combination therapy.
IMiD1 sensitizes MM.1S cells to Dex and PS-341
Our finding of complete abrogation of NF-κB activity in MM.1S cells treated with both Dex and IMiD1 prompted us to investigate the effect of this combination on MM cell survival. As seen in Figure 4B, pretreatment with IMiD1 enhanced the anti-MM effect of Dex. These data provide a molecular basis for the synergistic activity of Dex and Thal observed clinically in the setting of refractory30 or newly diagnosed2 MM.
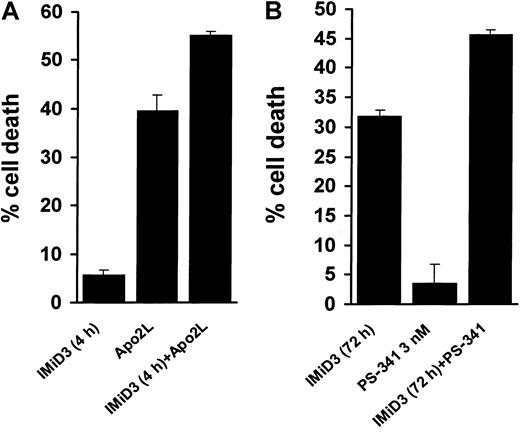
We also evaluated the in vitro anti-MM effect of IMiD1 in combination with PS-341, a novel proteasome inhibitor that blocks degradation of IκB inhibitory subunit and thus inhibits NF-κB activity.29 We found that pretreatment with IMiD1 enhanced the proapoptotic effect of PS-341 against MM.1S cells (Figure 4C) and primary MM patients' cells (Figure 4D). As with IMiD1, IMiD3 sensitized MM.1S cells to TRAIL/Apo2L (Figure5A) and PS-341 (Figure 5B), providing the framework for clinical use of IMiDs together with novel agents.
IMiD3 enhances MM cell death induced by TRAIL/Apo2L and PS-341.
(A) Combined proapoptotic effect of IMiD3 with TRAIL/Apo2L in MM.1S cells. Cells were pretreated with or without 1 μM IMiD3 for 4 hours, and a low concentration of TRAIL/Apo2L (50 ng/mL) was then added for an additional 24 hours. IMiD3 potentiated the apoptotic effect of TRAIL/Apo2L. Data shown (mean ± SD) are representative of 3 experiments. (B) Combined proapoptotic effect of IMiD3 with PS-341 in MM.1S cells. Cells were pretreated with or without 1 μM IMiD3 for 48 hours, and then a subtoxic concentration of PS-341 (3 nM) was added for an additional 24 hours. IMiD3 potentiated the apoptotic effect of PS-341. Data shown (mean ± SD) are representative of 3 experiments.
IMiD3 enhances MM cell death induced by TRAIL/Apo2L and PS-341.
(A) Combined proapoptotic effect of IMiD3 with TRAIL/Apo2L in MM.1S cells. Cells were pretreated with or without 1 μM IMiD3 for 4 hours, and a low concentration of TRAIL/Apo2L (50 ng/mL) was then added for an additional 24 hours. IMiD3 potentiated the apoptotic effect of TRAIL/Apo2L. Data shown (mean ± SD) are representative of 3 experiments. (B) Combined proapoptotic effect of IMiD3 with PS-341 in MM.1S cells. Cells were pretreated with or without 1 μM IMiD3 for 48 hours, and then a subtoxic concentration of PS-341 (3 nM) was added for an additional 24 hours. IMiD3 potentiated the apoptotic effect of PS-341. Data shown (mean ± SD) are representative of 3 experiments.
IMiD1 inhibits the activation of NF-κB by IGF-1 and TNF-α
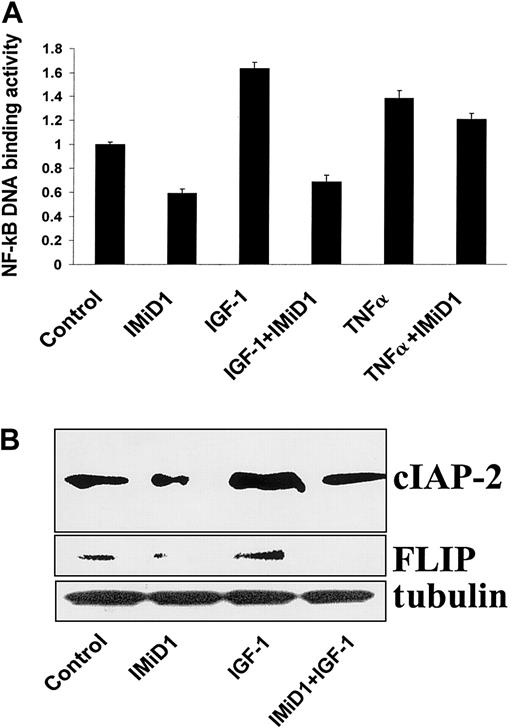
IGF-1 is a potent growth and survival factor for MM cells16-18 that activates the transcription factor NF-κB.15 NF-κB activity is also up-regulated in MM.1S cells by TNF-α.31 We therefore next investigated whether IMiD1 inhibited the activation of NF-κB by IGF-1 and TNF-α in MM.1S cells. As seen in Figure 6A, the stimulatory effect of IGF-1 on NF-κB DNA binding activity was completely inhibited by pretreatment with IMiD1; the stimulatory effect of TNF-α was also inhibited, but to a lesser degree.
IMiD1 blocks the antiapoptotic effect of IGF-1 in MM.
(A) DNA binding activity of the transcriptional factor NF-κB was quantified in MM.1S cells pretreated with or without 1 μM IMiD1 for 3 hours, and then treated for an additional 4 hours with or without 200 ng/mL IGF-1 or 50 ng/mL TNF-α. IMiD1 inhibited activation of NF-κB induced by IGF-1 and TNF-α in MM cells. (B) Immunoblotting analysis for the antiapoptotic molecules cIAP-2 and FLIP in MM.1S cells. Treatment with IGF-1 (200 ng/mL for 24 hours) increased the protein levels of the caspase-8 inhibitors cIAP-2 and FLIP; IMiD1 (1 μM) inhibited this effect.
IMiD1 blocks the antiapoptotic effect of IGF-1 in MM.
(A) DNA binding activity of the transcriptional factor NF-κB was quantified in MM.1S cells pretreated with or without 1 μM IMiD1 for 3 hours, and then treated for an additional 4 hours with or without 200 ng/mL IGF-1 or 50 ng/mL TNF-α. IMiD1 inhibited activation of NF-κB induced by IGF-1 and TNF-α in MM cells. (B) Immunoblotting analysis for the antiapoptotic molecules cIAP-2 and FLIP in MM.1S cells. Treatment with IGF-1 (200 ng/mL for 24 hours) increased the protein levels of the caspase-8 inhibitors cIAP-2 and FLIP; IMiD1 (1 μM) inhibited this effect.
We have recently found that IGF-1–induced activation of NF-κB in MM.1S cells was associated with up-regulation of the expression of several caspase inhibitors, including the caspase-8 inhibitors cIAP-2 and FLIP.15 Having demonstrated that the constitutive expression of cIAP-2 and FLIP protein levels is decreased by treatment with IMiD1 (Figure 3B), we next investigated whether IMiD1 can overcome the induction of these antiapoptotic proteins by IGF-1. As can be seen in Figure 6B, up-regulation of these caspase-8 inhibitors by IGF-1 was abrogated by IMiD1.
Discussion
In the present study, we investigated the molecular mechanism of the direct apoptotic effect exerted on MM cells by the class of immunomodulatory analogs of Thal, in particular IMiD1 (CC4047, Actimid) and IMiD3 (CC5013, Revimid). We found that the IMiDs induce caspase-8–dependent MM cell apoptosis, down-regulate NF-κB transcriptional activity, and sensitize MM cells to apoptosis induced by Fas cross-linking, TRAIL/Apo2L, Dex, and PS-341.
Several hypotheses have been proposed to explain the clinical effectiveness of Thal in MM, including antiangiogenic activity,3 direct induction of apoptosis or growth arrest in MM cells,4 inhibition of cytokine synthesis and secretion triggered by MM cell adhesion to bone marrow stromal cells,5 inhibition of TNF-α signaling,4 and stimulation of patients' natural killer cell anti-MM immunity.7 In the present study, we investigated the mechanism of the direct proapoptotic effect on MM cells, which we had identified previously.4 Because the IMiDs are severalfold more potent than Thal and are currently under clinical evaluation,9 they were the main focus of our investigation. We found that the IMiDs directly induce caspase-8–dependent apoptosis in MM cells and down-regulate NF-κB transcriptional activity in vitro. The latter protects against apoptosis in MM cells28,29; for example, the caspase inhibitor cIAP-2 is a target of NF-κB transcriptional activity,32,33 and we and others have demonstrated that NF-κB–dependent expression of cIAP-2 inhibits caspase-8 activation and caspase-8–dependent apoptosis.25,33 Additionally, NF-κB may regulate another caspase inhibitor, FLIP, in some models.26 Therefore, the down-regulation of the caspase-8 inhibitors cIAP-2 and FLIP by IMiD1 may contribute to the induction of caspase-8 activity, as well as the sensitization to Fas- and TRAIL/Apo2L–induced apoptosis. Because Fas-mediated apoptosis is a major mechanism of cell-mediated cytotoxicity, it is also possible that IMiD1 may sensitize MM cells to immune-mediated mechanisms of cell destruction in vivo. Our studies also show that Thal and IMiDs augment natural killer cell cytotoxicity against MM cells7 and that NF-κB inhibition increases the sensitivity of MM.1S cells to TRAIL/Apo2L.34 Therefore, IMiDs may both augment host anti-MM immunity and enhance tumor cell sensitivity.
The NF-κB inhibitory activity of the IMiDs suggests that they may have increased efficacy when combined with other anti-MM agents that block NF-κB. Increased NF-κB blockade and MM cell death were observed with Dex and the proteasome inhibitor PS-341 in our study. This observation may explain the enhanced clinical responses reported with the combined use of low-dose Thal and Dex in refractory30 or newly diagnosed2 MM patients,35 and further suggests that a combination of Dex with IMiDs could be clinically useful. Moreover, these findings support the future investigation of the effectiveness of PS-341 together with the IMiDs in MM patients.
IGF-1 has potent growth and survival effects on MM cells14,18 by activating PI-3K, as well as downstream Akt and MAPK signaling cascades.15,16 We have also demonstrated that IGF-1 activates NF-κB binding activity and up-regulates cIAP-2 and FLIP protein levels.15 In this study, we showed that short-term pretreatment (3 hours) with IMiD1 inhibited both NF-κB activation and induction of cIAP-2 and FLIP expression, suggesting that IMiD1 may exert its anti-MM effects in vivo by attenuating the prosurvival effect of paracrine factor(s), such as IGF-1, produced in the bone marrow microenvironment. Interestingly, one proposed mechanism for the teratogenic effects of Thal involves inhibition of IGF-1 function in the growing fetal limb bud.10 11 Furthermore, the ability of IMiDs to inhibit expression of adhesion molecules on MM cells suggests that they may also abrogate host bone marrow–MM cell interactions and resulting protection against apoptosis.
In conclusion, our findings delineate the intracellular signaling mechanisms whereby IMiDs induce MM cell apoptosis. They also show that the IMiDs potentiate the anti-MM activity of Fas cross-linking, TRAIL/Apo2L, Dex, and the proteasome inhibitor PS-341, providing the framework for derived clinical trials in MM.
Supported by the Multiple Myeloma Research Foundation (N.M. and C.S.M.), the Laurie Strauss Leukemia Foundation (N.M. and C.S.M.), the Bailey Family Research Fund (N.M. and C.S.M.), a National Institutes of Health Career Development Award (S.P.T.) and PO-1 78378 (K.C.A.), and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Department of Adult Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal