Thrombosis is the major mechanism underlying acute complications of atherosclerosis. Although thrombogenicity of atherosclerotic plaques has been ascribed to activation of the extrinsic pathway of blood coagulation, in the present study we investigated contribution of the intrinsic factor VIII (fVIII)–dependent pathway. We found that in vitro exposure of human macrophages and smooth muscle cells (SMCs) to atherogenic oxidized low-density lipoprotein (oxLDL) enhances their ability to support activity of 2 major complexes of the intrinsic pathway, Xase and prothrombinase, leading to a 20- and 10-fold increase in thrombin formation, respectively. In contrast, human aortic endothelial cells were less responsive to oxLDL. The increase in the intrinsic procoagulant activity was related to formation of additional fVIII binding sites due to enhanced translocation of phosphatidylserine to the outer surface of oxLDL-treated cells and a 5-fold higher affinity of interaction between components of the Xase complex, activated factors VIII and IX. Processes occurring at early apoptotic stages, including changes in the cell membrane induced by free radicals, may be related to activation of the intrinsic pathway as suggested by effects of inhibitors of early apoptosis on thrombin formation. Immunohistochemical studies on human atherectomy specimens revealed the presence of fVIII in the vicinity of macrophages and SMCs in atheromatous regions with massive deposits of oxLDL, supporting the possible involvement of the intrinsic pathway in thrombus formation in vivo. Our data predict that the intrinsic pathway significantly enhances thrombogenicity of atherosclerotic lesions after removal of the endothelial layer and exposure of SMCs and macrophages to blood flow.
Introduction
Human atherosclerosis involves 2 distinct pathologic processes—conventional atherogenesis at early stages, and atherothrombosis at advanced stages, which is responsible for acute manifestations of the disease.1,2 Atherogenesis is initiated by oxidation of low-density lipoproteins (LDL) and recruitment of monocytes to the intima of the vessel wall leading to accumulation of oxidized LDL (oxLDL) in macrophages and smooth muscle cells (SMCs) and their transformation into lipid-laden foam cells.2 This process is accompanied by extensive cell proliferation and elaboration of extracellular matrix components accounting for atheroma progression.3 However, severe clinical complications of atherosclerosis, including myocardial infarction, ischemic stroke, and sudden cardiac death, are caused by atherothrombosis manifested in thrombus formation primarily on ruptured advanced atherosclerotic plaques, which finally leads to occlusion of the vessel lumen.4 5
A major role in determining thrombogenicity of human atherosclerotic lesions has been ascribed to the extrinsic, tissue factor (TF)–dependent pathway of blood coagulation.6,7 TF is expressed at high levels in macrophages and SMCs within human atherosclerotic plaques and its activity correlates with plaque progression and thrombin generation.8-10 Membrane-bound complex of TF with activated factor VII (fVIIa) proteolytically activates factors IX (fIX) and X (fX). In its turn, activated fX (fXa) participates in conversion of a zymogen prothrombin into thrombin, the key enzyme of the coagulation cascade.11
Although the TF-dependent pathway is unequivocally responsible for initial generation of fXa, the intrinsic pathway catalyses fX activation approximately 50-fold more efficiently, thus dramatically amplifying coagulation events triggered by the TF-dependent pathway.11 In the intrinsic pathway, fX activation is provided by a membrane-bound Xase complex formed by activated factors VIII (fVIIIa) and IX (fIXa). The fVIII molecule consists of 3 homologous A domains, 2 homologous C domains, and the unique B domain, which are arranged in the order A1-A2-B-A3-C1-C2. Thrombin or fXa activates fVIII by intramolecular cleavages producing heterotrimeric fVIIIa (A1/A2/A3-C1-C2). Although the role of the intrinsic pathway in determining thrombogenicity of atherosclerotic plaque has not been studied, its possible contribution is suggested by a number of clinical studies, which demonstrated correlation between elevated levels of fVIII and fIX and the risk of coronary heart disease,12-14myocardial infarction,15 and ischemic stroke.16 On the other hand, several clinical surveys revealed a significantly reduced risk of myocardial infarction and 5-fold lower mortality from ischemic heart disease in fVIII-deficient patients with hemophilia A, suggesting that the lowering of fVIII level reduces development of thrombotic complications in atherosclerosis.17 18
The functioning of the intrinsic Xase complex requires its assembly on the phospholipid surface, the major role of which is to direct interaction between the components of the Xase complex from 3- to 2-dimensional space. This results in a dramatic acceleration of fXa generation due to decrease in the Michaelis constant (Km) for fX19 and increase in the catalytic constant of the reaction (kcat).20 Although the phospholipid surface is classically provided by membranes of activated platelets,21 3 major cell constituents of atherosclerotic lesion, that is, macrophages,22,23SMCs,23 and endothelial cells (ECs)23,24 also support Xase assembly in vitro, yet far less effectively than activated platelets. Within atherosclerotic lesions, all these cell types are exposed to oxLDL, which triggers transformation of macrophages and SMCs to lipid-laden foam cells.2 At later stages of atherogenesis, oxLDL induces apoptosis, as evidenced by an increased occurrence of apoptotic macrophages and SMCs in human atherosclerotic lesions,25,26 especially in ruptured plaques.27 Induction of apoptosis by oxLDL was also demonstrated in vitro for SMCs,28,29ECs,30,31 and macrophages.32,33 Because the complexes of both extrinsic and intrinsic pathways of coagulation are highly dependent on the presence of phosphatidylserine (PS) in cell membranes,20 translocation of this anionic phospholipid from the inner to the outer leaflet of the membrane during apoptosis is likely to be an important factor defining the procoagulant activity of the cells in atherosclerotic lesions. Apoptotic macrophages and SMCs were shown to have an increased procoagulant activity in the TF-dependent pathway,34 and induction of apoptosis in ECs by staurosporin was reported to increase the intrinsic Xase activity.35 However, the link between oxLDL-induced apoptosis and the activity of the intrinsic pathway in the environment of atherosclerotic lesion remains to be elucidated.
In the present study we investigated whether in vitro exposure of major cell constituents of the vessel wall to oxLDL alters their ability to support the activity of the intrinsic Xase and prothrombinase complexes. We compared relative susceptibility of SMCs, macrophages, and ECs to oxLDL and demonstrated that exposure of macrophages and SMCs to atherogenic levels of oxLDL resulted in a significant increase in the rates of fXa and thrombin formation.
Materials and methods
Materials
Human plasma–derived fVIII was purified from therapeutic fVIII concentrates (AHF concentrate, American Red Cross, Rockville, MD) as described.36 Human coagulation factors V, IXa, X, and Xa, human prothrombin, and thrombin were purchased from Enzyme Research Laboratories (South Bend, IN). Native LDL and fully oxidized LDL were from Intracel (Rockville, MD). A general caspase inhibitor (Z-VAD-FMK) was purchased from R & D Systems (Minneapolis, MN). An antioxidant butylated hydroxytoluene (BHT) and an inhibitor of acidic sphingomyelinase, desipramine, were from Sigma (St Louis, MO).
Antibodies
Mouse monoclonal antibody (mAb) ESH8 recognizing residues 2248-2285 of the C2 domain of fVIII37 was purchased from American Diagnostica (Greenwich, CT). The mAb NMC-VIII/10 recognizing residues 1675-1684 within the acidic region of the light chain of fVIII was produced as described.38 Biotinylation of mAbs ESH8 and NMC-VIII/10 was performed using EZ-Link Sulfo-NHS-LC-Biotinylation kit (Pierce, Rockford, IL). Mouse mAb OXL41.1 specifically recognizing oxLDL and not cross-reacting with native LDL was purchased from Neomarkers (Fremont, CA). Mouse mAb to vascular α-actin 1A4 conjugated with a red fluorophore Cy3 was from Sigma. Mouse mAb to macrophage-specific marker CD68 was purchased from Dako (Carpinteria, CA) and coupled with a red fluorophore Alexa Fluor 594 (Molecular Probes, Eugene, OR).
Cell culture
Primary cultures of human aortic SMCs and human aortic endothelial cells (HAECs) were purchased from Biowhittaker (Walkersville, MD) and used at passages 4 through 10. SMCs were propagated in SmGM-2 BulletKit Medium supplemented with 10% fetal bovine serum (FBS), human basic fibroblast growth factor (FGF), human epidermal growth factor (EGF), and insulin (Biowhittaker) at 37°C in 6% CO2. HAECs were propagated in modified endothelial cell basal medium-2 supplemented with 2% FBS, human basic FGF, human vascular endothelial growth factor (VEGF), human EGF, insulinlike growth factor 1 (IGF-1), heparin, ascorbic acid, and hydrocortisone (Biowhittaker) as above. SMCs and HAECs were used in experiments at 80% to 90% confluence. Human monocytes were isolated from mononuclear leukocyte preparations obtained by apheresis procedure performed by Research Blood Staff at the Holland Laboratory, American Red Cross under approved institutional review board protocol. The population of monocytes was enriched to 97% by positive selection on CD14 beads (Miltenyi, Auburn, CA). Differentiation of monocytes into macrophages was promoted by addition of macrophage colony-stimulating factor (Sigma) in RPMI supplemented with 10% human AB serum (Biowhittaker).
Prior to experiments, SMCs were transferred to low serum (0.5% FBS) SmGM-2 BulletKit Medium, HAECs to defined human endothelial serum-free growth medium supplemented with human basic FGF (20 ng/mL) and human EGF (10 ng/mL; Invitrogen, Carlsbad, CA) and macrophages to defined human macrophage serum-free growth medium (Invitrogen). Incubation of the cells with lipoproteins was performed under serum-free (for HAECs and macrophages) or low serum (for SMCs) conditions.
Measurement of the intrinsic pathway activities
Factor Xa generation assay.
Cells were incubated without LDLs or with 100 μg/mL of either oxLDL or native LDL for increasing time intervals, and the ability of the cell surface to support conversion of fX to fXa was measured in a chromogenic assay performed at 37°C as described.39 The reaction was performed in 20 mM Hepes buffer, pH 7.4, containing 0.15 M NaCl, 5 mM CaCl2, and 0.5% bovine serum albumin (HBS). FVIII and fIXa were added to final concentrations of 1 nM and 2 nM, respectively. After activation of fVIII by thrombin (0.5 U/mL) for 30 seconds, the reaction was initiated by addition of fX (170 nM). The aliquots were taken at defined time intervals, the reaction was stopped with 0.05 M EDTA, followed by determination of generated fXa from the rate of conversion of a chromogenic substrate S-2765 (Chromogenix, Milan, Italy). The increase in absorbance was read at 405 nm using a Labsystems multiscan microplate reader (Labsystems, Franklin, MA) in a kinetic mode and converted into fXa concentration using a purified fXa standard. Maximal rates of fX activation were calculated from individual kinetic curves by linear regression of fXa concentration over time using a SigmaPlot 1.02 (Jandel Scientific, Chicago, IL) computer program. In a control experiment, thrombin activation of fVIII was stopped by 1.5-molar excess of hirudin prior to addition to oxLDL-treated cells. In an additional control experiment, the Xase reaction was performed in wells incubated with oxLDL in the absence of cells.
Determination of parameters of interaction of the Xase components.
Following incubation of the cells in the absence or presence of oxLDL, fXa generation assays were performed as described above at a constant fVIII concentration (1 nM) and increasing concentrations of either fIXa or fX. In fIXa titration experiment, fX concentration was 170 nM, and fIXa concentrations increased from 0.05 to 5 nM; in fX titration experiment, fIXa concentration was 1 nM and fX concentrations ranged from 1 to 200 nM. Initial rates of fX activation for each condition were determined as described above and plotted versus concentration of fX or fIXa. The apparent affinity of fVIIIa/fIXa interaction (Kapp) was determined according to a standard equilibrium binding model described in the corresponding figure legend. Km and the maximal rate (Vmax) of fX conversion by fVIIIa/fXa complex were determined by fitting the initial rates of fX generation versus fX concentration using a Michaelis-Menton model.
Thrombin generation assay.
Activation of prothrombin to thrombin on the surface of control and oxLDL- or native LDL-treated cells was measured as described.40 The components of the Xase and prothrombinase complexes, fVIII, fIXa, and fV, were added in HBS to final concentrations of 1 nM, 2 nM, and 20 nM, respectively. The reaction was initiated by simultaneous addition of fX and prothrombin to final concentrations of 170 nM and 1.4 μM, respectively. FV was activated by fXa generated by the Xase complex. Thrombin formation was measured by the rate of conversion of a chromogenic substrate S-2238 specific for thrombin (Chromogenix) registered as an increase in absorbance at 405 nm and converted to thrombin concentration using purified thrombin standard. The maximal rates of thrombin generation were determined as in the fXa generation assay. In additional experiments, the cells were incubated with various inhibitors of apoptosis for 1 hour prior to addition of oxLDL. The inhibitors were used at the following concentrations: BHT, 50 μM for all cell types; desipramine, 1 μM for SMCs and 10 μM for macrophages and HAECs; Z-VAD-FMK, 100 μM for SMCs and HAECs and 10 μM for macrophages.
To examine the effect of oxLDL on the activity of the prothrombinase complex, thrombin formation on oxLDL-treated cells was determined in the presence of fV (20 nM), fXa (8 nM), and prothrombin (1.4 μM). The kinetics of thrombin formation were measured as described above.
Cell-mediated fVIII binding assay
Factor VIII was labeled with Na125I (100 mCi/mL [3700 MBq], Amersham Pharmacia Biotech, Uppsala, Sweden) using lactoperoxidase beads (Worthington Biochemical, Lakewood, NJ) as described.41 FV and annexin V (MBL International, Watertown, MA) were labeled with Na125I by IODOGEN method as described.42 43 The specific radioactivities of125I-fVIII, 125I-fV, and125I-annexin V were 8.7 × 106, 2.9 × 106, and 2.6 × 106 cpm/μg, respectively.
125I-FVIII binding assay was performed as generally described.44 After treatment of the cells with native LDL or oxLDL for increasing time intervals, the cells were incubated with125I-fVIII (0.5 nM) in 10 mM Hepes, pH 7.4 containing 137 mM NaCl, 4 mM KCl, 11 mM glucose, 5 mM CaCl2, and 2% BSA for 2 hours at 10°C. In some experiments, the cells were incubated with oxLDL in the presence of BHT, desipramine, or Z-VAD-FMK as above. After washing the cells with the binding buffer without125I-fVIII, surface-bound 125I-fVIII was determined as radioactivity released after treatment of the cells with 50 μg/mL trypsin (Invitrogen), 50 μg/mL proteinase K (Boehringer Mannheim, Mannheim, Germany), and 5 mM EDTA. The 125I-fVIII binding level was corrected for nonspecific 125I-fVIII binding measured in the presence of 500-fold excess of unlabeled fVIII. The binding experiments with 125I-fV and125I-annexin V were performed analogously.
Detection of PS by annexin V binding
Cells were grown in Lab-Tek II chamber slides (Nalge Nunc International, Naperville, IL) in the absence or presence of oxLDL for 12 hours. Translocation of PS from the inner to the outer leaflet of the cell membrane was tested with annexin V fused with enhanced green fluorescent protein (annexin V-EGFP) using ApoAlert annexin V-EGFP apoptosis kit (Clontech, Palo Alto, CA). The binding of annexin V-EGFP was performed at room temperature for 15 minutes on unfixed cells to avoid cell membrane perforation and possible annexin V penetration into the cells. In a positive control the cells were incubated with apoptosis-inducing agents, 20 nM staurosporin (Clontech) or with 20 ng/mL human recombinant tumor necrosis factor-α (R & D Systems). Annexin V+ cells were detected in an Eclipse E800 microscope (Nikon, Melville, NY) equipped with a set of fluorescent filter blocks and a digital SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI).
Immunohistochemistry
Coronary atherectomy specimens were obtained from patients (mean age, 64 ± 11 years) who underwent directional coronary atherectomy at the Washington Hospital Center (Washington, DC). The specimens were processed as described.45 Almost all specimens had regions of normal media, which served as built-in controls for quiescent SMCs. The atherectomy sections were stained for oxLDL or fVIII using mouse mAbs OXL41.1 and ESH8, respectively. The antibodies were visualized using horseradish peroxidase–based Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA), and sections were counterstained with hematoxylin. In double-staining experiments, oxLDL was detected as above, followed by staining for fVIII using ESH8 and alkaline phosphatase–based Vectastain ABC-AP kit (Vector Laboratories). The double-stained sections were counterstained with nuclear fast red.
In double-label immunofluorescence staining, tissue autofluorescence was quenched using sodium borohydride as described.46 Staining for fVIII was performed by incubating atherectomy sections with biotinylated mAb ESH8 or mAb NMC-VIII/10, followed by visualization with fluorescein-conjugated avidin DCS (5 μg/mL, Vector Laboratories). The fVIII-stained sections were subsequently stained for SMCs or macrophages using mouse anti-α-smooth muscle actin mAb 1A4 conjugated with Cy3 fluorophore or antihuman CD68 mAb conjugated with Alexa Fluor 594. Lipofuscinlike autofluorescence of double-labeled sections was eliminated using 1% Sudan black B in 70% ethanol.47 Air-dried sections were mounted in ProLong Antifade medium (Molecular Probes) and microscopy was performed as above using selective fluorescent filter blocks. Simultaneous visualization of fVIII and α-actin or CD68 was performed by merging single-dye images using SPOT Advance Program Mode (Diagnostic Instruments).
Results
Oxidized LDL increases activity of intrinsic Xase complex assembled on human SMCs and macrophages
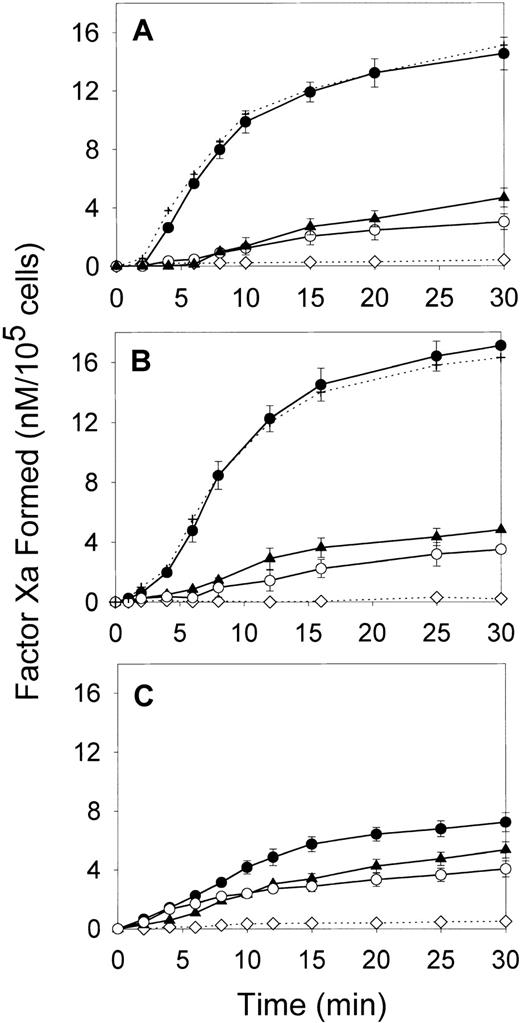
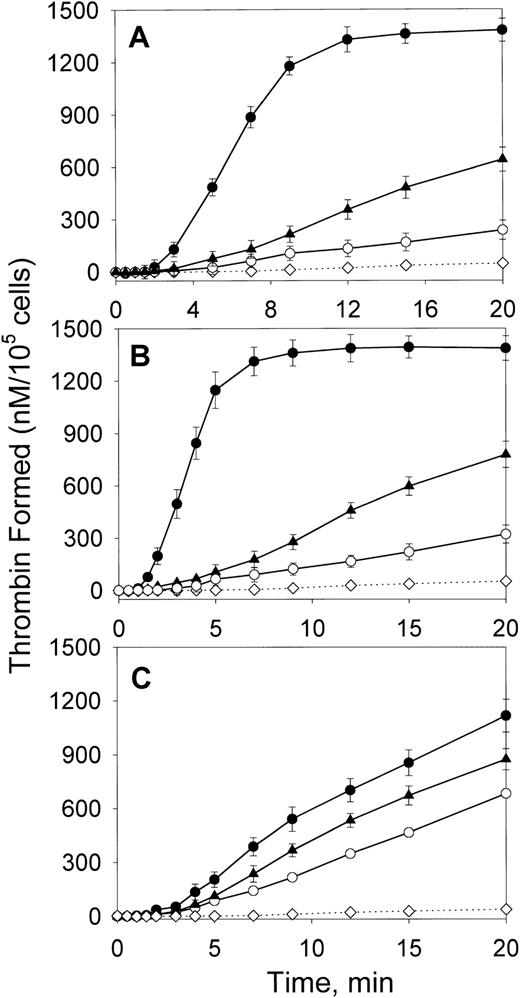
We first compared the effect of oxLDL on the ability of major cell types forming the arterial wall—SMCs, macrophages, and HAECs—to support fX activation by the intrinsic coagulation pathway. In the absence of lipoproteins, all cell types had a low ability to support fXa generation in the presence of thrombin-activated fVIII and fIXa (Figure 1). Incubation of SMCs and macrophages with oxLDL for increasing time intervals up to 24 hours led to a gradual increase in fXa formation (data not shown). The maximal effect was achieved at 12 hours of incubation resulting in a 6- and 8.1-fold increase in the maximal rates of fXa generation for oxLDL-treated SMCs and macrophages, respectively (Figure 1A,B and Table1). Notably, incubation of HAECs with oxLDL resulted only in a 1.6-fold increase in the rate of fXa generation (Figure 1C and Table 1). In contrast to oxLDL, native LDL had a slight effect on the procoagulant properties of the tested cell types. The control experiment confirmed that acceleration of Xase was mediated by the effect of oxLDL on the cells, because no generation of fXa was detected in the wells incubated with oxLDL in the absence of cells (Figure 1). In another control experiment, fVIII was activated prior to addition to the cells, and thrombin was subsequently inactivated by excess of hirudin. Because kinetics of fXa generation on oxLDL-treated cells were not affected by hirudin, the possible effect of thrombin on fXa formation by oxLDL-treated cells was excluded. OxLDL-treated cells stained negative with trypan blue, indicating that the registered increase in fXa generation was not due to the presence of necrotic cells. Thus, of 3 cell types comprising atherosclerotic lesion, exposure of SMCs and macrophages to oxLDL led to a significant acceleration of fVIII-dependent generation of fXa, whereas endothelial cells were far less responsive to oxLDL.
Effect of oxLDL on the procoagulant activity of human aortic SMCs, macrophages, and aortic ECs.
SMCs (A), macrophages (B), or HAECs (C) in the amount of 2 × 105/well were incubated in the absence of lipoproteins (○) or in the presence of 100 μg/ml oxLDL (●) or native LDL (▴) for 12 hours at 37°C. Following incubation, the conversion of fX (170 nM) into fXa catalyzed by fIXa (2 nM) and thrombin-activated fVIII (1 nM) was measured in a chromogenic assay as described in “Materials and methods.” In the control experiment (⋄), the Xase reaction was performed in wells incubated with oxLDL in the absence of cells. In the additional control experiment (+), the Xase reaction was performed using fVIII activated by thrombin, which was subsequently inactivated by excess of hirudin. Each data point represents the mean value ± SD of triplicates.
Effect of oxLDL on the procoagulant activity of human aortic SMCs, macrophages, and aortic ECs.
SMCs (A), macrophages (B), or HAECs (C) in the amount of 2 × 105/well were incubated in the absence of lipoproteins (○) or in the presence of 100 μg/ml oxLDL (●) or native LDL (▴) for 12 hours at 37°C. Following incubation, the conversion of fX (170 nM) into fXa catalyzed by fIXa (2 nM) and thrombin-activated fVIII (1 nM) was measured in a chromogenic assay as described in “Materials and methods.” In the control experiment (⋄), the Xase reaction was performed in wells incubated with oxLDL in the absence of cells. In the additional control experiment (+), the Xase reaction was performed using fVIII activated by thrombin, which was subsequently inactivated by excess of hirudin. Each data point represents the mean value ± SD of triplicates.
Maximal rates of generation of fXa and thrombin in the intrinsic pathway by oxLDL-treated cells
| Cell type . | Rate of fXa generation, V (nM/min) . | Rate of thrombin generation, Vthr (nM/min) . | ||||
|---|---|---|---|---|---|---|
| Control, V0 . | oxLDL-treated, V . | V/V0 ratio . | Control, Vthr0 . | OxLDL-treated, Vthr . | Vthr/Vthr0ratio . | |
| Macrophages | 0.218 ± 0.05 | 1.768 ± 0.022 | 8.1 ± 1.8 | 16.6 ± 0.43 | 326 ± 11.2 | 19.6 ± 0.84 |
| SMCs | 0.176 ± 0.016 | 1.06 ± 0.056 | 6.0 ± 0.63 | 18.3 ± 0.6 | 187.5 ± 4.7 | 10.2 ± 0.42 |
| HAECs | 0.33 ± 0.012 | 0.51 ± 0.035 | 1.55 ± 0.12 | 33.5 ± 0.82 | 74 ± 3.1 | 2.2 ± 0.11 |
| Cell type . | Rate of fXa generation, V (nM/min) . | Rate of thrombin generation, Vthr (nM/min) . | ||||
|---|---|---|---|---|---|---|
| Control, V0 . | oxLDL-treated, V . | V/V0 ratio . | Control, Vthr0 . | OxLDL-treated, Vthr . | Vthr/Vthr0ratio . | |
| Macrophages | 0.218 ± 0.05 | 1.768 ± 0.022 | 8.1 ± 1.8 | 16.6 ± 0.43 | 326 ± 11.2 | 19.6 ± 0.84 |
| SMCs | 0.176 ± 0.016 | 1.06 ± 0.056 | 6.0 ± 0.63 | 18.3 ± 0.6 | 187.5 ± 4.7 | 10.2 ± 0.42 |
| HAECs | 0.33 ± 0.012 | 0.51 ± 0.035 | 1.55 ± 0.12 | 33.5 ± 0.82 | 74 ± 3.1 | 2.2 ± 0.11 |
After incubation of the cells with oxLDL for 12 hours, generation of fXa and thrombin was determined as described in “Materials and methods.” Maximal rates were determined from individual kinetic curves shown in Figures 1 and 7 by linear regression of fXa or thrombin concentration over time using a SigmaPlot 1.02 computer program.
FVIII binding to oxLDL-treated cells is increased
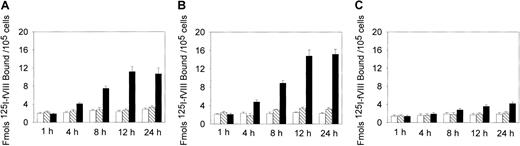
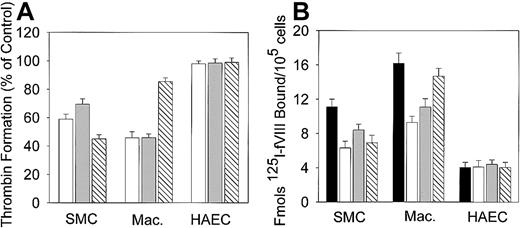
Because the binding of fVIII to the phospholipid membrane is required for assembly of the Xase complex, the increased procoagulant activity may be related to the increased binding of fVIII to oxLDL-treated cells. We, therefore, measured 125I-fVIII binding to the cells treated with oxLDL for increasing time intervals. Incubation of SMCs and macrophages with oxLDL for 12 hours led to a maximal 4-fold and 5-fold increase in 125I-fVIII binding, respectively, compared to untreated cells (Figure2A,B), whereas for HAECs this increase was only 1.5-fold (Figure 2C). In contrast, incubation of the cells with native LDL did not result in appreciable increase in fVIII binding (Figure 2).
Effect of oxLDL treatment on 125I-fVIII binding to the cell surface.
Human SMCs (A), macrophages (B), or HAECs (C) in the amount of 2 × 105/well were incubated in the absence of lipoproteins (open bars) or in the presence of 100 μg/mL native LDL (hatched bars) or 100 μg/mL oxLDL (black bars) for the time intervals indicated. Subsequently, 125I-fVIII was added to a final concentration of 0.5 nM and its binding was determined as described in “Materials and methods.” Each data point represents the mean value ± SD of triplicate determinations.
Effect of oxLDL treatment on 125I-fVIII binding to the cell surface.
Human SMCs (A), macrophages (B), or HAECs (C) in the amount of 2 × 105/well were incubated in the absence of lipoproteins (open bars) or in the presence of 100 μg/mL native LDL (hatched bars) or 100 μg/mL oxLDL (black bars) for the time intervals indicated. Subsequently, 125I-fVIII was added to a final concentration of 0.5 nM and its binding was determined as described in “Materials and methods.” Each data point represents the mean value ± SD of triplicate determinations.
Because oxLDL itself can support assembly of the intrinsic Xase complex,48 we verified that accelerated fXa generation and formation of additional fVIII-binding sites on macrophages and SMCs were due to changes in cell membrane caused by internalization of oxLDL. This was confirmed by lack of appreciable increase in the Xase activity and 125I-fVIII binding to the cells incubated with oxLDL at 10°C, when internalization was suppressed (data not shown). Thus, the increased ability of oxLDL-treated cells to support activity of the intrinsic Xase complex (Figure 1) correlated with the level of fVIII bound to their surface.
oxLDL induces translocation of PS in the cell membrane
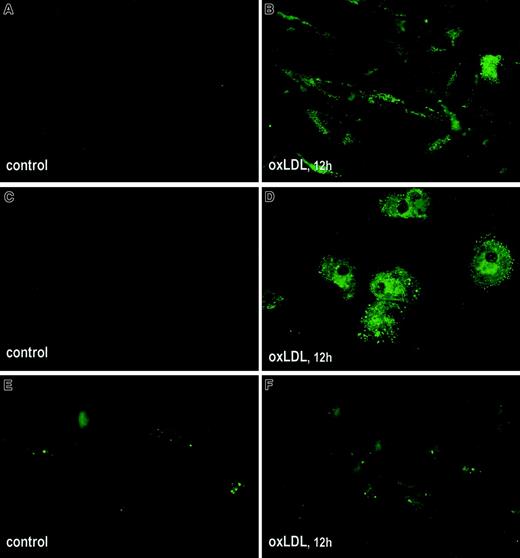
Binding of fVIII to phospholipid membranes is mediated by PS,49 which is ultimately required for the formation of fVIII binding sites.50 Because the number of fVIII binding sites sharply increases with an increase in PS content in both synthetic and physiologic membranes,20,50 the observed elevated fVIII binding to oxLDL-treated cells may be related to translocation of PS from the inner to the outer leaflet of the cell membrane. We compared exposure of PS on the surface of oxLDL-treated and untreated cells using annexin V–EGFP as a specific probe for PS.51 Microscopic analysis revealed that incubation of SMCs and macrophages with oxLDL for 12 hours led to a pronounced increase in fluorescence intensity, reflecting intensive translocation of PS to the outer membrane leaflet (Figure3A-D). For HAECs, surface-bound annexin V–EGFP was detected in a few untreated cells but the difference in fluorescence intensity of control and oxLDL-treated cells was less pronounced (Figure 3E,F). Thus, oxLDL-induced translocation of PS in macrophages and SMCs may account for the increased125I-fVIII binding to these cells.
Effect of oxLDL on translocation of PS to the outer leaflet of cell membranes.
Human SMCs (A,B), macrophages (C,D), and HAECs (E,F) were grown in Lab-Tek II chamber slides and incubated in the absence (A,C,E) or presence (B,D,F) of 100 μg/mL oxLDL for 12 hours. Exposure of PS on cell membranes was tested by immunofluorescence using annexin V coupled with EGFP as described in “Materials and methods.” Microscopy was performed using an Eclipse E800 microscope (Nikon) equipped with fluorescent filter blocks at magnification × 600.
Effect of oxLDL on translocation of PS to the outer leaflet of cell membranes.
Human SMCs (A,B), macrophages (C,D), and HAECs (E,F) were grown in Lab-Tek II chamber slides and incubated in the absence (A,C,E) or presence (B,D,F) of 100 μg/mL oxLDL for 12 hours. Exposure of PS on cell membranes was tested by immunofluorescence using annexin V coupled with EGFP as described in “Materials and methods.” Microscopy was performed using an Eclipse E800 microscope (Nikon) equipped with fluorescent filter blocks at magnification × 600.
FVIIIa interacts with fIXa with a higher affinity on oxLDL-treated cells
Because the PS content in membrane strongly affects the affinity of fVIIIa for fIXa and the ability of the assembled Xase complex to catalyze activation of fX into fXa,20 we examined the effect of oxLDL treatment of SMCs on the parameters characterizing fVIIIa/fIXa interaction and the activity of the resulting Xase complex. The dependence of fXa formation on fIXa concentration at constant concentration of fVIIIa and fX was adequately fitted to a standard equilibrium binding model (Figure 4A). The value of Kapp, characterizing the apparent affinity of fVIIIa for fIXa in the functional Xase assay, was 0.41 ± 0.17 nM for untreated and 0.08 ± 0.022 nM for oxLDL-treated SMC. Thus, treatment of the cells with oxLDL not only increases the concentration of cell surface-bound fVIII but also leads to a 5-fold increase in the affinity of fVIIIa/fIXa interaction, which provides a higher concentration of functional fVIIIa/fIXa complex on the surface of oxLDL-treated cells.
Effect of oxLDL treatment of SMC on assembly and activity of the Xase complex.
(A) Determination of the apparent affinity of fVIIIa/fIXa interaction. SMCs in the amount of 2 × 105/well were incubated in the absence (○) or presence (●) of 100 μg/mL oxLDL for 12 hours. The initial rates of fXa generation were determined in the chromogenic assay performed at constant concentrations of fVIII (1 nM) and fX (140 nM) and indicated concentrations of fIXa. Each data point represents the mean value ± SD of triplicates. The experimental data were fitted to an equation describing the equilibrium bindingV = Vsat[fIXa]/Kapp+ [fIXa], where V is the initial rate of fX activation, [fIXa] is the concentration of fIXa, Vsat is the rate of fX activation at the saturating [fIXa], and Kapp is the apparent affinity of fVIIIa for fIXa. The data were fitted to this equation using Marquart algorithm and SigmaPlot 1.02 computer program. (B) Determination of the kinetic parameters of fX activation. FVIII (1 nM), fIXa (1 nM), and indicated concentrations of fX were added to SMCs incubated in the absence (○) or presence (●) of oxLDL, which was followed by determination of the initial rates of fXa generation as in panel A. Each data point represents the mean value ± SD of triplicates. The curves show the best fit of the data to the Michaelis equationV = Vmax[fX]/Km+ [fX], where V is the initial rate of fX activation, [fX] is the concentration of fX, Vmax is the rate of fX activation at its saturating concentration, and Km is the Michaelis constant. The data were fitted to the above equation as in panel A.
Effect of oxLDL treatment of SMC on assembly and activity of the Xase complex.
(A) Determination of the apparent affinity of fVIIIa/fIXa interaction. SMCs in the amount of 2 × 105/well were incubated in the absence (○) or presence (●) of 100 μg/mL oxLDL for 12 hours. The initial rates of fXa generation were determined in the chromogenic assay performed at constant concentrations of fVIII (1 nM) and fX (140 nM) and indicated concentrations of fIXa. Each data point represents the mean value ± SD of triplicates. The experimental data were fitted to an equation describing the equilibrium bindingV = Vsat[fIXa]/Kapp+ [fIXa], where V is the initial rate of fX activation, [fIXa] is the concentration of fIXa, Vsat is the rate of fX activation at the saturating [fIXa], and Kapp is the apparent affinity of fVIIIa for fIXa. The data were fitted to this equation using Marquart algorithm and SigmaPlot 1.02 computer program. (B) Determination of the kinetic parameters of fX activation. FVIII (1 nM), fIXa (1 nM), and indicated concentrations of fX were added to SMCs incubated in the absence (○) or presence (●) of oxLDL, which was followed by determination of the initial rates of fXa generation as in panel A. Each data point represents the mean value ± SD of triplicates. The curves show the best fit of the data to the Michaelis equationV = Vmax[fX]/Km+ [fX], where V is the initial rate of fX activation, [fX] is the concentration of fX, Vmax is the rate of fX activation at its saturating concentration, and Km is the Michaelis constant. The data were fitted to the above equation as in panel A.
The parameters characterizing activity of the Xase complex assembled on untreated and oxLDL-treated cells were determined by varying fX concentration at constant concentrations of fVIIIa and fIXa. The kinetics of fX activation on both treated and untreated cells (Figure4B) were adequately described by the Michaelis equation. The obtained value of Vmax = 4.9 ± 0.4 nM/min for oxLDL-treated cells was 4.7 times higher than that for control cells, Vmax = 0.98 ± 0.108 nM/min, consistent with a higher concentration of membrane-bound fVIIIa/fIXa complex. In contrast, the difference between Km values for treated and control SMCs was insignificant (22.3 ± 4.7 nM and 19.42 ± 4.9 nM, respectively), suggesting that oxLDL does not affect the affinity of fX for the fVIIIa/fIXa complex.
FVIII is associated with macrophage- and SMC-derived foam cells in atherosclerotic lesions
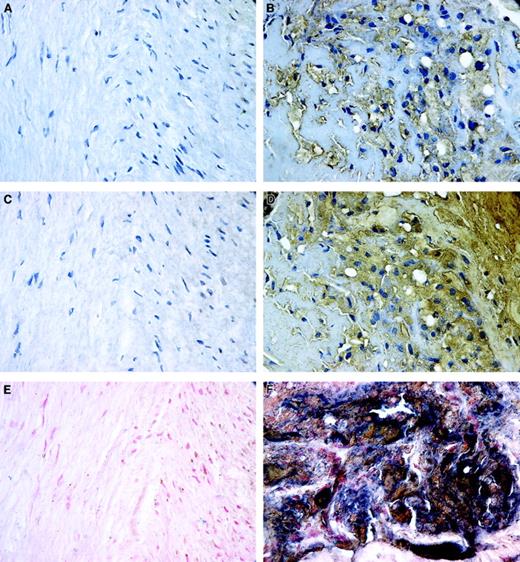
The elevated fVIII binding to oxLDL-treated macrophages and SMCs in vitro suggests that in human atherosclerotic lesions fVIII may be associated with lipid-laden macrophage- or SMC-derived foam cells. We first examined the patterns of distribution of fVIII and oxLDL in human atherosclerotic lesion by staining 19 human atherectomy specimens for oxLDL or fVIII. Figure 5 shows a representative atherectomy specimen containing regions of both normal tissue and atheroma. Normal tissue containing nonpathologic quiescent SMCs showed no staining for oxLDL (Figure 5A), whereas in the atheromatous region both intracellular and extracellular deposits of oxLDL were detected (Figure 5B), consistent with reported massive accumulation of oxLDL in advanced lesions.52 We next performed staining of serial sections of the same specimen for fVIII. We attempted to detect the A1/A3-C1-C2 dimer of fVIIIa, which remains membrane-associated on fVIIIa inactivation caused by dissociation of the A2 subunit.53 We used mAb ESH8, which binds to the C2 domain of fVIII and does not prevent its binding to the phospholipid membrane.37 Notably, intensive staining for fVIII was observed only in the atheromatous regions (Figure 5D), but not in the normal tissue (Figure 5C). Double staining for oxLDL and fVIII revealed large areas staining black in atheromatous regions (Figure 5F), which resulted from the superimposing of positive staining for oxLDL (brown) and fVIII (blue). In contrast, regions of normal tissue were negative for both markers (Figure 5E). These results indicate that fVIII is present in human atherosclerotic lesions and its accumulation correlates with deposition of oxLDL.
Patterns of localization of fVIII and oxLDL in atherosclerotic lesions.
Human coronary atherectomy sections containing normal media (A,C,E) and atheromatous regions (B,D,F) were stained for oxLDL (A,B) or fVIII (C,D) using mAbs OXL41.1 and ESH8, respectively, and double stained for both components (E,F). The single and double staining were performed as described in “Materials and methods.” Positive staining for oxLDL and fVIII in panels B and D is brown; cell nuclei in panels A through D are counterstained with hematoxylin. In double-stained images, panels E and F, accumulations of fVIII (blue) and oxLDL (brown) frequently overlap as evidenced by presence of areas stained black. Cell nuclei in panels E and F were counterstained with nuclear fast red. Microscopy was performed using an Eclipse E800 microscope at magnification × 600.
Patterns of localization of fVIII and oxLDL in atherosclerotic lesions.
Human coronary atherectomy sections containing normal media (A,C,E) and atheromatous regions (B,D,F) were stained for oxLDL (A,B) or fVIII (C,D) using mAbs OXL41.1 and ESH8, respectively, and double stained for both components (E,F). The single and double staining were performed as described in “Materials and methods.” Positive staining for oxLDL and fVIII in panels B and D is brown; cell nuclei in panels A through D are counterstained with hematoxylin. In double-stained images, panels E and F, accumulations of fVIII (blue) and oxLDL (brown) frequently overlap as evidenced by presence of areas stained black. Cell nuclei in panels E and F were counterstained with nuclear fast red. Microscopy was performed using an Eclipse E800 microscope at magnification × 600.
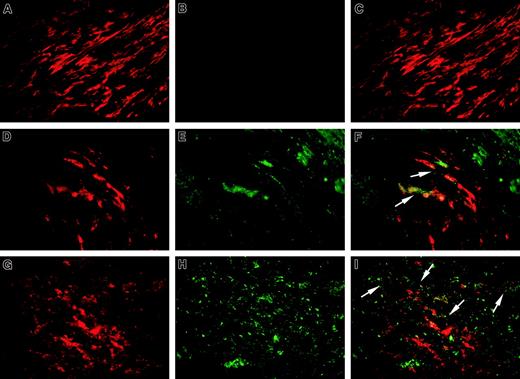
We next performed double-label immunofluorescence staining of 6 atherectomy specimens, where massive deposits of oxLDL were detected, for fVIII using mAb ESH8 and for α-actin or CD68, which are specific markers of SMCs and macrophages, respectively. A representative specimen is shown in Figure 6. Regions of normal media containing spindle-shaped α-actin–positive SMCs (red fluorescence, Figure 6A) did not show fVIII positivity (Figure 6B and merged image in panel C). In oxLDL-rich regions of advanced lesions we selected areas enriched in SMCs (Figure 6D) and macrophages (Figure6G), and fVIII presence in both regions was evidenced by green fluorescence (Figure 6E and 6H, respectively). Corresponding merged images (Figure 6F,I) revealed that fVIII was present in the vicinity of SMCs and macrophages. In the control experiment, we performed the staining for fVIII using mAb NMC-VIII/10 with the epitope within the acidic region of fVIII light chain, which is removed on fVIII activation. Lack of appreciable fVIII-related fluorescence in the serial section of the same specimen (data not shown) confirmed the specificity of ESH8 staining for fVIIIa. Thus, immunohistochemical studies of human atherosclerotic tissues revealed accumulation of fVIII in the vicinity of macrophages and SMCs within oxLDL- rich regions.
Detection of fVIII, macrophages, and SMCs in atherosclerotic lesions.
Sections of a human coronary atherectomy specimen containing normal media (A-C) and atheromatous regions (D-I) were double stained for fVIII (B,E,H, green fluorescence) and α-actin (A,D,red fluorescence) or CD68 (G, red fluorescence) as specific markers for SMCs and macrophages, respectively. Staining procedure is described in “Materials and methods. The single-stained images were obtained in an Eclipse E800 microscope at magnification × 1000 using selective fluorescent filter blocks. The merged images (C,F,I) were obtained by superimposing single-stained images using SPOT Advance Program Mode. Arrows in panels F and I point to fVIII staining (green fluorescence) on the periphery of selected SMCs and macrophages, respectively (red fluorescence). The staining patterns similar to those presented in this figure were found in all specimens analyzed.
Detection of fVIII, macrophages, and SMCs in atherosclerotic lesions.
Sections of a human coronary atherectomy specimen containing normal media (A-C) and atheromatous regions (D-I) were double stained for fVIII (B,E,H, green fluorescence) and α-actin (A,D,red fluorescence) or CD68 (G, red fluorescence) as specific markers for SMCs and macrophages, respectively. Staining procedure is described in “Materials and methods. The single-stained images were obtained in an Eclipse E800 microscope at magnification × 1000 using selective fluorescent filter blocks. The merged images (C,F,I) were obtained by superimposing single-stained images using SPOT Advance Program Mode. Arrows in panels F and I point to fVIII staining (green fluorescence) on the periphery of selected SMCs and macrophages, respectively (red fluorescence). The staining patterns similar to those presented in this figure were found in all specimens analyzed.
oxLDL dramatically increases thrombin formation on macrophages and SMCs
To assess the magnitude of the overall procoagulant effect of oxLDL, we determined the maximal rates of thrombin formation on the cells incubated with oxLDL for 12 hours, when maximal stimulation of the Xase activity was observed. Thrombin formation was measured in the presence of components of both Xase (fVIII, fIXa, and fX) and prothrombinase (fV and prothrombin) complexes. Incubation with oxLDL led to a 10-fold and 20-fold increase in the maximal rates of thrombin formation on the surface of SMC and macrophages, respectively (Figure7A,B and Table 1). The effect was much less pronounced for HAECs, where the increase was only 2.2-fold (Figure7C). Native LDL moderately increased the rates of thrombin generation on all cell types, which may be due to cell-mediated oxidation of LDL.54 55
Effect of oxLDL on thrombin generation via the intrinsic pathway.
Human SMCs (A), macrophages (B), or HAECs (C) in the amount of 2 × 105/well were incubated in the absence of lipoproteins (○) or in the presence of 100 μg/mL oxLDL (●) or native LDL (▴) for 12 hours at 37°C. Following incubation, the conversion of prothrombin (1.4 μM) into thrombin in the presence of fVIII (1 nM), fIXa (2 nM), fV (20 nM), and fX (170 nM) was measured in a chromogenic assay as described in “Materials and methods.” In the control experiment (⋄), the reaction was performed in wells incubated with oxLDL in the absence of cells. Each data point represents the mean value ± SD of triplicates.
Effect of oxLDL on thrombin generation via the intrinsic pathway.
Human SMCs (A), macrophages (B), or HAECs (C) in the amount of 2 × 105/well were incubated in the absence of lipoproteins (○) or in the presence of 100 μg/mL oxLDL (●) or native LDL (▴) for 12 hours at 37°C. Following incubation, the conversion of prothrombin (1.4 μM) into thrombin in the presence of fVIII (1 nM), fIXa (2 nM), fV (20 nM), and fX (170 nM) was measured in a chromogenic assay as described in “Materials and methods.” In the control experiment (⋄), the reaction was performed in wells incubated with oxLDL in the absence of cells. Each data point represents the mean value ± SD of triplicates.
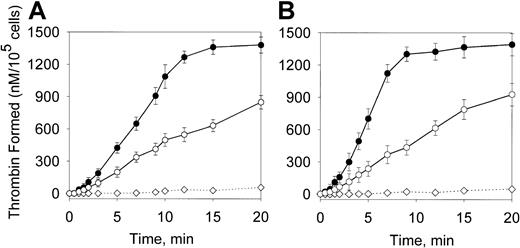
The contribution of prothrombinase in the overall increase of thrombin formation was assessed by performing the assay in the presence of components of the prothrombinase complex only. The concentrations of fV and prothrombin were as in Figure 7 and fXa concentration was 8 nM, which constitutes approximately 50% of maximal fXa concentration formed in the Xase assay (Figure 1). The maximal rates of thrombin formation on SMCs and macrophages were increased by oxLDL by 2.2-fold and 3.1-fold, respectively (Figure 8). This indicates that acceleration of thrombin formation on oxLDL-treated cells (Figure 7) results mainly from the increase of the intrinsic Xase activity and less from the increase of the prothrombinase activity. Comparison of 125I-fV binding to control and oxLDL-treated SMCs and macrophages revealed a moderate 1.6-fold and 2.1-fold increase in the binding (data not shown). Thus, oxLDL dramatically increases the ability of macrophages and SMCs to support thrombin formation in the intrinsic pathway and this effect is mainly due to activation of the intrinsic Xase.
Effect of oxLDL on the prothrombinase activity.
Human SMCs (A) or macrophages (B) were incubated in the absence (○) or in the presence (●) of oxLDL as described in Figure 7. Following incubation, the conversion of prothrombin (1.4 μM) into thrombin in the presence of fXa (8 nM) and fV (20 nM) was measured in a chromogenic assay as described in “Materials and methods.” In the control experiment (⋄), the reaction was performed in wells incubated with oxLDL in the absence of the cells. Each data point represents the mean value ± SD of triplicates.
Effect of oxLDL on the prothrombinase activity.
Human SMCs (A) or macrophages (B) were incubated in the absence (○) or in the presence (●) of oxLDL as described in Figure 7. Following incubation, the conversion of prothrombin (1.4 μM) into thrombin in the presence of fXa (8 nM) and fV (20 nM) was measured in a chromogenic assay as described in “Materials and methods.” In the control experiment (⋄), the reaction was performed in wells incubated with oxLDL in the absence of the cells. Each data point represents the mean value ± SD of triplicates.
Relationship between procoagulant activity of oxLDL-treated cells and apoptosis
Because oxLDL may induce apoptosis in SMCs,28,29macrophages,32,33 and ECs,30 31 we tested whether the observed increase in the procoagulant activity of oxLDL-treated cells is related to apoptosis. TUNEL assay did not reveal DNA fragmentation in macrophages and ECs and only 3% to 4% SMCs showed positive nuclear staining (data not shown). This indicates that fragmentation of chromatin, typical for advanced apoptotic stages, is not required for development of the maximal procoagulant activity of oxLDL-treated cells. We next tested the effect of inhibitors of early stages of apoptosis, including a scavenger of free radicals BHT, an inhibitor of acidic sphingomyelinase (the major enzyme involved in ceramide generation) desipramine, and a general caspase inhibitor Z-VAD-FMK, on thrombin generation. BHT and desipramine had comparable inhibitory effects on thrombin formation on oxLDL-treated macrophages and SMCs (Figure 9A), suggesting that free radical–mediated processes and ceramide generation are related to an increase in their procoagulant activity. Notably, a general caspase inhibitor was more effective on SMCs compared to macrophages, indicating that the maximal increase in the procoagulant activity of SMC may correspond to a more advanced stage of apoptosis. Neither of the inhibitors affected thrombin generation on oxLDL-treated HAECs.
Effects of inhibitors of early stage of apoptosis on thrombin generation and fVIII binding.
Human macrophages, SMCs, or HAECs in the amount of 2 × 105/well were incubated with 100 μg/mL oxLDL in the absence or presence of BHT (open bars), desipramine (gray bars), or a general caspase inhibitor Z-VAD-FMK (hatched bars) for 12 hours at 37°C as described in “Materials and methods.” (A) Thrombin generation in the presence of inhibitors. Following incubation, the conversion of prothrombin into thrombin in the intrinsic pathway was measured as described in Figure 7. The maximal rate of thrombin formation on oxLDL-treated cells in the absence of inhibitors was arbitrary defined as 100% (control). The maximal rate of thrombin formation on oxLDL-treated cells in the presence of inhibitors is expressed as percent of control. (B) fVIII binding in the presence of inhibitors. Binding of 125I-fVIII to oxLDL-treated cells in the absence (solid bars) or presence (open bars), of BHT desipramine (gray bars), or a general caspase inhibitor Z-VAD-FMK (hatched bars) was performed as described in Figure 2. Each bar in panels A and B represents the mean ± SD of 3 determinations.
Effects of inhibitors of early stage of apoptosis on thrombin generation and fVIII binding.
Human macrophages, SMCs, or HAECs in the amount of 2 × 105/well were incubated with 100 μg/mL oxLDL in the absence or presence of BHT (open bars), desipramine (gray bars), or a general caspase inhibitor Z-VAD-FMK (hatched bars) for 12 hours at 37°C as described in “Materials and methods.” (A) Thrombin generation in the presence of inhibitors. Following incubation, the conversion of prothrombin into thrombin in the intrinsic pathway was measured as described in Figure 7. The maximal rate of thrombin formation on oxLDL-treated cells in the absence of inhibitors was arbitrary defined as 100% (control). The maximal rate of thrombin formation on oxLDL-treated cells in the presence of inhibitors is expressed as percent of control. (B) fVIII binding in the presence of inhibitors. Binding of 125I-fVIII to oxLDL-treated cells in the absence (solid bars) or presence (open bars), of BHT desipramine (gray bars), or a general caspase inhibitor Z-VAD-FMK (hatched bars) was performed as described in Figure 2. Each bar in panels A and B represents the mean ± SD of 3 determinations.
Because the increase in the procoagulant activity of oxLDL-treated cells correlated with the increase in fVIII binding, we tested whether inhibitors of early apoptosis affect this binding. Noteworthy, BHT, desipramine, and a general caspase inhibitor attenuated fVIII binding in a pattern similar to their effects on thrombin formation as shown in panels B and A, respectively, of Figure 9. Binding of125I-annexin V to oxLDL-treated SMCs was inhibited by 35%, 22% and 30% by BHT, desipramine, and Z-VAD-FMK, respectively (data not shown). The above experiments suggest that the processes associated with early apoptotic stages (free radical formation, ceramide generation, and activation of caspases) may be related to development of the maximal procoagulant activity of oxLDL-treated cells in the intrinsic pathway.
Discussion
Although increased thrombogenicity of advanced atherosclerotic plaques has been linked to activation of the extrinsic, TF-dependent pathway of blood coagulation, the present study suggests that the intrinsic pathway significantly contributes to thrombus formation.
We found that exposure of macrophages and SMCs to oxLDL significantly enhanced their ability to support activity of 2 major complexes of the intrinsic pathway of blood coagulation, the Xase and prothrombinase complexes. This resulted in a 20- and 10-fold increase in thrombin formation for macrophages and SMCs, respectively, mainly due to activation of the intrinsic Xase complex. The magnitude of the effect of oxLDL on the procoagulant activity of macrophages and SMCs in the intrinsic pathway determined in our study is comparable to the reported increase in the activity of the extrinsic coagulation pathway, which constituted 30-fold for cultured human macrophages,5624-fold for macrophages isolated from atherosclerotic plaques,57 and 5- to 6-fold for cultured SMCs.58 The modest oxLDL-induced increase in the intrinsic procoagulant activity of HAECs determined by us is similar to that previously determined for human umbilical vein endothelial cells,23 however, less pronounced than the reported 4-fold increase in the extrinsic procoagulant activity.59Although oxLDL induces TF expression in macrophages60 and ECs61 and up-regulates its expression in SMCs,58 the effects observed in our study were solely due to oxLDL-induced activation of the intrinsic pathway, because fVIIa, the counterpart of TF, was not present in the assays. We demonstrated that the increase in the intrinsic procoagulant activity is related to formation of additional fVIII binding sites due to intensive translocation of PS to the outer surface of oxLDL-treated cells. This was evidenced by a pronounced increase in the binding of125I-fVIII and annexin V, a specific probe for PS, to the surface of oxLDL-treated cells. Furthermore, we found that interaction between Xase components, fVIII and fIXa, on the surface of oxLDL-treated SMC occurs with a 5-fold higher affinity. Both phenomena are consistent with the reported dependence of the number of fVIII binding sites50 and fVIIIa/fIXa affinity20 on the PS content in phospholipid membranes. Together, the increased fVIII binding and a higher affinity of fVIIIa for fIXa provide a higher concentration of functional Xase complex on the surface of oxLDL-treated cells. The detected modest 2-fold increase in fV binding to oxLDL-treated cells may be related to a lower sensitivity of fV binding to the PS content of membrane.50
Consistent with high levels of fVIII bound to the surface of oxLDL-treated macrophages and SMCs in vitro, immunohistochemical studies on human coronary atherectomy specimens revealed the presence of fVIII in the vicinity of macrophages and SMCs in oxLDL-enriched atheromatous regions but not in the normal tissue. These data are in favor of possible involvement of the intrinsic coagulation pathway in thrombus formation at the site of atherosclerotic lesion.
Redistribution of PS, which is likely responsible for the observed increase in the intrinsic procoagulant activity of oxLDL-treated cells, is also one of indicators of apoptosis.32,62 Several reports state that apoptotic cells become procoagulant.35,63,64 Because oxLDL can induce apoptosis in macrophages,32,33 SMCs,26,28 and ECs,30,31 we explored a link between the increased procoagulant activity of oxLDL-treated cells and apoptosis. We found that processes occurring at early apoptotic stages, including free radical–induced changes in the cell membrane and generation of ceramide, are related to activation of the intrinsic pathway. This was demonstrated by the ability of BHT (a scavenger of free radicals), desipramine (an inhibitor of acid sphyngomyelinase responsible for ceramide generation), and a general caspase inhibitor to decrease the rates of thrombin generation and fVIII binding to oxLDL-treated macrophages and SMCs. We did not detect, however, DNA fragmentation in oxLDL-treated cells, typical for the terminal apoptotic stage. We cannot exclude, therefore, that activation of the intrinsic pathway on macrophages and SMCs may be related to nonapoptotic translocation of PS induced by components of the oxLDL particle. This possibility is suggested by a recent report that oxidative derivatives of phosphatidylethanolamine, the active component of oxLDL, are responsible for augmentation of the platelet prothrombinase activity by inducing exposure of PS on their surface.65
Our results suggest that the intrinsic coagulation pathway, in addition to the TF-dependent pathway, accounts for the dramatic increase in thrombogenicity of atherosclerotic lesion in the course of its progression. The observed modest 2-fold increase in thrombin formation by oxLDL-treated HAECs in the intrinsic pathway implies that thrombogenicity of lesions with the preserved endothelial layer is mainly determined by oxLDL-induced TF expression in endothelial cells leading to a 4- to 7-fold increase in the extrinsic procoagulant activity.59 This moderate increase in the overall procoagulant activity of dysfunctional endothelium is consistent with its role as the protective barrier preventing plaque components from direct contact with blood. In light of our findings, a dramatic increase in plaque thrombogenicity with the loss of the endothelial layer integrity is determined by more than an order of magnitude increase in the procoagulant activity of SMCs and macrophages both in the intrinsic and extrinsic pathways. At the final stage, plaque rupture results in massive exposure to blood flow of plaque material depleted in SMCs and enriched in lipid-laden macrophage-derived foam cells. The highest oxLDL-induced increase in the procoagulant activity of this cell type in both the extrinsic pathway (24- to 30-fold56 57) and intrinsic pathway (20-fold in our experiments) may explain the burst of thrombogenicity of vulnerable atheromatous plaques, which is an acknowledged cause of sudden cardiac death.
In conclusion, we demonstrated that in vitro treatment of human macrophages and SMC with oxLDL dramatically increases their ability to support thrombin formation via the intrinsic pathway, which is likely to be an additional mechanism determining thrombogenicity of the atherosclerotic plaque.
We are indebted to Dr Christian Haudenschild (Department of Pathology, Holland Laboratory, American Red Cross, Rockville, MD) for providing human atherectomy specimens. We also express our gratitude to Dr Edward Tuddenham (MRC Clinical Sciences Center, Imperial College School of Medicine, London, United Kingdom), Drs Andrey Sarafanov, Larisa Cervenakova, Alexey Khrenov and Alexey Belkin (Holland Laboratory) for their critical review of the manuscript and helpful discussions.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-11-0140.
Supported by National Institutes of Health-National Heart, Lung and Blood Institute RO1 grant HL66101 awarded to E.L.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Evgueni L. Saenko, Department of Biochemistry, Holland Laboratory, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855; e-mail:saenko@usa.redcross.org.

![Fig. 4. Effect of oxLDL treatment of SMC on assembly and activity of the Xase complex. / (A) Determination of the apparent affinity of fVIIIa/fIXa interaction. SMCs in the amount of 2 × 105/well were incubated in the absence (○) or presence (●) of 100 μg/mL oxLDL for 12 hours. The initial rates of fXa generation were determined in the chromogenic assay performed at constant concentrations of fVIII (1 nM) and fX (140 nM) and indicated concentrations of fIXa. Each data point represents the mean value ± SD of triplicates. The experimental data were fitted to an equation describing the equilibrium bindingV = Vsat[fIXa]/Kapp+ [fIXa], where V is the initial rate of fX activation, [fIXa] is the concentration of fIXa, Vsat is the rate of fX activation at the saturating [fIXa], and Kapp is the apparent affinity of fVIIIa for fIXa. The data were fitted to this equation using Marquart algorithm and SigmaPlot 1.02 computer program. (B) Determination of the kinetic parameters of fX activation. FVIII (1 nM), fIXa (1 nM), and indicated concentrations of fX were added to SMCs incubated in the absence (○) or presence (●) of oxLDL, which was followed by determination of the initial rates of fXa generation as in panel A. Each data point represents the mean value ± SD of triplicates. The curves show the best fit of the data to the Michaelis equationV = Vmax[fX]/Km+ [fX], where V is the initial rate of fX activation, [fX] is the concentration of fX, Vmax is the rate of fX activation at its saturating concentration, and Km is the Michaelis constant. The data were fitted to the above equation as in panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/12/10.1182_blood-2001-11-0140/4/m_h81222725004.jpeg?Expires=1763528849&Signature=HtBPwGcjLjAUP5I~xodIxoq8dL-Ce4K18vucpegnqNTmLkoZmwGJPMYXk370X8wT8-44URne77jO84Za23hkZpuYcpcllkCBMiAiQ6dxjr-feZjl4VPiP~XJl2-pgiWyelybY9S0AbUPLhbi3SBveQUAF6mBSSm5QN~9-JMcTQQycfD1IY75e27OXESIdiI4FuMZEB3OiwfginQoEu-9VIp8P2r3RZ~HtInaMi5lLeQyvalY9vVdap8~AEUKz~rlXU2xarRhreORqecauF66wE4rkZF46nEc-hCBJM~mwbtgYXuRSiioK1BZ94osHXjWHLGMekL5CztwjxKIW5rUlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal