Second malignant neoplasms are a serious complication after successful treatment of childhood acute lymphoblastic leukemia (ALL). With improvement in survival, it is important to assess the impact of contemporary risk-based therapies on second neoplasms in ALL survivors. A cohort of 8831 children diagnosed with ALL and enrolled on Children's Cancer Group therapeutic protocols between 1983 and 1995 were observed to determine the incidence of second neoplasms and associated risk factors. The median age at diagnosis of ALL was 4.7 years. The cohort had accrued 54 883 person-years of follow-up. Sixty-three patients developed second neoplasms, including solid, nonhematopoietic tumors (n = 39: brain tumors n = 19, other solid tumors n = 20), myeloid leukemia or myelodysplasia (n = 16), and lymphoma (n = 8). The cumulative incidence of any second neoplasm was 1.18% at 10 years (95% confidence interval, 0.8%-1.5%), representing a 7.2-fold increased risk compared with the general population. The risk was increased significantly for acute myeloid leukemia (standardized incidence ratio [SIR] 52.3), non-Hodgkin lymphoma (SIR 8.3), parotid gland tumors (SIR 33.4), thyroid cancer (SIR 13.3), brain tumors (SIR 10.1), and soft tissue sarcoma (SIR 9.1). Multivariate analysis revealed female sex (relative risk [RR] 1.8), radiation to the craniospinal axis (RR 1.6), and relapse of primary disease (RR 3.5) to be independently associated with increased risk of all second neoplasms. Risk of second neoplasms increased with radiation dose (1800 cGy RR 1.5; 2400 cGy RR 3.9). Actuarial survival at 10 years from diagnosis of second neoplasms was 39%. Follow-up of this large cohort that was treated with contemporary risk-based therapy showed that the incidence of second neoplasms remains low after diagnosis of childhood ALL.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy, with an annual rate of 3 to 4 cases per 100 000 children.1 Population-based data indicate that children with ALL treated with contemporary therapy have a 5-year survival rate of 80%.2 With 2500 to 3000 children diagnosed with ALL annually, we expect approximately 2000 long-term survivors of childhood ALL each year. Long-term sequelae of treatment, such as impaired intellectual and psychomotor functioning,3 neuroendocrine abnormalities,4impaired reproductive capacity,5-8cardiotoxicity,9 and second malignant neoplasms10-17 are being reported with increased frequency in the growing cohort of survivors.
For survivors of childhood ALL, the estimated actuarial risk of developing a second neoplasm has been reported to be 2.5% at 15 years from diagnosis.11 Among second neoplasms observed after treatment of ALL, central nervous system (CNS) tumors in patients treated with cranial irradiation are the most common. Other commonly reported second neoplasms in this population include lymphoma, acute myeloid leukemia (AML), and thyroid cancer.10,11,13,14,18-31 However, most reports of second neoplasms are limited by relatively few patients treated on contemporary risk-based therapeutic protocols and observed for reasonable lengths of time. To address these concerns, we followed a cohort of 8831 children who had been treated on 1 of the 12 Children's Cancer Group (CCG) therapeutic protocols between 1983 and 1995 and had accrued 54 883 person-years of follow-up. Among 8831 patients, 3599 (41%) had been part of a previous report.11 The follow-up of these 3599 patients was 6 years (range, 3 to 8 years) at the time of that report.11 Therefore, this study is an update of the previous report (increased follow-up to a median of 15 years) and an extension of the cohort to include 5232 additional patients.
Patients and methods
The CCG conducted clinical trials in cooperation with member institutions throughout the United States and Canada. At the time of analysis, the group included 122 institutions, which were required to register all newly diagnosed cancer patients with the operations office, after which eligible patients were entered into active therapeutic clinical trials. The operations office was responsible for determining patient eligibility, randomized assignments to the appropriate therapeutic arms (if necessary), and follow-up of patients for all potential outcomes. Member institutions were required to submit follow-up reports on all patients enrolled in therapeutic protocols. Those reports included information on survival status, disease status, and development of second malignancies for all patients. The follow-up reports are submitted annually for as long as patients live.
Our cohort consisted of 8831 children with newly diagnosed ALL who were younger than 21 years at diagnosis and were enrolled in 1 of the 12 therapeutic protocols for untreated ALL conducted by CCG between 1983 and 1995. These twelve protocols were CCG-104, -105, -106, -107, -123, -139, -1881, -1882, -1883, -1891, -1901, and -1922. Informed consent was obtained from patients, parents, or guardians at the time of enrollment. The patients were randomized to 1 of 2 or more therapeutic schedules of chemotherapy or radiation. The total length of therapy ranged from 2 to 3 years. Clinical results of many of the trials, with the therapeutic plans, have been published.32-42
For each protocol, a therapeutic summary was prepared that included the dose of radiation therapy (and assigned fields) and chemotherapeutic exposures. Assigned cumulative doses were calculated for cyclophosphamide and the anthracyclines (daunomycin and doxorubicin). The total assigned cumulative doses ranged from 0 to 16 200 (females) and 24 000 (males) mg/m2 of body surface area for cyclophosphamide and from 0 to 500 mg/m2 of body surface area for anthracyclines. Assigned radiation doses ranged from 0 to 1800 cGy to the cranium (for CNS prophylaxis) and 2400 cGy to the cranium and 600 to 1200 cGy to the spine (for treatment of CNS disease). The authors reviewed the records of patients with second neoplasms to assess the actual doses of chemotherapeutic agents given to them. Most patients received more than 95% of their prescribed doses of parenteral chemotherapeutic agents. The actual doses given to the patients of oral drugs such as methotrexate and 6-MP were lower (73%), but exposure to oral antimetabolites was not evaluated in our analyses.
Patients also were categorized into “early” and “recent” treatment eras. The early treatment era corresponded to the period when patients were treated according to 1 of 6 therapeutic protocols open between 1983 and 1989 (CCG-104, -105, -106, -107, -123, and -139 [n = 3713]). The recent treatment era corresponded to the period when patients were treated according to the remaining 6 therapeutic protocols open between 1989 and 1995 (CCG-1881, -1882, -1883, -1891, -1901, and -1922 [n = 5118]).
For patients in whom second neoplasms developed, date of diagnosis, histologic characteristics, and tumor site were recorded. Pathology reports were obtained from treating institutions and reviewed to verify diagnoses.
The time at risk for second neoplasms was computed from the date of diagnosis of ALL to the date of diagnosis of second neoplasm, date of death, or date of last contact, whichever came first. The end of follow-up for the study was December 1999. Overall and event-free survival was calculated using actuarial methods. Cumulative incidence of second malignancy over time was calculated using competing risk methods. To estimate the risk of second neoplasms, the number of person-years of observation were compiled for subgroups of the cohort, defined by age and sex. Rates of incidence of cancer (obtained from the registry of Surveillance, Epidemiology, and End Results Program of the National Institutes of Health2) were used to calculate the expected number of cases of cancer. Standardized incidence ratios (SIRs) were calculated as the ratios of observed to expected cases. Cox regression techniques were used for calculating relative risk (RR) estimates.43 Variables examined in the regression model included age at diagnosis, sex, cumulative cyclophosphamide and anthracycline doses, and radiation dose delivered to the craniospinal axis. Age at diagnosis of ALL was analyzed as a categorical variable (less than or equal to 5 years vs more than 5 years). Exposures to cyclophosphamide and anthracyclines also were analyzed as categorical variables (cyclophosphamide dose 0 mg/m2, 1-2000 mg/m2, and more than 2000 mg/m2; anthracyclines 0 mg/m2, 1-200 mg/m2, and more than 200 mg/m2). Epipodophyllotoxins were not used in any of the CCG therapeutic protocols. Although all patients in our cohort were treated according to therapeutic protocols, we did not have information regarding the treatment received by patients in the event of relapse, which potentially could have influenced development of second malignancies. Therefore, we included relapse of primary disease as a variable in the multivariate analysis. All tests of statistical significance were 2-sided.
Excess absolute risk was calculated as an additional indicator of impact of cancer diagnosis and therapy on the cohort when compared with the general population. Excess absolute risk was determined by subtracting the expected number of malignancies in the cohort from the observed number, dividing the difference by person-years of follow-up, and multiplying that value by 10 000.
Results
The cohort was observed for a median of 5.5 years (range, 0 to 16.1 years). During the study, there was documented contact with 90% of patients within the previous 5 years and 78% within the previous 2 years. The estimated overall survival at 9 years was 76.9% ± 0.5%, and the event-free survival was 67.2% ± 0.6%. Sixty-three patients developed second neoplasms, including 39 patients with solid, nonhematopoietic tumors, 16 with acute and chronic myeloid leukemia or myelodysplasia (MDS), 6 with non-Hodgkin lymphoma (NHL), and 2 with Hodgkin disease. Characteristics of the patient population and treatment received are summarized in Table 1. The median length of follow-up among the patients who survive second cancers is 3 years (range, 0 to 12 years).
Characteristics of the patient population
| Characteristics . | Patients with second cancer . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total cohort . | Brain tumor . | AML/MDS* . | HD . | NHL . | Sarcoma . | Thyroid cancer . | Parotid tumors . | Other solid tumors . | |
| No. of patients | 8831 | 19 | 14 | 2 | 6 | 4 | 4 | 4 | 8 |
| Male sex, % of cohort | 57% | 37% | 71% | 50% | 67% | 0% | 50% | 50% | 50% |
| Age at diagnosis of ALL | |||||||||
| Median, y | 4.7 | 3.8 | 4.8 | 9.0 | 5.6 | 8.0 | 2.9 | 2.9 | 11.8 |
| Range, y | 0-20.8 | 1.3-15.5 | 1.6-15.8 | 3.8-14.3 | 1.3-8.1 | 1.9-14.1 | 1.5-4.0 | 1.8-14.9 | 0.2-17.4 |
| 5 y or less, % of cohort | 54% | 68% | 57% | 50% | 50% | 50% | 100% | 75% | 13% |
| Time to 2nd neoplasms, y | |||||||||
| Median | — | 7.1 | 3.1 | 2.3 | 3.1 | 3.6 | 9.7 | 8.9 | 7.9 |
| Range | — | 3.9-13.0 | 0.9-10.7 | 1.9-2.6 | 1.5-12.7 | 2.4-5.6 | 5.5-11.8 | 5.2-15.8 | 1.1-15.7 |
| Treatment, % of cohort | |||||||||
| Cyclophosphamide | 79% | 68% | 85% | 100% | 67% | 100% | 100% | 75% | 86% |
| Anthracyclines | 79% | 68% | 85% | 100% | 67% | 100% | 100% | 75% | 86% |
| Radiation | 38% | 63% | 50% | 50% | 17% | 50% | 50% | 75% | 63% |
| Characteristics . | Patients with second cancer . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total cohort . | Brain tumor . | AML/MDS* . | HD . | NHL . | Sarcoma . | Thyroid cancer . | Parotid tumors . | Other solid tumors . | |
| No. of patients | 8831 | 19 | 14 | 2 | 6 | 4 | 4 | 4 | 8 |
| Male sex, % of cohort | 57% | 37% | 71% | 50% | 67% | 0% | 50% | 50% | 50% |
| Age at diagnosis of ALL | |||||||||
| Median, y | 4.7 | 3.8 | 4.8 | 9.0 | 5.6 | 8.0 | 2.9 | 2.9 | 11.8 |
| Range, y | 0-20.8 | 1.3-15.5 | 1.6-15.8 | 3.8-14.3 | 1.3-8.1 | 1.9-14.1 | 1.5-4.0 | 1.8-14.9 | 0.2-17.4 |
| 5 y or less, % of cohort | 54% | 68% | 57% | 50% | 50% | 50% | 100% | 75% | 13% |
| Time to 2nd neoplasms, y | |||||||||
| Median | — | 7.1 | 3.1 | 2.3 | 3.1 | 3.6 | 9.7 | 8.9 | 7.9 |
| Range | — | 3.9-13.0 | 0.9-10.7 | 1.9-2.6 | 1.5-12.7 | 2.4-5.6 | 5.5-11.8 | 5.2-15.8 | 1.1-15.7 |
| Treatment, % of cohort | |||||||||
| Cyclophosphamide | 79% | 68% | 85% | 100% | 67% | 100% | 100% | 75% | 86% |
| Anthracyclines | 79% | 68% | 85% | 100% | 67% | 100% | 100% | 75% | 86% |
| Radiation | 38% | 63% | 50% | 50% | 17% | 50% | 50% | 75% | 63% |
HD indicates Hodgkin disease.
Does not include the patients with chronic myelogenous leukemia (n = 1) and chronic myelomonocytic leukemia (n = 1).
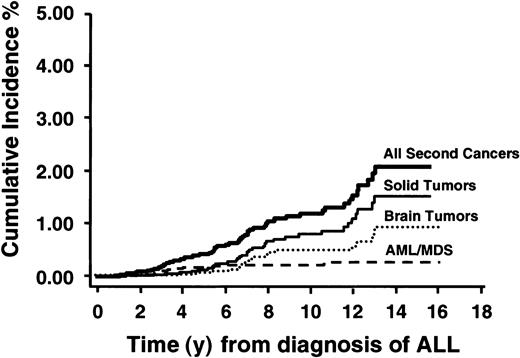
Figure 1 shows the cumulative incidence of all second neoplasms, AMLs, all solid cancers, and brain tumors. The cumulative incidence of any second malignant neoplasm was 1.18% (95% confidence interval [CI], 0.8%-1.5%) at 10 years from diagnosis of ALL and rose to 2.08% (95% CI, 1.4%-2.8%) at 15 years. Most of this risk was from solid nonhematopoietic malignancies including brain tumors (cumulative incidence 0.82% at 10 years). Among patients who remained in first continued remission, the cumulative incidence of any second malignancy was 0.91% (95% CI, 0.6%-1.2%) at 10 years from diagnosis. The cumulative incidence by type of second malignancy for the entire cohort and for those in first continued remission is shown in Table 2.
Cumulative incidence of all second neoplasms, AML/MDS, all solid tumors, and brain tumors in 8831 children with ALL.
Cumulative incidence of all second neoplasms, AML/MDS, all solid tumors, and brain tumors in 8831 children with ALL.
Cumulative incidence of second neoplasms among patients treated for childhood ALL
| Type/site of second malignancies . | Cumulative incidence (95% CI) . | |
|---|---|---|
| Entire cohort . | Patients remaining in initial remission of ALL . | |
| All second malignancies | 10 y: 1.18% (0.8%-1.5%) | 10 y: 0.91% (0.6%-1.2%) |
| 15 y: 2.08% (1.4%-2.8%) | 15 y: 1.48% (0.9%-2.1%) | |
| All solid malignancies | 10 y: 0.82% (0.5%-1.1%) | 10 y: 0.63% (0.3%-0.9%) |
| 15 y: 1.55% (0.9%-2.2%) | 15 y: 1.0% (0.5%-1.5%) | |
| Brain tumors | 10 y: 0.47% (0.2%-0.6%) | 10 y: 0.40% (0.2%-0.6%) |
| 15 y: 0.90% (0.4%-1.4%) | 15 y: 0.61% (0.2%-1.0%) | |
| AML/MDS | 10 y: 0.21% (0.1%-0.3%) | 10 y: 0.14% (0.03%-0.2%) |
| 15 y: 0.27% (0.1%-0.4%) | 15 y: 0.21% (0.03%-0.4%) | |
| NHL | 10 y: 0.08% (0.01%-0.2%) | 10 y: 0.05% (0%-0.1%) |
| 15 y: 0.19% (0.01%-0.4%) | 15 y: 0.18% (0%-0.5%) | |
| Type/site of second malignancies . | Cumulative incidence (95% CI) . | |
|---|---|---|
| Entire cohort . | Patients remaining in initial remission of ALL . | |
| All second malignancies | 10 y: 1.18% (0.8%-1.5%) | 10 y: 0.91% (0.6%-1.2%) |
| 15 y: 2.08% (1.4%-2.8%) | 15 y: 1.48% (0.9%-2.1%) | |
| All solid malignancies | 10 y: 0.82% (0.5%-1.1%) | 10 y: 0.63% (0.3%-0.9%) |
| 15 y: 1.55% (0.9%-2.2%) | 15 y: 1.0% (0.5%-1.5%) | |
| Brain tumors | 10 y: 0.47% (0.2%-0.6%) | 10 y: 0.40% (0.2%-0.6%) |
| 15 y: 0.90% (0.4%-1.4%) | 15 y: 0.61% (0.2%-1.0%) | |
| AML/MDS | 10 y: 0.21% (0.1%-0.3%) | 10 y: 0.14% (0.03%-0.2%) |
| 15 y: 0.27% (0.1%-0.4%) | 15 y: 0.21% (0.03%-0.4%) | |
| NHL | 10 y: 0.08% (0.01%-0.2%) | 10 y: 0.05% (0%-0.1%) |
| 15 y: 0.19% (0.01%-0.4%) | 15 y: 0.18% (0%-0.5%) | |
At the time of analysis, the cohort had accrued 54 883 person-years of follow-up. The number of observed and expected second neoplasms, calculated on the basis of age- and sex-specific rates for all cancers and various groupings of cancers, are shown in Table3. Overall, a total of 8.5 neoplasms would have been expected, and 61 (excluding the 2 patients with meningioma) were observed (SIR 7.2; 95% CI, 5.5-9.1). In addition, significantly elevated risks were observed for AML or MDS (AML/MDS) (SIR 52.3), NHL (SIR 8.3), brain tumors (SIR 10.1), soft tissue sarcoma (SIR 9.1), thyroid cancer (SIR 13.3), and parotid gland tumors (SIR = 33.4). The absolute risk for all cancers was 9.6 cases per 10 000 patients per year and ranged from 0.2 cases per 10 000 patients per year for Hodgkin disease to 2.8 cases for brain tumors (Table 3).
Observed and expected rates of second neoplasms, according to type or site
| Type or site . | Observed/expected cases . | SIR (95% CI) . | Absolute risk per 104 y . |
|---|---|---|---|
| All cancers3-150 | 61/8.5 | 7.2 (5.5-9.1) | 9.6 |
| Leukemia | 16/0.3 | 59.4 (33.9-96.4) | 2.9 |
| AML/MDS3-151 | 14/0.3 | 52.3 (28.6-87.7) | 2.5 |
| Lymphoma | |||
| NHL3-152 | 5/0.6 | 8.3 (2.6-17.2) | 0.8 |
| Hodgkin disease | 2/0.7 | 2.7 (0.3-9.7) | 0.2 |
| Solid cancers3-153 | |||
| Brain tumors | 17/1.7 | 10.1 (5.9-16.2) | 2.8 |
| Soft tissue sarcoma | 4/0.4 | 9.1 (2.4-20.2) | 0.7 |
| Thyroid cancer | 4/0.3 | 13.3 (3.6-34.1) | 0.7 |
| Parotid gland tumors | 4/0.1 | 33.4 (9.1-85.6) | 0.7 |
| Type or site . | Observed/expected cases . | SIR (95% CI) . | Absolute risk per 104 y . |
|---|---|---|---|
| All cancers3-150 | 61/8.5 | 7.2 (5.5-9.1) | 9.6 |
| Leukemia | 16/0.3 | 59.4 (33.9-96.4) | 2.9 |
| AML/MDS3-151 | 14/0.3 | 52.3 (28.6-87.7) | 2.5 |
| Lymphoma | |||
| NHL3-152 | 5/0.6 | 8.3 (2.6-17.2) | 0.8 |
| Hodgkin disease | 2/0.7 | 2.7 (0.3-9.7) | 0.2 |
| Solid cancers3-153 | |||
| Brain tumors | 17/1.7 | 10.1 (5.9-16.2) | 2.8 |
| Soft tissue sarcoma | 4/0.4 | 9.1 (2.4-20.2) | 0.7 |
| Thyroid cancer | 4/0.3 | 13.3 (3.6-34.1) | 0.7 |
| Parotid gland tumors | 4/0.1 | 33.4 (9.1-85.6) | 0.7 |
Excludes 2 patients with meningioma.
Excludes the 2 patients with chronic leukemia.
Excludes 1 patient with Epstein-Barr virus–associated B-cell lymphoproliferative disease.
Excludes lymphatic and hematopoietic tumors. The sum of the solid tumors does not equal the total number given because only types for which the risk was significantly elevated were included.
We also estimated the SIR of second neoplasms according to period of observation (ie, the interval from first treatment to diagnosis of second neoplasm) (Table 4). The SIR was highest during the first 10 years of follow-up and declined thereafter. This phenomenon is consistent with the increase in the expected incidence of cancer with increasing age. For leukemia, the excess risk appeared within the first 5 years of treatment and declined over the next 5 years. No cases of leukemia were observed beyond 10 years after diagnosis of ALL.
SIRs for second neoplasms, according to length of follow-up
| Type of cancer . | Length of follow-up . | |||
|---|---|---|---|---|
| 0-5 y . | 6-10 y . | 11-15 y . | More than 15 y . | |
| No. of patients | 3 905 | 3 007 | 1 761 | 156 |
| Person-years of observation | 11 218 | 19 953 | 21 340 | 2 371 |
| All second cancers4-150 | ||||
| Observed | 35 | 18 | 8 | 0 |
| Observed:expected (95% CI) | 20.6 (5.8-28) | 6.1 (3.6-9.3) | 2.3 (1.0-4.3) | — |
| Solid tumors4-150,4-151 | ||||
| Observed | 14 | 16 | 7 | 0 |
| Observed:expected (95% CI) | 12.7 (6.9-20.3) | 8.9 (5.1-13.8) | 3.3 (1.3-6.3) | — |
| Leukemia | ||||
| Observed | 13 | 3 | 0 | 0 |
| Observed:expected (95% CI) | 233.9 (137.9-350.5) | 31.6 (5.9-77.4) | — | — |
| Lymphoma | ||||
| Observed | 4 | 0 | 1 | 0 |
| Observed:expected (95% CI) | 20.0 (5.2-44.4) | — | — | — |
| CNS tumors4-150 | ||||
| Observed | 4 | 11 | 2 | 0 |
| Observed:expected (95% CI) | 10.8 (2.8-24) | 17.5 (8.7-29.3) | 3.2 (0.3-9.1) | — |
| Type of cancer . | Length of follow-up . | |||
|---|---|---|---|---|
| 0-5 y . | 6-10 y . | 11-15 y . | More than 15 y . | |
| No. of patients | 3 905 | 3 007 | 1 761 | 156 |
| Person-years of observation | 11 218 | 19 953 | 21 340 | 2 371 |
| All second cancers4-150 | ||||
| Observed | 35 | 18 | 8 | 0 |
| Observed:expected (95% CI) | 20.6 (5.8-28) | 6.1 (3.6-9.3) | 2.3 (1.0-4.3) | — |
| Solid tumors4-150,4-151 | ||||
| Observed | 14 | 16 | 7 | 0 |
| Observed:expected (95% CI) | 12.7 (6.9-20.3) | 8.9 (5.1-13.8) | 3.3 (1.3-6.3) | — |
| Leukemia | ||||
| Observed | 13 | 3 | 0 | 0 |
| Observed:expected (95% CI) | 233.9 (137.9-350.5) | 31.6 (5.9-77.4) | — | — |
| Lymphoma | ||||
| Observed | 4 | 0 | 1 | 0 |
| Observed:expected (95% CI) | 20.0 (5.2-44.4) | — | — | — |
| CNS tumors4-150 | ||||
| Observed | 4 | 11 | 2 | 0 |
| Observed:expected (95% CI) | 10.8 (2.8-24) | 17.5 (8.7-29.3) | 3.2 (0.3-9.1) | — |
Excludes 2 patients with meningioma.
Excludes lymphatic and hematopoietic tumors.
Twenty-one of 63 second malignancies developed among patients who had relapses of their original diseases before diagnosis of second malignancy. The records of patients who developed relapses of their original diseases before development of second neoplasms were reviewed for details of the treatment given to them. However, the records did not include details of doses and durations of therapy received for relapses; hence, it was difficult to include postrelapse therapeutic exposures in the analyses. Because detailed information on therapy after relapse of ALL was not available at the time of this analysis, presence of relapse was included as a variable in the multivariate analysis (Table 5). Multivariate analysis found female sex (RR 1.8; 95% CI, 1.1-2.8), radiation to the craniospinal axis (RR 1.6; 95% CI, 1.0-2.6), and relapse of primary disease (RR 3.5; 95% CI, 2.1-5.8) to be independently associated with increased risk of all second neoplasms. Risk of second neoplasms increased with radiation dose (1800 cGy: RR 1.5; 95% CI, 0.9-2.6; and 2400 cGy: RR 3.9; 95% CI, 1.4-11.2).
Risk of a second neoplasm associated with selected characteristics of patients and therapeutic exposures (multivariate analysis)
| Characteristics . | RR (95% CI) . | ||||
|---|---|---|---|---|---|
| All second neoplasms (n = 63) . | Solid tumors (n = 39) . | Brain tumors (n = 19) . | AML/MDS (n = 14) . | NHL (n = 6) . | |
| Age at diagnosis of ALL | |||||
| 0-5 y | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| More than 5 y | 1.1 (0.7-1.7) | 0.9 (0.5-1.7) | 0.6 (0.2-1.5) | 1.3 (0.5-3.7) | 1.4 (0.3-6.8) |
| Sex | |||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.8 (1.1-2.8)5-150 | 2.9 (1.5-5.8)5-150 | 2.5 (0.9-6.4) | 0.8 (0.3-2.3) | 0.6 (0.1-3.5) |
| Cyclophosphamide dose | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1-2000 mg/m2 | 1.2 (0.6-2.2) | 1.8 (0.8-4.3) | 0.7 (0.2-2.6) | 5.7 (0.7-46.9) | 0.8 (0.1-5.6) |
| More than 2000 mg/m2 | 1.4 (0.7-2.7) | 1.6 (0.6-3.9) | 0.9 (0.3-2.9) | 5.8 (0.7-48.3) | 0.9 (0.1-6.3) |
| Anthracycline dose | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1-200 mg/m2 | 1.2 (0.4-2.3) | 1.5 (0.7-3.6) | 0.6 (0.2-1.9) | 5.7 (0.7-44.6) | 0.6 (0.1-1.5) |
| More than 200 mg/m2 | 1.4 (0.6-3.3) | 2.4 (0.8-6.9) | 1.8 (0.5-6.5) | 5.9 (0.5-65.7) | Nonconvergent |
| Radiation | |||||
| No radiation given | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Radiation given | 1.6 (1.0-2.6)5-150 | 2.5 (1.2-5.5)5-150 | 2.4 (1.1-5.2)5-150 | 1.3 (0.5-3.8) | 0.3 (0.1-2.8) |
| Radiation dose | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1800 cGy | 1.5 (0.9-2.6) | 2.8 (1.5-5.6)5-150 | 2.1 (0.7-6.3) | 1.6 (0.5-4.8) | 0.6 (0.2-2.3) |
| 2400 cGy | 3.9 (1.4-11.2)5-150 | 5.8 (1.3-25.3)5-150 | 4.2 (0.5-37.7) | 4.4 (0.6-36.1) | 3.6 (0.5-28.2) |
| Relapse of primary disease | |||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 3.5 (2.1-5.8)5-150 | 3.1 (1.6-6.1)5-150 | 2.5 (0.9-7.6)5-151 | 3.4 (1.2-9.8)5-150 | 2.2 (0.4-11.8) |
| Characteristics . | RR (95% CI) . | ||||
|---|---|---|---|---|---|
| All second neoplasms (n = 63) . | Solid tumors (n = 39) . | Brain tumors (n = 19) . | AML/MDS (n = 14) . | NHL (n = 6) . | |
| Age at diagnosis of ALL | |||||
| 0-5 y | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| More than 5 y | 1.1 (0.7-1.7) | 0.9 (0.5-1.7) | 0.6 (0.2-1.5) | 1.3 (0.5-3.7) | 1.4 (0.3-6.8) |
| Sex | |||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.8 (1.1-2.8)5-150 | 2.9 (1.5-5.8)5-150 | 2.5 (0.9-6.4) | 0.8 (0.3-2.3) | 0.6 (0.1-3.5) |
| Cyclophosphamide dose | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1-2000 mg/m2 | 1.2 (0.6-2.2) | 1.8 (0.8-4.3) | 0.7 (0.2-2.6) | 5.7 (0.7-46.9) | 0.8 (0.1-5.6) |
| More than 2000 mg/m2 | 1.4 (0.7-2.7) | 1.6 (0.6-3.9) | 0.9 (0.3-2.9) | 5.8 (0.7-48.3) | 0.9 (0.1-6.3) |
| Anthracycline dose | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1-200 mg/m2 | 1.2 (0.4-2.3) | 1.5 (0.7-3.6) | 0.6 (0.2-1.9) | 5.7 (0.7-44.6) | 0.6 (0.1-1.5) |
| More than 200 mg/m2 | 1.4 (0.6-3.3) | 2.4 (0.8-6.9) | 1.8 (0.5-6.5) | 5.9 (0.5-65.7) | Nonconvergent |
| Radiation | |||||
| No radiation given | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Radiation given | 1.6 (1.0-2.6)5-150 | 2.5 (1.2-5.5)5-150 | 2.4 (1.1-5.2)5-150 | 1.3 (0.5-3.8) | 0.3 (0.1-2.8) |
| Radiation dose | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1800 cGy | 1.5 (0.9-2.6) | 2.8 (1.5-5.6)5-150 | 2.1 (0.7-6.3) | 1.6 (0.5-4.8) | 0.6 (0.2-2.3) |
| 2400 cGy | 3.9 (1.4-11.2)5-150 | 5.8 (1.3-25.3)5-150 | 4.2 (0.5-37.7) | 4.4 (0.6-36.1) | 3.6 (0.5-28.2) |
| Relapse of primary disease | |||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 3.5 (2.1-5.8)5-150 | 3.1 (1.6-6.1)5-150 | 2.5 (0.9-7.6)5-151 | 3.4 (1.2-9.8)5-150 | 2.2 (0.4-11.8) |
Risk estimates in bold identify the significant associations.
P ≤ .05.
.05 < P < .1.
Solid cancers
Solid nonhematopoietic cancers developed in 39 patients and included 19 with brain tumors; 4 each with thyroid cancer, parotid gland tumors, and soft tissue sarcoma; 2 each with malignant melanoma, colon cancer, and osteogenic sarcoma; and 1 each with breast and ovarian cancer. The cumulative incidence of a second solid nonhematopoietic malignancy was 0.82% (95% CI, 0.5%-1.1%) at 10 years (Figure 1; Table 2) and rose to 1.55% (95% CI, 0.9%-2.2%) at 15 years from diagnosis of ALL. The median time to development of second solid malignancies was 7.1 years (range, 1.1-15.8 years). Multivariate analysis revealed relapse of primary disease (RR 3.1; 95% CI, 1.6-6.1), female sex (RR 2.9; 95% CI, 1.5-5.8), and exposure to radiation (RR 2.5; 95% CI, 1.2-5.5) to be independently associated with an increased risk of a second solid malignancy (Table 5). The risk increased with increasing dose of radiation (1800 cGy: RR 2.8; 95% CI, 1.5-5.6; and 2400 cGy: RR 5.8; 95% CI, 1.3-25.3). Seventy-five percent of solid tumors developed within radiation fields. Seventeen of 39 patients with solid malignancies have died at the time of this report.
Brain tumors.
Brain tumors developed in 19 patients and included 9 with glioblastoma multiforme, 4 with anaplastic astrocytoma, 3 with primitive neuroectodermal tumors of the brain, 2 with meningioma, and 1 with medulloblastoma. The median age at diagnosis of ALL for these 19 patients was 3.8 years, and the median time to development of brain tumors was 7.1 years. The cumulative incidence of brain tumors approached 0.47% (95% CI, 0.2-0.6) at 10 years (Figure1), and the cohort was at a 10-fold increased risk for developing brain tumors when compared with the general population. Multivariate analysis revealed radiation to the craniospinal axis (RR 2.4; 95% CI, 1.1-5.2) to be associated with increased risk of brain tumors after ALL (Table5). Age at diagnosis of ALL was not identified as a significant risk factor. Eleven of 19 patients with brain tumors have died.
Soft tissue sarcoma.
Soft tissue sarcoma developed in 4 patients. These included rhabdomyosarcoma involving the eye, uterus (embryonal), and urinary bladder (undifferentiated) in 1 patient each and a high-grade pleomorphic sarcoma of the pelvis in the fourth patient. The patient with the uterine rhabdomyosarcoma had received total body irradiation for unrelated donor bone marrow transplantation before development of rhabdomyosarcoma. Two other patients received prophylactic radiation to the brain, but sarcomas developed outside the radiation fields. The fourth patient did not receive radiation therapy. The median time to development of secondary sarcoma was 3.6 years (range, 2.4 to 5.6 years). The cohort was at a 9.1-fold increased risk for developing soft tissue sarcoma compared with the general population. All 4 patients were females, with a significantly increased risk of developing sarcomas among the female compared with male cohort (P = .02). No other host or therapy-related risk factors were identified. Three of 4 patients with soft tissue sarcoma have died.
Parotid gland tumors.
Mucoepidermoid tumors of the parotid gland developed in 4 patients. This cohort was at a 33.4 times increased risk for developing a parotid gland tumor compared with the general population. The median time to development of those tumors was 8.9 years (range, 5.2 to 15.8 years), and no risk factors were identified. All 4 patients were alive at the time of this report.
Thyroid cancer.
Papillary carcinoma of the thyroid gland developed in 4 patients at a median of 9.7 years (range, 5.5 to 11.8 years) from diagnosis of ALL. The cohort was at a 13.3-fold increased risk for development of secondary thyroid cancers when compared with the general population. Exposure to 2400 cGy of radiation was associated with a significantly increased risk of developing thyroid cancer (RR 30.8; 95% CI, 1.2-62.9). All 4 patients with thyroid cancer were alive at the time of this report.
Leukemia
Secondary leukemia developed in 16 patients. Fourteen developed AML/MDS, 1 developed chronic myelogenous leukemia, and 1 was diagnosed with chronic myelomonocytic leukemia. Marker studies were very carefully reviewed to determine that the secondary leukemias were true second neoplasms and not relapses of original diseases. Of 14 patients with AML/MDS, 10 were characterized morphologically with AML (M0 [n = 1], M1 [n = 4], M2 [n = 2], M4 [n = 1], M5 [n = 1], M6 [n = 1]), 3 had morphologic features of MDS, and 1 developed myelofibrosis. The median time to development of AML/MDS was 3.1 years (range, 0.9-10.7 years) from diagnosis of ALL. The cumulative incidence of developing AML/MDS approached 0.21% (95% CI, 0.1-0.3) at 10 years, with 13 of 14 events reported in the first 5 years and no events reported after 10 years from diagnosis (Figure 1). Exposure to cyclophosphamide or anthracyclines was not identified as a significant risk factor for the development of AML/MDS (Table 5). However, patients with relapses of their primary diseases were at significantly higher risk for developing secondary AML/MDS (RR 3.4; 95% CI, 1.2-9.8) (Table 5). Twelve of 14 patients with secondary AML/MDS have died.
Lymphoma
NHL developed in 6 patients, including 1 with Epstein-Barr virus–associated B-cell lymphoproliferative disease. The median time to the development of NHL was 3.1 years (range, 1.5-2.7 years). The cumulative incidence of secondary NHL reached 0.08% (95% CI, 0.01-0.2) at 10 years from diagnosis of ALL (Figure 1). The cohort was at an 8-fold increased risk for developing a lymphoma when compared with the general population.
Hodgkin disease developed in 2 patients within the first 3 years of follow-up. The cohort was at a 2.7-fold increased risk for developing Hodgkin disease, but that risk was not statistically significant when compared with the general population. Both patients with Hodgkin disease were alive at the time of this report.
Treatment eras
A comparison of the patient characteristics, therapeutic exposures, treatment outcomes, and second malignancies is summarized in Table 6. Patients treated in the early treatment era were observed for a median of 10.3 years (range, 0.1 to 16.1 years). The cumulative incidence of second neoplasms among patients treated in the early era approached 0.94% (95% CI, 0.6%-1.3%) at 10 years and 1.84% (95% CI, 1.2%-2.5%) at 15 years.
Comparison of patient populations by treatment eras
| . | Early era . | Recent era . | P . |
|---|---|---|---|
| Cohort size | 3 713 | 5 118 | |
| Median length of follow-up, y | 10.3 | 4.6 | |
| Range | 0.1-16.1 | 0-9.1 | < .001 |
| Person-years of follow-up | 31 558 | 23 325 | |
| Median age at diagnosis of ALL, y | 4.6 | 4.7 | .8 |
| Range | 0.0-20.7 | 0-20.8 | |
| Sex, % males | 59% | 55% | < .001 |
| Cyclophosphamide dose | |||
| None | 35% | 8% | |
| 1-2 000 mg/m2 | 22% | 63% | |
| More than 2 000 mg/m2 | 43% | 29% | < .001 |
| Anthracycline dose | |||
| None | 35% | 8% | |
| 1-200 mg/m2 | 47% | 86% | |
| More than 200 mg/m2 | 18% | 6% | < .001 |
| Radiation dose | |||
| None | 49% | 72% | |
| 1 800 cGy | 49% | 25% | |
| 2 400 cGy | 2% | 3% | < .001 |
| Survival | |||
| Overall survival at 5 y | 78.7 ± 0.7% | 84.7 ± 0.5% | < .001 |
| Event-free survival at 5 y | 65.5 ± 0.8% | 74.8 ± 0.7% | < .001 |
| Cumulative incidence of second neoplasms at 5 y (95% CI) | |||
| All second neoplasms | 0.37% (0.2-0.5) | 0.43% (0.2-0.5) | .2 |
| AML | 0.17% (0.02-0.3) | 0.15% (0.03-0.3) | .4 |
| NHL | 0.06% (0-0.4) | 0.05% (0-0.1) | .2 |
| . | Early era . | Recent era . | P . |
|---|---|---|---|
| Cohort size | 3 713 | 5 118 | |
| Median length of follow-up, y | 10.3 | 4.6 | |
| Range | 0.1-16.1 | 0-9.1 | < .001 |
| Person-years of follow-up | 31 558 | 23 325 | |
| Median age at diagnosis of ALL, y | 4.6 | 4.7 | .8 |
| Range | 0.0-20.7 | 0-20.8 | |
| Sex, % males | 59% | 55% | < .001 |
| Cyclophosphamide dose | |||
| None | 35% | 8% | |
| 1-2 000 mg/m2 | 22% | 63% | |
| More than 2 000 mg/m2 | 43% | 29% | < .001 |
| Anthracycline dose | |||
| None | 35% | 8% | |
| 1-200 mg/m2 | 47% | 86% | |
| More than 200 mg/m2 | 18% | 6% | < .001 |
| Radiation dose | |||
| None | 49% | 72% | |
| 1 800 cGy | 49% | 25% | |
| 2 400 cGy | 2% | 3% | < .001 |
| Survival | |||
| Overall survival at 5 y | 78.7 ± 0.7% | 84.7 ± 0.5% | < .001 |
| Event-free survival at 5 y | 65.5 ± 0.8% | 74.8 ± 0.7% | < .001 |
| Cumulative incidence of second neoplasms at 5 y (95% CI) | |||
| All second neoplasms | 0.37% (0.2-0.5) | 0.43% (0.2-0.5) | .2 |
| AML | 0.17% (0.02-0.3) | 0.15% (0.03-0.3) | .4 |
| NHL | 0.06% (0-0.4) | 0.05% (0-0.1) | .2 |
Despite a significantly larger proportion of patients exposed to chemotherapeutic agents and a smaller proportion of patients exposed to prophylactic cranial irradiation in the recent era, there was no statistically significant difference in incidence of second neoplasms in the first 5 years of follow-up in the 2 treatment eras (Table 6).
Discussion
Among the 8831 patients who were treated for childhood ALL since 1983 on CCG therapeutic protocols, we found the cumulative incidence of second malignancies to be low (1.18% at 10 years after the initial diagnosis). However, this report provides evidence that the risk of a second neoplasm is increased 7-fold among long-term survivors of childhood ALL when compared with the age- and sex-matched general population. The risk was highest among patients who had suffered a relapse of their primary disease, among patients who had received radiation therapy, and among females, with the sex preference primarily accounted for by secondary soft tissue sarcoma.
ALL is the most common malignancy in childhood and is associated with excellent outcomes, resulting in an increasing population of long-term survivors, so attention is being focused on potential therapy-related long-term complications such as second neoplasms.10-17,44-46 In a CCG report of 9720 patients diagnosed between 1972 and 1988 and observed for a median of 4.7 years, Neglia et al estimated a 2.5% cumulative risk of second neoplasms 15 years after diagnosis of ALL.11 Similarly, Dalton et al reported a cumulative risk of second neoplasms of 2.7% in a cohort of 1597 patients diagnosed between 1972 and 1995 who were treated according to Dana-Farber Cancer Institute protocols and observed for a median of 7.6 years.14 A population-based study of 981 children with ALL from the Nordic countries reported a cumulative risk of second neoplasms of 2.9% at 20 years from diagnosis.44Pratt et al estimated an 8% cumulative risk 15 years after diagnosis in a group of 1815 patients, diagnosed and treated on one of the St Jude protocols between 1962 to 1988, all of whom had received radiation.45 Recently, Loning et al reported a cumulative risk of 3.3% at 15 years in a cohort of 5006 patients enrolled in 5 ALL–Berlin-Frankfurt-Munster trials between 1979 and 1995 and followed for a median of 5.7 years.10
Intrathecal chemotherapy can be effective in selected patient populations as prophylaxis against CNS leukemia47 48; therefore, radiation therapy now tends to be used primarily for children with CNS disease at diagnosis or with characteristics that indicate a high risk of CNS disease. On the other hand, chemotherapy regimens are considerably more intensive than those used in previous cohorts. Thus, our cohort of patients treated more recently and more aggressively with chemotherapy, yet more conservatively with radiation therapy, allows for assessment of incidence of second cancers in a cohort of patients who received contemporary, risk-based therapy.
Nineteen patients with brain tumors were identified in this cohort, with the cumulative incidence of brain tumors approaching 0.5% at 10 years from diagnosis of ALL. An increased risk of brain tumors has been observed among long-term survivors of childhood ALL.10,11,14,19,23-31,49 Similar to previous studies, brain tumors were more likely to develop among patients who had received radiation, and the risk increased with increasing radiation dose.10,11,14 Previous studies reported increased risk of brain tumors among patients who were less than 5 years old at the time of radiation.10,11,14,16 Thirteen of the 19 secondary brain tumors in our study developed among patients who were less than 5 years old at the time of diagnosis of ALL, but the risk of developing a secondary brain tumor did not differ statistically in the 2 age categories. An unusually high incidence of secondary brain tumors was reported among children treated on a therapeutic protocol at St Jude Children's Research Hospital.28 The St Jude protocol differed from previous protocols in that more intensive systemic antimetabolite therapy was given before and during radiotherapy. An assessment of clinical, biologic, and pharmacokinetic features revealed higher erythrocyte concentrations of thioguanine nucleotide metabolites and a higher proportion of defective thiopurine methyl transferase phenotype among patients with brain tumors compared with those without brain tumors, indicating that underlying genetic characteristics and treatment variables may contribute to the excess of brain tumors in this population.
The patients in our cohort were at a 9-fold increased risk for developing soft tissue sarcoma when compared with the general population. There was no correlation with therapeutic exposures, but the risk was significantly increased among females. Brain tumor, sarcoma, and leukemia are part of the familial Li-Fraumeni syndrome.50 Epidemiologic studies have shown that families with members who have brain tumors have increased incidence of leukemia.51 A recent report by Hisada et al shows that, compared with the general population, members of Li-Fraumeni syndrome families have an exceptionally high risk of developing multiple cancers.52 The excess risk of additional primary cancers is mainly for cancers that are characteristic of Li-Fraumeni syndrome, with the highest risk observed for survivors of childhood cancer. It is possible that there is an interaction between genetic susceptibility and radiation, resulting in a subpopulation at increased risk for developing brain tumor and soft tissue sarcoma that needs to be explored further. Unfortunately, information on family history of cancer was not available for this cohort of patients.
Case reports of parotid gland tumors as second malignancies after treatment of childhood ALL have been described,53 with patients presenting with painless swelling 6 to 7 years after treatment for ALL. Our cohort was at a 33-fold increased risk for mucoepidermoid carcinoma of the parotid gland, which developed a median of 9 years from diagnosis of ALL, and all 4 patients were alive at the time of this report.
There are several case reports of thyroid carcinoma after treatment for ALL with prophylactic cranial irradiation, total body irradiation and, in one report, with chemotherapy alone.54-58 The reports indicate that thyroid cancer develops 12 to 13 years after treatment, the risk is highest in younger children, and the secondary thyroid cancer is associated with an excellent long-term outcome. Four patients developed papillary carcinoma of the thyroid in our cohort at a median of 9.7 years from treatment, placing this patient population at a 13-fold increased risk when compared with the general population. Radiation to the craniospinal axis was associated with an increased risk, and all 4 patients were alive at the time of this report, indicating that after cranial irradiation patients require long-term clinical monitoring of the thyroid and cervical areas for nodules.
We found that, after a relatively short latent period (0.9 years), the cumulative incidence of secondary AML/MDS rose sharply, but it appeared to reach a plateau after 5.5 years, with a median time to development of secondary AML/MDS of 3.1 years. This is consistent with data from other studies.59,60 Pui et al reported the risk of epipodophyllotoxin-related secondary AML in patients with ALL to be 3.8% at 6 years.13 They also demonstrated that the risk of epipodophyllotoxin-related AML depends largely on the schedule of drug administration. The cumulative incidence of secondary AML of 0.2% in our cohort is much lower than that reported by Pui et al, in part because epipodophyllotoxins were not used in any of the therapeutic protocols used in this cohort of patients.
Hodgkin disease15,61,62 and NHL11 15 as second neoplasms have been reported in previous studies. In our cohort, the risks of developing secondary Hodgkin disease and NHL were increased 3-fold and 8-fold, respectively, compared with the general population.
Comparison of the 2 treatment eras (1983-1989 vs 1989-1995) showed that a significantly larger proportion treated in the recent era received anthracyclines and cyclophosphamide. Patients treated in the early era were more likely to have received prophylactic cranial radiation compared with those treated in the recent era. Despite these differences in exposure, there is no statistically significant difference in the incidence of second malignancies in the 2 eras within the first 5 years of follow-up. The median length of follow-up was shorter for the recent era (4.6 years vs 10.3 years for the early era) and so may not allow a meaningful comparison of late-occurring malignancies such as brain tumors and other solid cancers typically associated with radiation. However, secondary AML typically has a short latency period, with most events within the first 4 to 7 years and relatively few events described after the first decade. Therefore, in our cohort, we can say that incidence of secondary AML appears comparable in the 2 treatment eras and has remained constant over the last 15 years.
This study has many strengths. First, the large number of patients treated between 1983 and 1995 allowed us to describe the incidence of second malignant neoplasms among patients treated on contemporary therapeutic protocols. Second, treatment of patients according to CCG therapeutic protocols ensured uniform access to standard therapy, giving us the opportunity to explore risk factors associated with second malignant neoplasms identified in this cohort.
Several limitations to our report also need to be discussed. Although all the patients in our cohort were treated according to therapeutic protocols, we do not have detailed information regarding actual doses of therapeutic exposures given to patients for relapses, which potentially could have influenced the development of second malignancies. Therefore, we included history of relapse as a variable in the multivariate analysis to serve as a surrogate marker for further therapy received. Relapse of primary disease was associated with increased risk of all second neoplasms, AML/MDS, and all solid tumors.
Another limitation of this study was the relatively short overall follow-up period for this cohort, the median length of follow-up of this cohort being 5.5 years. However, the cohort has contributed 54 883 person-years of observation. In addition, 3713 patients from the early era were observed for a median of 10.3 years, contributing 31 558 person-years of observation. The cumulative incidence of second neoplasms developing among patients treated in the early era approached 0.9% at 10 years from diagnosis and represents a fairly accurate estimate of the incidence of second neoplasms among patients treated on contemporary risk-based therapy. However, although most previous studies reported a median follow-up ranging from 4.6 years11 to 7.6 years,14 we anticipate that longer follow-up will likely result in identification of more second neoplasms, especially those associated with radiation therapy, such as brain tumors. Furthermore, longer follow-up of the recent era will give us a more accurate estimate of the impact of the conservative use of prophylactic radiation on the incidence of radiation-associated second malignancies.
The current therapeutic approach to childhood ALL is risk stratification, with aggressive chemotherapy and radiation therapy reserved for patients considered at high risk for relapses and adverse outcomes. Follow-up of this large cohort shows that, with contemporary risk-based therapies, the incidence rate of second neoplasms remains low throughout the first decade after diagnosis of childhood ALL, although it is higher than expected in the general population.
Supported in part by 5 U10 CA13539-26S2.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
S. Bhatia, Children's Oncology Group, PO Box 60012, Arcadia, CA 91006-0012; e-mail: sbhatia@coh.org; cc:smason@childrensoncologygroup.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal