The capacity of an adenovirus encoding the mature form of vascular endothelial growth factor (VEGF)–D, VEGF-DΔNΔC, to induce angiogenesis, lymphangiogenesis, or both was analyzed in 2 distinct in vivo models. We first demonstrated in vitro that VEGF-DΔNΔC encoded by the adenovirus (Ad-VEGF-DΔNΔC) is capable of inducing endothelial cell proliferation and migration and that the latter response is primarily mediated by VEGF receptor-2 (VEGFR-2). Second, we characterized a new in vivo model for assessing experimental angiogenesis, the rat cremaster muscle, which permits live videomicroscopy and quantitation of functional blood vessels. In this model, a proangiogenic effect of Ad-VEGF-DΔNΔC was evident as early as 5 days after injection. Immunohistochemical analysis of the cremaster muscle demonstrated that neovascularization induced by Ad-VEGF-DΔNΔC and by Ad-VEGF-A165 (an adenovirus encoding the 165 isoform of VEGF-A) was composed primarily of laminin and VEGFR-2–positive vessels containing red blood cells, thus indicating a predominantly angiogenic response. In a skin model, Ad-VEGF-DΔNΔC induced angiogenesis and lymphangiogenesis, as indicated by staining with laminin, VEGFR-2, and VEGFR-3, whereas Ad-VEGF-A165 stimulated the selective growth of blood vessels. These data suggest that the biologic effects of VEGF-D are tissue-specific and dependent on the abundance of blood vessels and lymphatics expressing the receptors for VEGF-D in a given tissue. The capacity of Ad-VEGF-DΔNΔC to induce endothelial cell proliferation, angiogenesis, and lymphangiogenesis demonstrates that its potential usefulness for the treatment of coronary artery disease, cerebral ischemia, peripheral vascular disease, restenosis, and tissue edema should be tested in preclinical models.
Introduction
The vascular endothelial growth factor (VEGF) family of secreted glycoproteins plays a prominent role in angiogenesis, the formation of blood vessels from pre-existing ones, and lymphangiogenesis, the growth of the lymphatics.1Therapeutic angiogenesis2 is likely to have a major impact on the management of clinical disorders characterized by tissue ischemia.2,3 VEGFs can promote the growth of blood vessels,4-6 and angiogenic factors can be delivered as purified protein or through plasmids and viruses.7 The appeal of a genetic vector, such as the adenovirus, is its provision of an efficient burst of gene expression with a single administration that subsequently diminishes without integration of genetic material into the patient's genome.
The mammalian VEGF family is composed of VEGF-A,8 the first member identified, and VEGF-B, VEGF-C, VEGF-D, and placental growth factor.1,9 VEGF-A has at least 4 isoforms generated by alternative RNA splicing,1 each containing a common region that mediates interaction with 2 receptor tyrosine kinases expressed on vascular endothelium in developing tissues and tumors, VEGF receptor-1 (VEGFR-1; flt-1),8 and VEGFR-2 (KDR/flk-1).1,10 The ability to promote endothelial cell (EC) proliferation, migration, and angiogenesis is attributed primarily to signaling through VEGFR-2.8 Other VEGF family members contain a receptor-binding region that is highly conserved in primary structure, designated the VEGF homology domain (VHD).11 12
Recent studies have characterized the biologic responses induced by VEGF-C. Overexpression of full-length VEGF-C in skin during embryonic development caused hyperplasia of lymphatic vessels.13 This response is in contrast to that induced by VEGF-A, which stimulated the development of blood vessels.14 In agreement with these findings, in vivo studies using full-length VEGF-C delivered by adenovirus demonstrated that the subcutaneous expression of VEGF-C induced primarily lymphangiogenesis, whereas VEGF-A stimulated angiogenesis.15 Overall, in vivo studies have implicated VEGF-C and its receptor, VEGFR-3, in the process of lymphatic vessel development.16 However, VEGF-C induced the formation of blood vessels in a rabbit model of hindlimb ischemia,5mouse cornea, and chick embryo17 indicating potential usefulness for therapeutic angiogenesis. These findings suggest that the response to VEGF-C could be tissue-specific.
The most recently characterized mammalian member of the VEGF family, VEGF-D,11,18,19 is closely related in primary structure to VEGF-C. Receptor specificities of human VEGF-C and VEGF-D are the same because both bind and activate VEGFR-2 and VEGFR-311,12; however, mouse VEGF-D binds to only one mouse receptor, VEGFR-3,20 expressed on lymphatic endothelium, whereas mouse VEGF-C activates human VEGFR-2 and VEGFR-3.21 These differences do not permit reliable extrapolation of in vivo studies using VEGF-C in mice to VEGF-D. Both VEGF-C and VEGF-D are produced as precursor molecules that have poor affinity for VEGF receptors.22,23 Subsequent cleavage of the carboxyl and amino terminal propeptides liberates the mature, truncated form of VEGF-D, VEGF-DΔNΔC, consisting of the VHD.22 Human VEGF-DΔNΔC binds with high affinity to VEGFR-2 and VEGFR-3.11,22 Given the central role of VEGFR-2 in angiogenesis8 and VEGFR-3 in lymphangiogenesis,24 it would be expected that human VEGF-DΔNΔC might be an effective inducer of angiogenesis and lymphangiogenesis in vivo. In an initial study, human VEGF-D was shown to be angiogenic in a rabbit retina assay,25 though lymphangiogenesis was not analyzed. In contrast, VEGF-D induced lymphatic growth but not angiogenesis in the skin of transgenic mice during embryonic development.16 In a mouse tumor model, human VEGF-D induced angiogenesis, lymphangiogenesis, and metastatic spread through the lymphatics.6 However, the capacity of VEGF-D to promote angiogenesis or lymphangiogenesis in normal adult tissues has not been assessed.
The aim of this study was to generate an adenovirus encoding human VEGF-DΔNΔC (Ad-VEGF-DΔNΔC) and to determine its ability to induce angiogenesis or lymphangiogenesis in 2 distinct in vivo rat models. A common problem with most existing animal models of angiogenesis is their limited ability to provide an adequate assessment of a complex 3-dimensional network of blood vessels using 2-dimensional tissue sections. Therefore, an additional purpose of this study was to develop a new experimental model to assess angiogenesis, namely the rat cremaster muscle.
Materials and methods
Adenovirus construction and purification
cDNA encoding the VHD of human VEGF-D tagged at the N-terminus with the FLAG octapeptide (VEGF-DΔNΔC/FLAG)11 was ligated into the BamHI site of the pCA13 adenoviral shuttle vector (Microbix Biosystems, Toronto, ON, Canada). The adenovirus was generated by cotransfection of the VEGF-D vector and pJM17 into 293 cells (American Type Culture Collection, Manassas, VA). Single plaque-forming colonies were isolated and expanded using naive 293 cells. Three cycles of plaque purification and virus expansion were performed to ensure a single viral clone. The virus was designated Ad-VEGF-DΔNΔC. We also used Ad-VEGF-A165, kindly provided by Genvec (Gettysburg, MD) and an adenovirus-expressing green fluorescence protein (Ad-GFP; Quantum Biotechnologies, Montreal, Quebec, Canada). For viral purification, subconfluent monolayers of 293 cells were exposed to 100 pfu virus until a moderate cytopathic effect (CPE) was observed. Cell pellets were suspended in 5 mL Dulbecco modified essential medium (DMEM)/F12 medium (BioWhittaker, Walkersville, MD), exposed to 5 freeze-thaw cycles, and centrifuged at 3000g for 5 minutes. Adenoviruses were purified using a double cesium chloride banding procedure and were dialyzed extensively against TBS buffer (50 mM Tris, 150 mM NaCl, pH 8.0) at 4°C.
Purification of VEGF-DΔNΔC/FLAG
Subconfluent 293 cells were transfected with Ad-VEGF-DΔNΔC in the presence of 2% fetal calf serum (FCS; BioWhittaker). After overnight incubation, the medium was replaced with fresh DMEM/F12 medium containing 5 mM glutamine and 10% FCS. Once a near-complete cytopathic effect was observed, the cell supernatants were centrifuged at 1000g for 10 minutes and sterilized using a 0.22 μm filter. VEGF-DΔNΔC/FLAG was purified by affinity chromatography with anti-FLAG–agarose (Sigma, St Louis, MO) and was analyzed by SDS-PAGE followed by silver staining (Bio-Rad, Hercules, CA), Coomassie blue staining, or Western blotting with anti-FLAG or anti–VEGF-D antibodies (R&D Systems, Minneapolis, MN).
Endothelial cell migration and proliferation assays
Primary human umbilical vein endothelial cells (HUVECs) were grown to confluence in DMEM/F12 medium supplemented with 15% fetal bovine serum, 150 μg/mL endothelial growth factor (Clonetics, San Diego, CA), and 90 μg/mL heparin (Sigma).26 Cells were maintained in 1% serum for 20 hours before the experiments. Cell migration assays were performed as previously described27using transwells (8-μm pore size; Corning Costar, Acton, MA). Before placement in transwells, HUVECs were preincubated for 10 minutes, with or without blocking antibody against β3 integrin (c7E3 at 20 μg/mL; Centocor, Malvern, PA), VEGFR-2/Fc chimera, or anti–VEGFR-2 neutralizing antibodies (10 μg/mL each) (R&D Systems) in the presence of an additional 1 mM CaCl2 before stimulation with growth factors. Migration was quantified by performing microscopic cell counts at ×200 magnification on 10 to 12 random fields in each well as described.27 Each experiment was performed in triplicate and was repeated at least 3 times. Cell proliferation assays were performed as described.28
Rat cremaster muscle model
Externalization of the cremasteric sac from Sprague-Dawley male rats (Harlan, Indianapolis, IN) was achieved under pentobarbital as previously described.29 Testicles were not removed. The cremasteric sac was visualized, and 75 injections (108 pfu adenovirus/injection or phosphate-buffered saline [PBS]) were made directly into the cremasteric muscle through the adventitial sac under direct visualization. Ethilon sutures (6-0) were placed 5-mm cephalad to each injection site for subsequent localization. The sac was replaced within the scrotal cavity, and the skin was sutured. Animals were returned to their cages for 5 days or 3 weeks (n = 6/group). At those times, the animals were sedated with pentobarbital anesthesia, and cremasteric dissection was performed for live videomicroscopy as previously described.29 Number of functional blood vessels per field was determined by a person blinded to the treatments. Following live videomicroscopy, the cremaster muscles were transected and formalin fixed for 2 to 10 days. Cremaster muscles were paraffin-embedded in a horizontal orientation and sectioned throughout their entire lengths, and 6-μm sections were obtained.
Skin model of angiogenesis
Sprague-Dawley rats were injected in the left mammary line in the subcutaneous space with 109 pfu adenovirus or PBS in a volume of 0.5 mL (n = 7/group). The epigastric skin and muscle were carefully dissected, and representative areas were excised and placed in formalin. Tissue was paraffin-embedded, and 6-μm sections were cut.
Immunohistochemistry
Tissue sections (6 μm) were stained using polyclonal antibodies against von Willebrand factor (VWF; DAKO, Carpinteria, CA), laminin (Sigma), VEGFR-2 and VEGFR-3 (Santa Cruz Biotechnology, Santa Cruz, CA) or VEGF-D (R&D). Formalin-fixed and paraffin-embedded sections were digested with 0.1% Trypsin in PBS supplemented with 0.1% calcium chloride for 20 minutes at 37°C and were exposed to 3% hydrogen peroxide diluted in 0.02 M PBS (pH 7.4) for 5 minutes at room temperature to block endogenous peroxidase activity. Sections were incubated in a blocking solution (PBS containing 10% normal goat serum and 2% bovine serum albumin) for 1 hour at 37°C. Primary antibody diluted with PBS containing 2% goat serum was applied and incubated overnight at 4°C. After washing with PBS, the slides were treated with biotinylated secondary antibody, washed again, and treated with streptavidin-conjugated horseradish peroxidase according to the manufacturer's protocol (Vector Laboratories, Burlingame, CA). The signal was visualized using 0.05% 3.3′-diaminobenzidine tetrahydrochloride (DAKO) in 0.05 M Tris buffer (pH 7.6) containing 0.003% hydrogen peroxide. Sections were counterstained with hematoxylin (Vector). A negative control was performed to ensure the specificity of peroxidase immunostaining by replacing primary antibody with a nonimmune rabbit IgG. Sections were examined with a Leica DML microscope, and representative areas were photographed using a ×20 and a ×40 objective and the “MagnaFire” program. Vessels were quantified at ×200 using an ocular lens with grid. At least 20 fields were quantified in 4 different cremasters. The relative area of vessels was quantified using ×10 eyepieces equipped with 10 × 1 mm by 10 × 1 mm graticule and was expressed as a percentage of area of the grid. For controls, 30 fields were analyzed in 4 different skin samples. At least 40 fields were counted in 3 cremasters for each experimental point, and significance was analyzed using the paired t test in SigmaPlot.
Results
Characterization of VEGF-D adenovirus
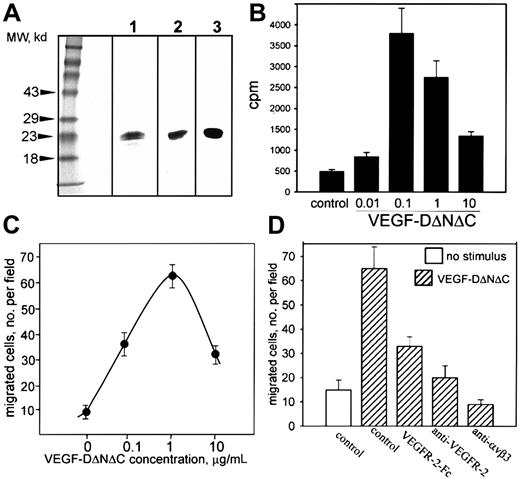
A nonreplicating adenovirus encoding FLAG-tagged human VEGF-DΔNΔC (Ad-VEGF-DΔNΔC) was generated as described in “Materials and methods.” Cells (293) were infected with Ad-VEGF-DΔNΔC, and VEGF-DΔNΔC/FLAG was purified from cell lysate by affinity chromatography. VEGF-DΔNΔC/FLAG migrated as a doublet with Mr 20 to 22 kd (Figure 1A, lane 1).11 The identity of the protein was confirmed by Western blot analysis with anti-FLAG and anti–VEGF-D antibodies (Figure 1A, lanes 2 and 3). Therefore, Ad-VEGF-DΔNΔC induced the production of VEGF-D in vitro.
Characterization of VEGF-D.
(A) VEGF-DΔNΔC/FLAG (20 μg purified protein) was analyzed by SDS-PAGE under reducing conditions and was detected by silver staining (lane 1) or Western blotting with anti-FLAG2 or anti–VEGF-D3 antibodies. (B) Thymidine incorporation into endothelial cells induced by VEGF-DΔNΔC/FLAG. (C) Effect of VEGF-DΔNΔC/FLAG on endothelial cell migration toward vitronectin (10 μg/mL) in a modified Boyden chamber. (D) Specificity of endothelial cell migration induced by VEGF-DΔNΔC/FLAG. Effects of VEGFR-2-Fc chimera and anti–VEGFR-2 or anti-αVβ3 blocking antibodies (10 μg/mL each) are shown. Data are means ± SD of 3 separate experiments.
Characterization of VEGF-D.
(A) VEGF-DΔNΔC/FLAG (20 μg purified protein) was analyzed by SDS-PAGE under reducing conditions and was detected by silver staining (lane 1) or Western blotting with anti-FLAG2 or anti–VEGF-D3 antibodies. (B) Thymidine incorporation into endothelial cells induced by VEGF-DΔNΔC/FLAG. (C) Effect of VEGF-DΔNΔC/FLAG on endothelial cell migration toward vitronectin (10 μg/mL) in a modified Boyden chamber. (D) Specificity of endothelial cell migration induced by VEGF-DΔNΔC/FLAG. Effects of VEGFR-2-Fc chimera and anti–VEGFR-2 or anti-αVβ3 blocking antibodies (10 μg/mL each) are shown. Data are means ± SD of 3 separate experiments.
The capacity of VEGF-DΔNΔC/FLAG to induce proliferation and migration of ECs in vitro was assessed using HUVECs. VEGF-DΔNΔC/FLAG stimulated the proliferation of HUVECs with a maximum activity at 100 ng/mL (Figure 1B), similar to that reported for VEGF-D in an in vitro angiogenesis assay.25 The response was reduced at higher VEGF-D concentrations, as has been observed previously with VEGF.30 VEGF-DΔNΔC/FLAG was an effective stimulator of EC migration in vitro (Figure 1C). Using vitronectin as an extracellular matrix ligand, a substantial number of ECs migrated onto the substratum in response to 100 ng/mL VEGF-DΔNΔC/FLAG; the maximum effect was with 1 μg/mL. Higher concentrations of ligand blunted migration, similar to the effect observed in proliferation assays. FACS analysis (not shown) and previously published reports31 32 demonstrated that HUVECs express receptors for VEGF-D, VEGFR-2, and VEGFR-3. To verify the specificity of VEGF-D action on EC migration, soluble VEGFR-2–IgG chimeric protein was included and produced approximately 66% inhibition (Figure 1D). Neutralizing VEGFR-2 antibodies also inhibited the migratory response, demonstrating a primary role for VEGFR-2 (Figure 1D).
VEGF-DΔNΔC expression in vivo
A rat cremaster muscle model was selected for in vivo assessment of the angiogenic effect of Ad-VEGF-DΔNΔC. This model is widely used to investigate the process of leukocyte rolling by live video microscopy. The flat, thin cremaster muscle provides a unique opportunity to quantify functional blood vessels—vessels with flowing red and white cells—by videomicroscopy.
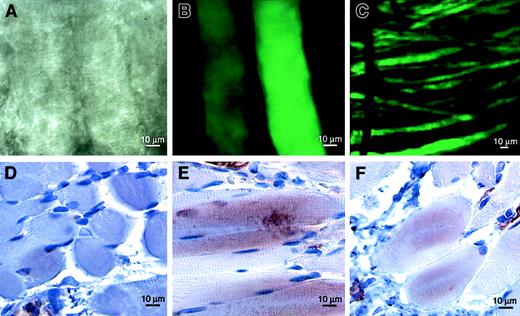
To confirm that adenovirus is capable of effective gene transfer in the cremaster muscle, we used Ad-GFP as a reporter. Five days after 108 pfu Ad-GFP were injected directly into the muscle, intense green fluorescence could be visualized using confocal microscopy (Figure 2A-C). Although most fluorescence was localized in the region of the injection, occasional coursing fluorescent muscle fibers could be tracked over distances of nearly 2 cm. Fluorescence within the tissue was greatly diminished by 3 weeks after Ad-GFP injection but was still detectable (not shown). To document the persistence of VEGF-D expression resulting from treatment with Ad-VEGF-DΔNΔC, we stained tissue sections of cremasters with anti–VEGF-D antibodies at 3, 5, 14, and 21 days after injection. The most intense staining for VEGF-D was 5 days after injection; more than 80% of muscle fibers and fibroblasts were positively stained in the area of angiogenesis (Figure 2E). Staining of approximately 50% of cells was still observed 21 days after injection of Ad-VEGF-DΔNΔC (Figure 2F) but not of Ad-GFP (Figure 2D). A similar expression level was observed with Ad-VEGF-A165. The percentages of cells positively stained with anti-VEGF polyclonal antibodies 5 and 21 days after injection were 75% and more than 60%. Therefore, VEGF-D was expressed in tissue for a considerable period of time.
VEGF-D expression in vivo.
In situ gene transfer to rat cremaster muscle was confirmed by direct visualization of GFP using confocal fluorescence microscopy. Discrete muscle fibers were visualized using phase-contrast (A) and fluorescence (B, C) microscopy 5 days after the injection of Ad-GFP. Immunostaining for VEGF-D revealed maximum expression 5 days (E) and detectable levels 21 days (F) after the injection of Ad-VEGF-DΔNΔC but not of control Ad-GFP (D). Positive staining was observed in muscle fibers, fibroblasts, and blood vessel walls.
VEGF-D expression in vivo.
In situ gene transfer to rat cremaster muscle was confirmed by direct visualization of GFP using confocal fluorescence microscopy. Discrete muscle fibers were visualized using phase-contrast (A) and fluorescence (B, C) microscopy 5 days after the injection of Ad-GFP. Immunostaining for VEGF-D revealed maximum expression 5 days (E) and detectable levels 21 days (F) after the injection of Ad-VEGF-DΔNΔC but not of control Ad-GFP (D). Positive staining was observed in muscle fibers, fibroblasts, and blood vessel walls.
Analysis of angiogenesis induced by Ad-VEGF-DΔNΔC in the cremaster muscle
Live videomicroscopy allowed direct visualization and quantitation of functional capillaries, as demonstrated by flowing red and white blood cells (for comparison of blood vessel density in Ad-VEGF-DΔNΔC–treated cremasters vs control, see the attached video). Comparison of the vessel density at 7 days after injection, made by counting the number of vessels within 5 randomly chosen high-power fields surrounding an injection site, demonstrated a statistically significant increase in the number of vessels in the cremasters treated with Ad-VEGF-DΔNΔC compared with the cremasters injected with either Ad-GFP (P < .001) or PBS control (P < .005) (Figure 3A). To compare the angiogenic activity of VEGF-DΔNΔC with that of VEGF-A165, we used an adenovirus encoding VEGF-A165 in the same model. Figure 3A summarizes the results of 6 separate experiments. Ad-VEGF-DΔNΔC injection resulted in a 2-fold increase in the density of blood vessels, whereas Ad-VEGF-A165 stimulated a 2.2-fold increase compared with Ad-GFP. Differences between the effects of Ad-VEGF-DΔNΔC and Ad-VEGF-A165 were not statistically significant.
Ad-VEGF-DΔNΔC induces angiogenesis in cremaster muscle.
(A) Results of live videomicroscopy. The density of blood vessels was quantified 7 days after injections with Ad-VEGF-DΔNΔC, Ad-VEGF-A165, Ad-GFP, and PBS. Paired t test revealed that the increase in the density of functional vessels was statistically significant for Ad-VEGF-DΔNΔC and Ad-VEGF-A165 rather than for PBS and Ad-GFP (P < .001 in all cases). The difference in the effects of Ad-VEGF-DΔNΔC and Ad-VEGF-A165 was not statistically significant. An average of 3 to 7 capillaries per field was observed in the Ad-GFP group. Data are means ± SE of 6 separate experiments. (B) Quantification of angiogenesis stimulated by Ad-VEGF-DΔNΔC 7 days after injection. Serial tissue sections were stained for VWF and laminin, and positively stained vessels per grid were counted using a ×20 objective. All fields in 4 different cremasters for each group were analyzed. A 100% value was assigned to VWF-positive vessels, and percentages denote the proportion of vessels that were also laminin-positive. Data are means ± SE. (C) Tissue sections stained for laminin and VEGFR-2. A 100% value was assigned to laminin-positive vessels, and percentages in the panel denote the proportion of vessels that were also VEGFR-2–positive. Data are means ± SE.
Ad-VEGF-DΔNΔC induces angiogenesis in cremaster muscle.
(A) Results of live videomicroscopy. The density of blood vessels was quantified 7 days after injections with Ad-VEGF-DΔNΔC, Ad-VEGF-A165, Ad-GFP, and PBS. Paired t test revealed that the increase in the density of functional vessels was statistically significant for Ad-VEGF-DΔNΔC and Ad-VEGF-A165 rather than for PBS and Ad-GFP (P < .001 in all cases). The difference in the effects of Ad-VEGF-DΔNΔC and Ad-VEGF-A165 was not statistically significant. An average of 3 to 7 capillaries per field was observed in the Ad-GFP group. Data are means ± SE of 6 separate experiments. (B) Quantification of angiogenesis stimulated by Ad-VEGF-DΔNΔC 7 days after injection. Serial tissue sections were stained for VWF and laminin, and positively stained vessels per grid were counted using a ×20 objective. All fields in 4 different cremasters for each group were analyzed. A 100% value was assigned to VWF-positive vessels, and percentages denote the proportion of vessels that were also laminin-positive. Data are means ± SE. (C) Tissue sections stained for laminin and VEGFR-2. A 100% value was assigned to laminin-positive vessels, and percentages in the panel denote the proportion of vessels that were also VEGFR-2–positive. Data are means ± SE.
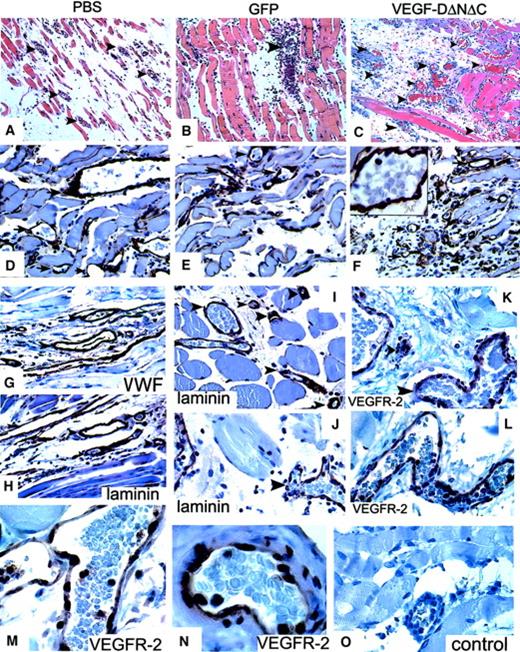
The induction of vascular structures by Ad-VEGF-DΔNΔC was clearly demonstrable by routine hematoxylin and eosin staining. Vascular channels were visualized by day 5 and consisted of small slitlike channels and larger cavernous structures (Figure4C). At 3 weeks fewer, but still persistent, thin-walled vessels were seen in the cremasters treated with Ad-VEGF-DΔNΔC, but no vessel formation was observed in those treated with Ad-GFP (Figure 4E-F). Ad-VEGF-DΔNΔC and Ad-GFP were associated with persistent mononuclear infiltrate, but this was more intense in the Ad-GFP group, demonstrating that the inflammatory response did not induce angiogenesis (Figures 3, 4). Because videomicroscopy allows the detection of only relatively large functional vessels and underestimates the number of newly formed capillaries, we performed staining of tissue sections using VWF as a marker of ECs. As can be seen from the results of counting VWF-positive vessels (Figure 3B), Ad-VEGF-DΔNΔC and Ad-VEGF165 induced 2.5- and 3-fold increases in blood vessel density, respectively. To further characterize the pattern of neovascularization induced by Ad-VEGF-DΔNΔC, we stained tissue sections for laminin, a component of blood vessel basement membrane. The increase in the number of laminin-containing vessels after Ad-VEGF-DΔNΔC injection is evident in Figure 3B and Figure 4D-F. We sought to further characterize vascular structures using specific markers of the vascular system (Figure 4 G-O). Quantitative analyses showed that most VWF-positive vessels also contained laminin (Figure3B). In the control Ad-GFP group, 91.7% of VWF-positive vessels were also positive for laminin; for the Ad-VEGF-DΔNΔC and Ad-VEGF-A165 groups, the values were 87% and 100%, respectively. Because VEGFR-2 is primarily expressed by ECs of blood vessels,33 we performed similar analysis for laminin and VEGFR-2–stained serial tissue sections. Of laminin-positive vessels 87.4%, 76.1%, and 93.7% were clearly blood vessels in the GFP, VEGF-DΔNΔC, and VEGF-A165 groups, respectively, as indicated by VEGFR-2 staining (Figure 3C). Virtually all VEGFR-2–positive vessels contained erythrocytes (Figure 4L-M).
Analysis of the response in the cremaster muscle to Ad-VEGF-DΔNΔC.
(A-C) Tissue sections of cremaster muscle 5 days after injection (hematoxylin and eosin staining; ×20 objective). (A) PBS induced moderate interstitial edema surrounding skeletal muscle. No vascular proliferation was seen. (B) Ad-GFP induced chronic inflammation consisting of lymphocytes and tissue monocytes with mild interstitial edema. No significant vascular proliferation. (C) Ad-VEGF-DΔNΔC induced interstitial edema and a central focus of vessels with the appearance of venous channels. Blood vessels are indicated by arrowheads. (D-F) Sections taken 3 weeks after injections were stained for laminin. Ad-VEGF-DΔNΔC induced a distinct increase in the number of laminin-positive blood capillaries; ×40 objective. (F) Higher magnification (×100 objective) is shown in the inset. Arrows indicate blood vessels. (G-L) Pairs of serial tissue sections from cremaster muscle 21 days after Ad-VEGF-DΔNΔC injection were stained for VWF (G) and laminin (H) and for VEGFR-2 (K, L) and laminin (I, J). A wide range of vascular, blood-filled, irregular venous channels could be identified between muscle fibers. Some were thin-walled with a single EC layer between the lumen and adjacent tissue. Blood vessels (bv) are shown and in some cases are denoted by arrowheads. Panels G-L, ×50 objective. (M, N) VEGFR-2 expression 21 days after treatment with Ad-VEGF-DΔNΔC. (O) Negative control. Panels M-O, ×100 objective.
Analysis of the response in the cremaster muscle to Ad-VEGF-DΔNΔC.
(A-C) Tissue sections of cremaster muscle 5 days after injection (hematoxylin and eosin staining; ×20 objective). (A) PBS induced moderate interstitial edema surrounding skeletal muscle. No vascular proliferation was seen. (B) Ad-GFP induced chronic inflammation consisting of lymphocytes and tissue monocytes with mild interstitial edema. No significant vascular proliferation. (C) Ad-VEGF-DΔNΔC induced interstitial edema and a central focus of vessels with the appearance of venous channels. Blood vessels are indicated by arrowheads. (D-F) Sections taken 3 weeks after injections were stained for laminin. Ad-VEGF-DΔNΔC induced a distinct increase in the number of laminin-positive blood capillaries; ×40 objective. (F) Higher magnification (×100 objective) is shown in the inset. Arrows indicate blood vessels. (G-L) Pairs of serial tissue sections from cremaster muscle 21 days after Ad-VEGF-DΔNΔC injection were stained for VWF (G) and laminin (H) and for VEGFR-2 (K, L) and laminin (I, J). A wide range of vascular, blood-filled, irregular venous channels could be identified between muscle fibers. Some were thin-walled with a single EC layer between the lumen and adjacent tissue. Blood vessels (bv) are shown and in some cases are denoted by arrowheads. Panels G-L, ×50 objective. (M, N) VEGFR-2 expression 21 days after treatment with Ad-VEGF-DΔNΔC. (O) Negative control. Panels M-O, ×100 objective.
In summary, immunohistochemical analysis of cremaster muscle demonstrated that neovascularization induced by Ad-VEGF-DΔNΔC and by Ad-VEGF-A165 is composed primarily of laminin and VEGFR-2–positive vessels containing red blood cells, thus providing convincing evidence of a predominantly angiogenic response.
Effects of Ad-VEGF-DΔNΔC in the skin
The absence of significant lymphangiogenesis in the cremaster muscle in response to Ad-VEGF-DΔNΔC was in contrast to previous observations of the effect of an adenovirus encoding VEGF-C,15 and it prompted us to assess effects in a second model. Because the skin is the most widely used in vivo system, we injected Ad-GFP, Ad-VEGF-DΔNΔC, and Ad-VEGF-A165 in rats subcutaneously and analyzed the patterns of neovascularization. One week after injection, intense hypervascularity was observed in the hypodermis of animals treated with Ad-VEGF-DΔNΔC compared with animals treated with Ad-GFP or PBS (Figure5). Blood vessels were identified by the presence of intraluminal red blood cells (Figure 5A-C) and by positive staining for laminin (Figure 5D-F) and VEGFR-2 (Figure 5G-I). There was prominent infiltration by lymphocytes, tissue monocytes, eosinophils, and mast cells in the connective tissue of skin treated with Ad-GFP and Ad-VEGF-DΔNΔC (Figure 5B-C), consistent with a chronic inflammatory response caused by adenovirus injection. The intense inflammatory infiltrate surrounding vessels was markedly diminished in the Ad-VEGF-DΔNΔC group by the third week (not shown).
Vasculature induced by Ad-VEGF-DΔNΔC in skin.
Focal areas of hypodermis taken from rat 1 week after subdermal injection with PBS (A, D, G), Ad-GFP (B, E, H), or Ad-VEGF-DΔNΔC (C, F, I). Hematoxylin and eosin–stained tissue sections are shown (A-C); ×20 objective. (A) Small tuft of vessels primarily composed of veins with surrounding monocytes, lymphocytes, and macrophages. (B) Irregular, small veins with some signs of inflammation. (C) Venous channels with extremely irregular branching, consistent with an area of vascular proliferation. Tissue sections were stained for laminin (D-F) or VEGFR-2 (G-I). Dramatic increase in the density of laminin and VEGFR-2–positive blood vessels as a result of Ad-VEGF-DΔNΔC injection is evident. Panels D-I, ×40 objective.
Vasculature induced by Ad-VEGF-DΔNΔC in skin.
Focal areas of hypodermis taken from rat 1 week after subdermal injection with PBS (A, D, G), Ad-GFP (B, E, H), or Ad-VEGF-DΔNΔC (C, F, I). Hematoxylin and eosin–stained tissue sections are shown (A-C); ×20 objective. (A) Small tuft of vessels primarily composed of veins with surrounding monocytes, lymphocytes, and macrophages. (B) Irregular, small veins with some signs of inflammation. (C) Venous channels with extremely irregular branching, consistent with an area of vascular proliferation. Tissue sections were stained for laminin (D-F) or VEGFR-2 (G-I). Dramatic increase in the density of laminin and VEGFR-2–positive blood vessels as a result of Ad-VEGF-DΔNΔC injection is evident. Panels D-I, ×40 objective.
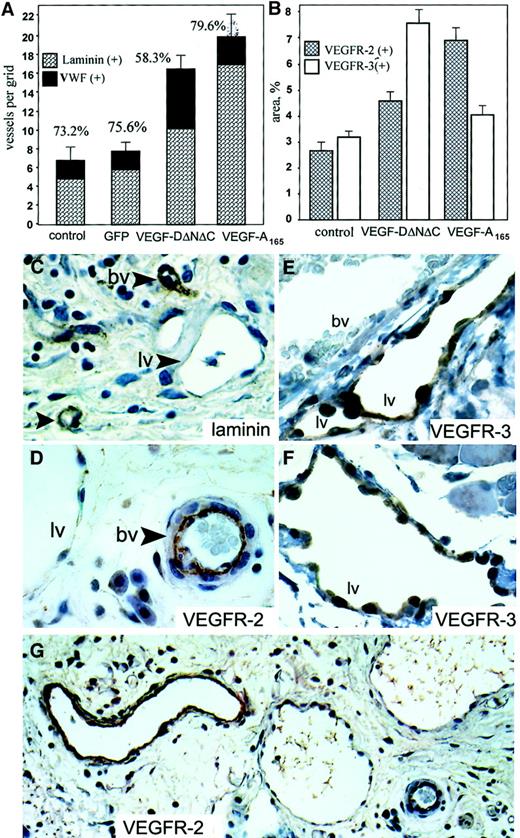
In the subcutaneous fat of animals treated with Ad-VEGF-DΔNΔC, there was a higher density of slitlike structures that did not contain red blood cells, consistent with lymphatic growth. Lymphatic channels were identified as numerous irregular, large, cavernous structures with a single cell lining, little to no muscle layer, absence of VEGFR-2 staining (Figure 6D-G) and weak or absent staining for laminin (Figure 6C). Most of these structures also stained positively for VWF even though the staining was weak compared with blood vessels (not shown). This pattern is consistent with previously published characteristics of lymphatic vessels.34
Angiogenesis and lymphangiogenesis induced by Ad-VEGF-DΔNΔC in skin.
Tissue sections were taken 3 weeks after injection of PBS, Ad-GFP, Ad-VEGF-DΔNΔC, or Ad-VEGF-A165. (A) Serial sections were stained for VWF, and laminin and positive vessels per field were quantified. A 100% value was assigned to VWF-positive vessels, and percentages denote the proportion of vessels that were also laminin positive. Bars represent means ± SE for 3 separate tissue samples in each group. (B) Sections were stained for VEGFR-2 and VEGFR-3, and vessel areas were measured using a 10 × 10 graticule. All areas in 4 × 20-mm tissue samples were analyzed. Bars represent percentages of area covered by positively stained vessels ± SE. Note the predominant formation of VEGFR-2–positive vessels after Ad-VEGF-A165 injection and VEGFR-3–positive vessels induced by Ad-VEGF-DΔNΔC. (C-G) Samples 3 weeks after injection of Ad-VEGF-DΔNΔC. Tissue sections stained for laminin (C), VEGFR-2 (G; higher magnification of the same area in D; ×20 objective), and VEGFR-3 (E, F) are shown. Blood vessels (bv) and lymphatic vessels (lv) are indicated. Lymphatic channels with single endothelial cell layer were not stained for laminin (C) or for VEGFR-2 (D), but they demonstrated significant expression of VEGFR-3 (F). Note the absence of VEGFR-3 staining of vessels filled with red blood cells (E). Panels C-F, ×50 objective.
Angiogenesis and lymphangiogenesis induced by Ad-VEGF-DΔNΔC in skin.
Tissue sections were taken 3 weeks after injection of PBS, Ad-GFP, Ad-VEGF-DΔNΔC, or Ad-VEGF-A165. (A) Serial sections were stained for VWF, and laminin and positive vessels per field were quantified. A 100% value was assigned to VWF-positive vessels, and percentages denote the proportion of vessels that were also laminin positive. Bars represent means ± SE for 3 separate tissue samples in each group. (B) Sections were stained for VEGFR-2 and VEGFR-3, and vessel areas were measured using a 10 × 10 graticule. All areas in 4 × 20-mm tissue samples were analyzed. Bars represent percentages of area covered by positively stained vessels ± SE. Note the predominant formation of VEGFR-2–positive vessels after Ad-VEGF-A165 injection and VEGFR-3–positive vessels induced by Ad-VEGF-DΔNΔC. (C-G) Samples 3 weeks after injection of Ad-VEGF-DΔNΔC. Tissue sections stained for laminin (C), VEGFR-2 (G; higher magnification of the same area in D; ×20 objective), and VEGFR-3 (E, F) are shown. Blood vessels (bv) and lymphatic vessels (lv) are indicated. Lymphatic channels with single endothelial cell layer were not stained for laminin (C) or for VEGFR-2 (D), but they demonstrated significant expression of VEGFR-3 (F). Note the absence of VEGFR-3 staining of vessels filled with red blood cells (E). Panels C-F, ×50 objective.
We quantified the percentage of vascular structures containing laminin-positive basement membranes by staining serial tissue sections with VWF and laminin. In groups of mice treated with PBS, Ad-GFP, and Ad-VEGF-A165, 73.2%, 75.6%, and 79.6% of VWF-positive vessels were positive for laminin, respectively. In contrast, we observed a significant shift in the percentage of laminin-positive structures from 75.6% (Ad-GFP) to 58.3% (P < .01) after Ad-VEGF-DΔNΔC injection. This difference implies that the remaining 41.7% of vascular structures that have no laminin could be lymphatic vessels. To further characterize the specificity of the Ad-VEGF-DΔNΔC–induced vascular response, we performed staining of tissue sections for VEGFR-2, specific for blood vessels (reviewed in1), and for VEGFR-3, specific for lymphatic endothelium.10,34 Anti–VEGFR-3 antibodies stained primarily large, thin-walled vascular structures that can be identified as lymphatic vessels (Figure 6E-F). Consistent with previous reports,35 we observed weak VEGFR-3 staining of some blood-filled capillaries, though most blood vessels were VEGFR-3–negative (Figure 6E). Quantitation of vessel density revealed that Ad-VEGF-DΔNΔC induced a 1.72-fold increase in the number of VEGFR-3–positive lymphatic vessels compare with Ad-GFP (P < .005) and a 1.85-fold increase in the number of VEGFR-2–positive vessels (P < .001). We also observed that in skin tissue the density of pre-existing VEGFR-3–positive vessels was approximately one third the total number of capillaries compared with cremaster muscle, whereas lymphatic vessels represented only 12% to 15% (Figure 6A).
To demonstrate the ratio of the area covered by VEGFR-2–positive vascular structures to that for vessels stained for VEGFR-3, we used an approach similar to that described by Enholm et al15because, though lymphatic vessels could be fewer than blood capillaries, their volume or area could be significantly greater because of hyperplasticity. After staining of parallel tissue sections for VEGFR-2 and VEGFR-3, the relative areas of vascular structures were quantified. As shown in Figure 6B, Ad-VEGF-DΔNΔC induced a 1.6-fold increase in the area covered by VEGFR-2–positive blood vessels and a 2.5-fold increase for VEGFR-3–positive lymphatic vessels. In contrast, Ad-VEGF165 stimulated predominantly the development of VEGFR-2–positive vessels, which is in agreement with previously published findings.36
Discussion
Human VEGF-D binds VEGFR-2 and VEGFR-3 on ECs,11 but the mature form, VEGF-DΔNΔC, has a much higher binding affinity for both receptors than the unprocessed form.11,22 Our in vitro studies demonstrated that VEGF-DΔNΔC is an effective stimulator of the proliferation and migration of blood vessel ECs and that the migratory response of ECs that express VEGFR-2 and VEGFR-3 in vitro is almost completely blocked by anti–VEGFR-2 antibodies, demonstrating a key role for VEGFR-2 in this process. This result is consistent with the finding of Kadambi et al,37 showing that neutralizing anti–VEGFR-2 antibodies inhibit the vascularization of VEGF-C overexpressing tumors in vivo.
It has been reported that persistent expression of an angiogenic growth factor is required to promote the formation of a mature vascular network.38,39 In the present study, we used a new model of experimental angiogenesis, the cremaster muscle, in which the expression of VEGF-DΔNΔC and the control GFP reached a maximum 3 to 5 days after adenoviral injection but was still detectable for at least 3 weeks. Ad-VEGF-DΔNΔC injection doubled the density of fully functional blood vessels and was similar to the effect of Ad-VEGF-A165. The proangiogenic effect of Ad-VEGF-DΔNΔC was evident as early as 5 days after injection. Treatment with Ad-VEGF-DΔNΔC and Ad-VEGF-A165 was associated with an inflammatory infiltrate; however, a similar inflammatory response was observed with a control adenovirus, Ad-GFP, which did not induce an angiogenic response. Most vessels that formed in response to Ad-VEGF-DΔNΔC were blood vessels because they stained positively for VEGFR-2, contained red blood cells, and had basement membranes positive for laminin. In fact, only 20% of vessels in Ad-VEGF-DΔNΔC–treated muscle did not express VEGFR-2. This proportion was even lower in Ad-VEGF-A165–treated tissue. Overall, we observed a predominantly angiogenic response in the cremaster muscle to Ad-VEGF-DΔNΔC and to Ad-VEGF-A165, in agreement with studies using VEGF-D in the rabbit cornea assay.25
The capacity of VEGF-C to induce angiogenesis in some tissues and lymphangiogenesis in others5,13,15 and the lack of information about VEGF-D prompted us to assess and compare the biologic activity of Ad-VEGF-DΔNΔC in different tissues. We used the common skin model of angiogenesis and observed 2.4- and 3-fold increases in the density of VWF-positive vessels 1 week after Ad-VEGF-DΔNΔC and Ad-VEGF-A165 injection, respectively, but no changes in response to control Ad-GFP. Increases in the number of laminin and VEGFR-2–stained vessels induced by Ad-VEGF-DΔNΔC was evident; however, approximately half the vascular channels lacked laminin-containing basement membranes and VEGFR-2 staining. These channels were positively stained for VEGFR-3, which is expressed on lymphatic ECs.34 Quantitation of VEGFR-2– and VEGFR-3–positive vessels demonstrated that Ad-VEGF-DΔNΔC induced lymphangiogenesis and angiogenesis in the skin model, whereas Ad-VEGF-A165 stimulated the selective growth of blood vessels. Given that Ad-VEGF-DΔNΔC stimulated a predominantly angiogenic response in muscle, these results demonstrate that the tissue environment influences the biologic effects of VEGF-DΔNΔC. We have used the fully processed form, VEGF-DΔNΔC, which, in contrast to full-length VEGF-D, binds to VEGFR-2 and, therefore, is likely to be more angiogenic. We also observed a significantly higher density of pre-existing lymphatic vessels in skin than in cremaster muscle. Thus, it is possible that the availability of ECs expressing one or another receptor (VEGFR-2 or VEGFR-3) in the region of treatment with adenovirus could, in part, determine the outcome of therapy with VEGF-D or VEGF-C. However, we cannot exclude the possibility that the differential recruitment of circulating progenitors of ECs40 could also contribute to this process. Our findings suggest that to adequately assess the potential of an angiogenic–lymphangiogenic compound in a given therapeutic setting, an appropriate in vivo model must be selected. The differential functional consequences in different tissues do not permit, for example, the extrapolation of results obtained in a skin model to the treatment of ischemic conditions in the heart.
In summary, our studies provide the first demonstration that VEGF-DΔNΔC, when administered in adenoviral form, induces the formation of functional vasculature in adult tissues. In muscle tissue, Ad-VEGF-DΔNΔC stimulated primarily an angiogenic response, similar to that of Ad-VEGF-A165. In contrast, in skin we observed not only angiogenesis but also the formation of lymphatic vessels. Ad-VEGF-DΔNΔC clearly has potential usefulness for therapeutic angiogenesis and lymphangiogenesis in the context of coronary artery disease, cerebral ischemia, peripheral vascular disease, restenosis, and tissue edema.2 41
Supported in part by an American Heart Association Grant-in Aid (T.V.B.) and by National Institutes of Health grants HL29582 and HL34727 (P.E.D.). M.G.A. and S.A.S. are supported by the National Health and Medical Research Council of Australia and the Anti-Cancer Council of Victoria. Human umbilical vein endothelial cells were obtained from cords collected through the Birthing Services Department at the Cleveland Clinic Foundation and the Perinatal Clinical Research Center (National Institutes of Health GCRC award RR-00080) at the Cleveland Metrohealth Hospital.
T.V.B. and C.K.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tatiana V. Byzova, Joseph J. Jacobs Center for Thrombosis and Vascular Biology, Departments of Molecular Cardiology, Cardiology and Taussig Cancer Center, NB50, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195; e-mail:byzovat@ccf.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal