Incidence rates of follicular lymphoma (FL) inexplicably vary markedly between Western and Asian countries. A hallmark of FL is thebcl-2 translocation, characterized by 1 of 2 common breakpoints known as major breakpoint region (MBR) and minor cluster region (mcr). We analyzed previously published data to compare rates ofbcl-2 translocation in FL across geographic regions. Available data from the literature suggest that the incidence ofbcl-2 in healthy persons in the absence of FL may be as high as 50% in Western and Asian populations. However, in FL our results show that the frequency of bcl-2 positivity was significantly higher for US than for Asian populations (P < .0001). This pattern persisted for MBR and mcr subgroups. We conclude that a significant gradient exists in thebcl-2 frequency between these FL populations. We therefore suggest that the relatively low incidence of FL in Asian populations is caused not by a lower frequency of bcl-2 rearrangements in healthy populations but by distinct molecular pathways developing in different geographic regions that nonetheless culminate in FL, which is morphologically similar but molecularly distinct. Studies demonstrating differences in clinical characteristics according to the presence or absence of bcl-2 rearrangements support this concept. Thus we hypothesize that FL may in fact be a heterogeneous malignancy encompassing entities with distinct molecular pathogenesis and potentially distinct clinical manifestations. If these findings were confirmed in prospective studies, it would imply that different etiologic or genetic factors might influence the development of FL across separate regions.
Introduction
It has been repeatedly observed that there are marked differences in the incidence rates of follicular lymphoma (FL) across geographic regions. Extremely low incidence rates are reported among most Asian countries, and comparatively high rates are reported in North America and Western Europe. Although widely accepted, this observation remains unexplained.
The t(14;18)(q32;q21) translocation, with downstream overexpression of the antiapoptotic bcl-2 protein, is the hallmark of FL. However, this potentially oncogenic mutation has also been identified in a significant proportion of healthy persons. In the absence of clinical disease, the significance of this finding is uncertain but may suggest that the translocation is an early, initiating event, with subsequent cumulative oncogenic alterations necessary to manifest as the clinical entity FL.
Whether there are differences in the incidence of bcl-2translocations in FL across geographic regions has not been thoroughly explored. We have analyzed previously published data to propose that the incidence of the t(14;18) translocation may be significantly lower in patients with FL from Asia than in patients from Western countries. By implication, there may be distinct molecular pathways operating in different geographic regions that nonetheless culminate in FL that is morphologically similar but molecularly distinct. Recently published comparative studies demonstrating differences in clinical characteristics according to the presence or absence ofbcl-2 gene rearrangements support this concept. Based on these observations, we have developed the hypothesis that FL may in fact be a heterogeneous disorder encompassing entities with distinct molecular pathogenesis, in particular bcl-2 germline versus rearranged, and potentially distinct clinical manifestations and natural history.
Geographic variation in FL
For many years it has been recognized that FL makes up a relatively small proportion of all cases of non-Hodgkin lymphoma (NHL) diagnosed within most Asian countries (Table1).1-15,17,19 Summary data from many of the larger recent studies reveal that across most nations in this region, only 8% to 12% of consecutive series of newly diagnosed NHL are of follicular histology. Two series with a total of 1756 patients suggest that the proportion may be even lower in Korea—3.8% and 6.2%, respectively.11 12
Relative frequency of histologic subtypes of NHL
| Region . | Year . | No. cases . | Incidence by histologic subtype . | All follicular (%) . | DLCL (%) . | ||
|---|---|---|---|---|---|---|---|
| FSC (%) . | FM (%) . | FLC (%) . | |||||
| Africa/Asia/India | |||||||
| Japan10 | 1983 | 604 | – | – | – | 11.6 | – |
| Japan2 | 1985 | 294 | – | – | – | 10.7 | 46.2 |
| Hong Kong8 | 1984 | 267 | 6.4 | 3.4 | 0.4 | 10.1 | 18.7 |
| Hong Kong3 | 1998 | 197 | – | – | – | 8.0 | 39.0 |
| Thailand9 | 1996 | 1391 | 1.9 | 0.9 | 1.0 | 3.8 | 39.9 |
| Thailand15 | 1998 | 389 | 2.8 | 1.8 | 6.4 | 11.1 | 31.3 |
| Korea11 | 1992 | 290 | 0.3 | 1.4 | 2.1 | 3.8 | 58.3 |
| Korea12 | 1998 | 1466 | – | – | – | 6.2 | 43.7 |
| Korea5 | 1985 | 298 | 0 | 0.7 | 2.7 | 3.4 | 53.7 |
| China7 | 1987 | 192 | 5.2 | 3.6 | 5.2 | 14.0 | 24.5 |
| Philippines4 | 1986 | 262 | 4.2 | 3.8 | 0 | 8.0 | 29.7 |
| India6 | 1985 | 238 | 5.9 | 2.5 | 0.4 | 8.8 | 39.1 |
| India13 | 1985 | 1050 | – | – | – | 10.0 | – |
| Taiwan14 | 1991 | 636 | 2.9 | 1.8 | 1.8 | 6.3 | 54.9 |
| Pakistan17 | 1992 | 495 | – | – | – | 8.1 | 41.0 |
| Gabon19 | 1991 | 72 | 0 | 0 | 1.5 | 1.5 | 35.8 |
| North America/Europe/South Africa | |||||||
| United States3 | 1998 | 200 | – | – | – | 32 | 28 |
| Canada3 | 1998 | 200 | – | – | – | 31 | 31 |
| South Africa3 | 1998 | 188 | – | – | – | 33 | 31 |
| Britain3 | 1998 | 119 | – | – | – | 28 | 29 |
| Germany3 | 1998 | 203 | – | – | – | 18 | 30 |
| France3 | 1998 | 192 | – | – | – | 17 | 39 |
| Italy3 | 1998 | 79 | – | – | – | 11 | 45 |
| United States1 | 1982 | 1153 | 22.5 | 7.7 | 3.8 | 34.0 | 27.6 |
| Region . | Year . | No. cases . | Incidence by histologic subtype . | All follicular (%) . | DLCL (%) . | ||
|---|---|---|---|---|---|---|---|
| FSC (%) . | FM (%) . | FLC (%) . | |||||
| Africa/Asia/India | |||||||
| Japan10 | 1983 | 604 | – | – | – | 11.6 | – |
| Japan2 | 1985 | 294 | – | – | – | 10.7 | 46.2 |
| Hong Kong8 | 1984 | 267 | 6.4 | 3.4 | 0.4 | 10.1 | 18.7 |
| Hong Kong3 | 1998 | 197 | – | – | – | 8.0 | 39.0 |
| Thailand9 | 1996 | 1391 | 1.9 | 0.9 | 1.0 | 3.8 | 39.9 |
| Thailand15 | 1998 | 389 | 2.8 | 1.8 | 6.4 | 11.1 | 31.3 |
| Korea11 | 1992 | 290 | 0.3 | 1.4 | 2.1 | 3.8 | 58.3 |
| Korea12 | 1998 | 1466 | – | – | – | 6.2 | 43.7 |
| Korea5 | 1985 | 298 | 0 | 0.7 | 2.7 | 3.4 | 53.7 |
| China7 | 1987 | 192 | 5.2 | 3.6 | 5.2 | 14.0 | 24.5 |
| Philippines4 | 1986 | 262 | 4.2 | 3.8 | 0 | 8.0 | 29.7 |
| India6 | 1985 | 238 | 5.9 | 2.5 | 0.4 | 8.8 | 39.1 |
| India13 | 1985 | 1050 | – | – | – | 10.0 | – |
| Taiwan14 | 1991 | 636 | 2.9 | 1.8 | 1.8 | 6.3 | 54.9 |
| Pakistan17 | 1992 | 495 | – | – | – | 8.1 | 41.0 |
| Gabon19 | 1991 | 72 | 0 | 0 | 1.5 | 1.5 | 35.8 |
| North America/Europe/South Africa | |||||||
| United States3 | 1998 | 200 | – | – | – | 32 | 28 |
| Canada3 | 1998 | 200 | – | – | – | 31 | 31 |
| South Africa3 | 1998 | 188 | – | – | – | 33 | 31 |
| Britain3 | 1998 | 119 | – | – | – | 28 | 29 |
| Germany3 | 1998 | 203 | – | – | – | 18 | 30 |
| France3 | 1998 | 192 | – | – | – | 17 | 39 |
| Italy3 | 1998 | 79 | – | – | – | 11 | 45 |
| United States1 | 1982 | 1153 | 22.5 | 7.7 | 3.8 | 34.0 | 27.6 |
FSC indicates follicular small-cleaved cell; FM, follicular mixed; FLC, follicular large cell; and DLCL, diffuse large-cell lymphoma.
These figures contrast dramatically with the relative proportion of 20% to 33% reported for most industrialized North American and Western European countries. In this context, it is interesting to note that data reported from Cape Town, South Africa conform to the pattern of the Western European centers, with 33% of all lymphomas having follicular histology. Notably, northern Italy appears to provide an exception to this observation, with only 11% of all lymphomas reported as of follicular histology.3
Although the available data in developing nations outside Asia are less complete, the relative frequency of FL is similar to that of the Asian countries, with proportions of 16.7% reported in Oman,168.1% in Pakistan,17 13.3% in Nigeria,18 and 1.5% in Gabon, equatorial Africa.19 These similar frequencies occur despite marked variation in the ethnic background and geographic localization of these regions, consistent with a dominant effect for environmental rather than genetic influences. This is also consistent with the data derived from Asian emigrants to the United States and their descendants,20 in which emigrants to the United States appeared to maintain the disease risk of their country of birth and early development, whereas the incidence among the children born in the United States approached that of US-born whites.
Absolute incidence rates
Although the above figures allow comparisons of therelative proportion of various lymphoma types between countries, they do not provide data on the absoluteincidence rates. Unfortunately, most of the available population-based incidence figures do not include specific histologic subtyping. Much of the following discussion therefore relies on application of the above relative proportions observed in the above hospital-based series to population-based overall incidence figures and thus assumes that the relative distribution of histologic types is representative of that seen in the country as a whole. An important factor, which will have an impact on these international comparisons, is the age structure of the populations under scrutiny. However, even allowing for an extreme effect of population demographics such as that presented by Hakulinen for Quito, Ecuador, where the median age of the population is just 20 years, such adjustment to “world standard” population resulted in an increase in less than 50% in the crude incidence rates.21 The application of such extreme adjustment factors to the presented data for most Asian countries would not significantly alter the conclusions presented herein.
Reliable population-based data on specific incidence rates for FL are available for the United States. The most recent direct data available for histologic subtypes is from SEER registry for 1986 to 1988,22 in which the annual incidence rate (per 100 000 population) for follicular small-cleaved cell (FSC) lymphoma was 1.3, follicular mixed (FM) lymphoma was 0.7, and follicular large (FLC) lymphoma was 0.4. The cumulative annual incidence rate for all forms of FL steadily increased from 1978 to 1995: it was 2.0 for 1978 to 1981, 2.3 for 1982 to 1985, 2.4 for 1986 to 1988, and 2.6 for the overall period from 1978 to 1995. Within the United States, distinct from the increased incidence related to HIV lymphomas, the overall incidence of NHL has been increasing by approximately 3.4% per year, a trend that is apparent worldwide.23 (The rate of increase, however, might have slowed in the last decade, as evidenced by a 0.4% annual increase in US statistics from 1991 to 1998.24) Aggressive histologic types have a higher rate of increase, such that the overall rate of increase for FL is less at approximately 2% per year.22,25,26 An updated SEER registry analysis to 1995 suggests the increase in FL incidence is attributed mainly to an increase in incidence of FM and FLC subtypes.27 With the recent introduction of the World Health Organization classification system,28 the criteria for the cytologic subclassification of FL will be changed to that initially proposed by Mann and Berard,29 which yields a higher proportion of FL subtypes than previously used cytologic classification systems.30 31
Applying population-based overall incidence figures for NHL to the estimated proportion of follicular NHL from the available studies (if more than one study from a given country was available, the mean figure was used), yields estimated annual incidence rates for FL in Asian countries in the range of 0.15 to 0.38 per 100 000 population per year (Table 2).18,22,25 32-34This is clearly much lower than the rate of 1.3 to 3.0 per 100 000 population per year seen in the industrialized Western European and North American countries. Thus it is clear that the relative infrequency of FL reported from Asian countries results from a truly low incidence rate; averaging less than 10% of that seen in North America and most industrialized Western European nations.
Incidence rates for overall NHL and follicular histologic types by country
| Country-region . | Overall NHL rate . | Derived incidence follicular NHL . |
|---|---|---|
| Japan-Miyagi22 | 2.5* | 0.28 |
| Japan-Osaka22 | 3.4* | 0.38 |
| India-Bombay22 | 2.3* | 0.20 |
| Thailand-Khon Kaen34 | 3.1* | 0.23 |
| Thailand32 | 2.1* | 0.15 |
| China-Qidong34 | 2.4* | 0.34 |
| Philippines32 | 3.5* | 0.28 |
| Nigeria-Jos18 | 9.1‡ | 1.20 |
| Canada-Quebec34 | 11.1* | 3.44 |
| Canada-British Columbia22 | 7.8* | 2.42 |
| Germany-Hamburg33 | 2.9* | 0.52 |
| United Kingdom25 | 12.2† | 3.42 |
| France25 | 13.2† | 2.24 |
| Italy32 | 6.53* | 0.72 |
| United States22 | 13.9* | 2.4 |
| Country-region . | Overall NHL rate . | Derived incidence follicular NHL . |
|---|---|---|
| Japan-Miyagi22 | 2.5* | 0.28 |
| Japan-Osaka22 | 3.4* | 0.38 |
| India-Bombay22 | 2.3* | 0.20 |
| Thailand-Khon Kaen34 | 3.1* | 0.23 |
| Thailand32 | 2.1* | 0.15 |
| China-Qidong34 | 2.4* | 0.34 |
| Philippines32 | 3.5* | 0.28 |
| Nigeria-Jos18 | 9.1‡ | 1.20 |
| Canada-Quebec34 | 11.1* | 3.44 |
| Canada-British Columbia22 | 7.8* | 2.42 |
| Germany-Hamburg33 | 2.9* | 0.52 |
| United Kingdom25 | 12.2† | 3.42 |
| France25 | 13.2† | 2.24 |
| Italy32 | 6.53* | 0.72 |
| United States22 | 13.9* | 2.4 |
Adjusted to “world standard” population.
Adjusted to similar age-standardized population. See reference for details.
Not population-adjusted.
Molecular characteristics of FL
Bcl-2 oncogene structure and function
Many excellent thorough reviews of this area have been published in recent years.35-37 In summary, bcl-2 is the archetypal member of a growing family of apoptosis-regulating genes. More than 15 members of this family are known, and these fall into either apoptotic death-inducing or death-inhibitory classes. The ultimate cellular response to a potentially proapoptotic extracellular signal is determined by the ratio of antiapoptotic to proapoptotic bcl-2 family proteins. This dynamic balance has been termed the apoptotic rheostat, and it is mediated by competitive dimerization between selective pairs of antagonists and agonists.36 37In this context, clearly any genetic changes that alter the level of expression of one or another of this dynamically balanced system will profoundly influence the likelihood of a cell undergoing programmed cell death in response to a given physiologic stimulus.
Bcl-2 gene rearrangement in FL
The most common nonrandom chromosomal translocation identified to date in FL (as reported in the United States) is the balanced translocation between the immunoglobulin heavy-chain gene on chromosome 18 and the bcl-2 gene on chromosome 14. The resultant t(14;18)(q32;q21) leads to constitutive overexpression of a structurally intact and functional bcl-2 protein38-40 and is the hallmark of FL. Functionally, this results in a prolongation of cell survival and expansion of follicular lymphoid tissue. The survival advantage that bcl-2 mutations confer on lymphocytes may predispose these cells to accumulation of further DNA damage.41 42
Most of the observed t(14;18) translocations have breakpoints clustered in 1 of 2 sites on chromosome 18. The major breakpoint region (MBR), which is involved in approximately 70% of observed translocations, is in the untranslated region 3′ of the last exon of the bcl-2gene. The minor cluster region (mcr), which accounts for 10% to 15% of observed translocations, is 30 kb downstream of the bcl-2gene. The tight physical clustering of breakpoints within the 2 defined regions of the bcl-2 gene facilitates effective screening for the presence of the t(14;18) translocation by polymerase chain reaction (PCR) using paired primers for an immunoglobulin heavy chain JH consensus sequence together with either MBR or mcr sequence, the methods for which are detailed elsewhere.43Additional but less common breakpoint regions lying between the MBR and mcr have also been identified but constitute a trivial proportion of detected translocations.44 45
In the remaining 15% to 20% of patients with FL, the bcl-2gene appears to be physically intact using all available methods.46 Despite this apparent germline configuration, overexpression of the bcl-2 protein evident in 90% of these cases.47-49 Thus, through known genetic translocations, as yet unidentified structural chromosomal changes, or alternative mechanisms, as many as 98% of all cases of FSC (grade 1) lymphoma are associated with bcl-2 protein overexpression.50 The frequency of overexpression in histologic grades 2 and 3 (FM and FLC) FL is slightly lower, in the 75% to 85% range.51 These observations provide overwhelming support for the hypothesis thatbcl-2 overexpression is critically involved in the pathogenesis of FL.
Incidence of bcl-2 gene rearrangement in healthy persons
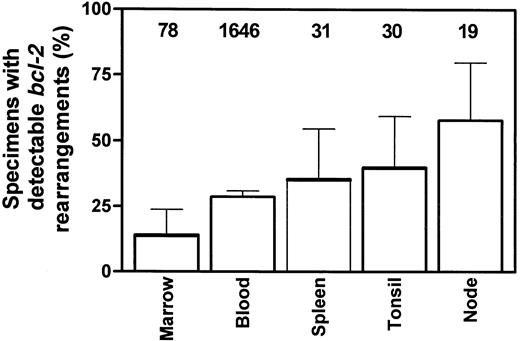
Based on the above data clearly linking bcl-2 gene rearrangements with FL, many investigators had previously suggested that the detection of such a translocation was diagnostic of NHL and most frequently was associated with follicular disease. The first study demonstrating conclusively that clonal bcl-2 rearrangements could be detected in the absence of clinically manifest FL appeared in 1991, by Limpens et al52 at the University of Leiden. Using sensitive nested PCR capable of detecting one clonal cell in 200 000, they demonstrated bcl-2–JH gene rearrangements in 9 (56%) of 16 lymph nodes with follicular hyperplasia and in 4 (50%) of 8 of tonsils with follicular hyperplasia. Since that time additional studies have confirmed this initially unexpected result (Table3).52-65 Excluding the study by Fuscoe et al,56 which used a significantly more sensitive technique (able to detect one clonal cell in 10 000 000), and the study by Ohshima,60 which used a low-sensitivity, single-step methodology, the overall positivity rates for the various tissues tested are bone marrow, 14% (11/78)52,61; spleen, 35% (11/31)59; tonsils, 40% (12/30)52,53; peripheral blood, 28.7% (473/1646)54,55,57-59,61,62-65; and follicular hyperplasia nodes, 58% (11/19)52 53(Figure 1).
Overview of studies of bcl-2 rearrangement in persons without FL
| Author/region . | PCR method (no. cycles) . | Amount DNA . | Tissue samples . | Median age, y (range) . | Percentage positive . | Frequency (per 106 cells) . | Comments . |
|---|---|---|---|---|---|---|---|
| Limpens et al/Europe52 | Nested (50) | 1 μg | Follicular hyperplasia | 33 (7-78) | 56 (9/16) | 1-100 | — |
| — | — | Reactive tonsils | 4-8 | 50 (4/8) | 1-1000 | — | |
| — | — | Reactive nodes | NR | 0 (0/19) | NR | — | |
| — | — | Normal marrow | NR | 0 (0/30) | NR | — | |
| Aster et al/United States53 | Seminested (60) | 2 μg | Tonsils | 6.5 (2-32) | 25 (3/12) | 0.4-2.4 | — |
| — | — | Follicular hyperplasia | 34 (25-64) | 67 (2/3) | 0.5-4.6 | — | |
| Aster et al/Japan53 | — | — | Tonsils | 6 (3-40) | 50 (5/10) | 0.3-60.2 | — |
| Ohshima et al/Japan60 | Single-step (NR) | NR | Reactive nodes | NR | 11 (2/18) | NR | — |
| Liu et al/United States59 | Nested (60) | 2 μg | Sorted PB B cells | 50 (0-75) | 55 (29/53) | 0.8-32 | Frequency ∝ age |
| — | — | Autopsy spleen | 50 (0-85) | 35 (11/31) | 2.7-853 | Liver + 1/10; other tissues negative | |
| Bell et al/United States54 | Nested (60) | 2 μg | Sorted PB B-cells | Younger than 52 | 48 (59/122) | NR | ↑ In smokers, no association with age |
| Limpens et al/Europe58 | Seminested (56) | 1 μg | Sorted PB B-cells | NR | 67 (6/9) | ≈ 10 | — |
| Ji et al/United States57 | Seminested (46) | ≥ 7 μg | Normal PB | 6-60 and older | 63 (79/125) | 0.2-180 | Higher with age, M > F |
| Dolken et al/Europe55 | Nested (60) | 0.01-1 μg | PB mononuclear cells | 38 (23-90) | 46 (26/57) | 1-100 | No association with age |
| Fuscoe et al/United States56 | Nested (60) | 2.5 μg | PB lymphocytes | 27 (17-48) | 88 (30/34) | 0.08-960 | No association with age. M = F |
| Rauzy et al/Europe61 | Seminested (70) | ∼ 0.5 μg | Marrow | 68 (1-89) | 23 (11/48) | NR | Association with smoking |
| — | — | PB | 70 (1-100) | 44 (60/137) | NR | No association with age, sex, or smoking | |
| Delage et al/Canada63 | Nested (25 + 35) | 1 μg | PB mononuclear cells | 41 (38-64) | 100 (6/6) | 1 K-10 K | Multiple MBR, mcr in 5/6 |
| Summers et al/United Kingdom62 | RT-PCR (40) | 1.5 μg | PB | 42 (21-69) | 23 (112/481) | 104 | No association with age |
| Yasukawa et al65 | Nested (30) | 10 μg | PB mononuclear cells | NR | 44 (17/39) | NR | German population |
| — | — | PB mononuclear cells | NR | 15 (6/39) | NR | Japanese population | |
| Paltiel et al/Israel64 | Nested (NR) | NR | Sorted PB B cells | NR | 6.9 (24/346) | NR | No association with age, sex, smoking, or rural residence |
| Author/region . | PCR method (no. cycles) . | Amount DNA . | Tissue samples . | Median age, y (range) . | Percentage positive . | Frequency (per 106 cells) . | Comments . |
|---|---|---|---|---|---|---|---|
| Limpens et al/Europe52 | Nested (50) | 1 μg | Follicular hyperplasia | 33 (7-78) | 56 (9/16) | 1-100 | — |
| — | — | Reactive tonsils | 4-8 | 50 (4/8) | 1-1000 | — | |
| — | — | Reactive nodes | NR | 0 (0/19) | NR | — | |
| — | — | Normal marrow | NR | 0 (0/30) | NR | — | |
| Aster et al/United States53 | Seminested (60) | 2 μg | Tonsils | 6.5 (2-32) | 25 (3/12) | 0.4-2.4 | — |
| — | — | Follicular hyperplasia | 34 (25-64) | 67 (2/3) | 0.5-4.6 | — | |
| Aster et al/Japan53 | — | — | Tonsils | 6 (3-40) | 50 (5/10) | 0.3-60.2 | — |
| Ohshima et al/Japan60 | Single-step (NR) | NR | Reactive nodes | NR | 11 (2/18) | NR | — |
| Liu et al/United States59 | Nested (60) | 2 μg | Sorted PB B cells | 50 (0-75) | 55 (29/53) | 0.8-32 | Frequency ∝ age |
| — | — | Autopsy spleen | 50 (0-85) | 35 (11/31) | 2.7-853 | Liver + 1/10; other tissues negative | |
| Bell et al/United States54 | Nested (60) | 2 μg | Sorted PB B-cells | Younger than 52 | 48 (59/122) | NR | ↑ In smokers, no association with age |
| Limpens et al/Europe58 | Seminested (56) | 1 μg | Sorted PB B-cells | NR | 67 (6/9) | ≈ 10 | — |
| Ji et al/United States57 | Seminested (46) | ≥ 7 μg | Normal PB | 6-60 and older | 63 (79/125) | 0.2-180 | Higher with age, M > F |
| Dolken et al/Europe55 | Nested (60) | 0.01-1 μg | PB mononuclear cells | 38 (23-90) | 46 (26/57) | 1-100 | No association with age |
| Fuscoe et al/United States56 | Nested (60) | 2.5 μg | PB lymphocytes | 27 (17-48) | 88 (30/34) | 0.08-960 | No association with age. M = F |
| Rauzy et al/Europe61 | Seminested (70) | ∼ 0.5 μg | Marrow | 68 (1-89) | 23 (11/48) | NR | Association with smoking |
| — | — | PB | 70 (1-100) | 44 (60/137) | NR | No association with age, sex, or smoking | |
| Delage et al/Canada63 | Nested (25 + 35) | 1 μg | PB mononuclear cells | 41 (38-64) | 100 (6/6) | 1 K-10 K | Multiple MBR, mcr in 5/6 |
| Summers et al/United Kingdom62 | RT-PCR (40) | 1.5 μg | PB | 42 (21-69) | 23 (112/481) | 104 | No association with age |
| Yasukawa et al65 | Nested (30) | 10 μg | PB mononuclear cells | NR | 44 (17/39) | NR | German population |
| — | — | PB mononuclear cells | NR | 15 (6/39) | NR | Japanese population | |
| Paltiel et al/Israel64 | Nested (NR) | NR | Sorted PB B cells | NR | 6.9 (24/346) | NR | No association with age, sex, smoking, or rural residence |
NR indicates not recorded; PB, peripheral blood.
Summary of literature results for percentage of samples from various tissues positive for bcl-2 gene rearrangements from persons without evidence of lymphoma.
Data are presented as percentages and 95% CI, with total number of samples examined indicated above each tissue. See text for details and references.
Summary of literature results for percentage of samples from various tissues positive for bcl-2 gene rearrangements from persons without evidence of lymphoma.
Data are presented as percentages and 95% CI, with total number of samples examined indicated above each tissue. See text for details and references.
Similar results have been obtained using fluorescence in situ hybridization in 4 of 32 lymph nodes involved by nonneoplastic lymphoproliferative disorders, with estimates of the frequency ofbcl-2–rearranged cells of 2% to 5%.66 In comparison, using PCR, the frequency of such cells containing thebcl-2 translocation appears to be approximately 1 in 106 to 1 in 104 cells in most of these studies, but there have been some suggestions that the rate of positivity within the whole study population and the frequency of clonal cells within a single person may be associated with other known risk factors for FL, consistent with the premise that cells carrying abcl-2–JH rearrangement in healthy persons may be precursor cells that subsequently give rise to clinical disease.
Comparison of breakpoint sites in the presence and absence of FL
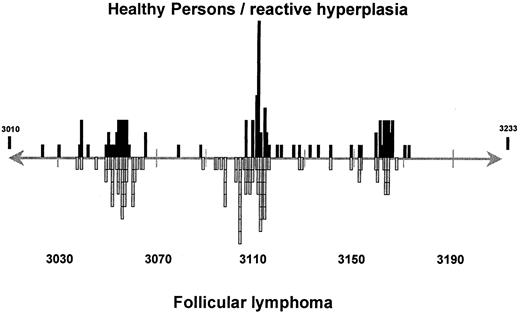
The next issue to be considered is whether these sporadicbcl-2 rearrangements detected in healthy persons have a similar clustering of breakpoints, as is known to occur in patients with FL. At this time, such a comparison is limited to examination of the approximately 500–base pair (bp) MBR region, because studies in healthy persons have been limited to this region. Even with this caveat, it is still of interest to compare the distribution of breakpoint sites within the MBR region in patients with FL and in healthy persons. Such an analysis requires not just detection of abcl-2 gene rearrangement but the generation of adequate PCR product for full sequencing. This has been achieved and reported for 126 breakpoint sites in persons without FL,53,55,56,58,61-63 and it is compared with the breakpoint sites from 71 patients with FL.53,67-75Although there are minor discrepancies, such as a slight excess of breakpoints clustered in the area of bp 3165 among healthy persons (20% vs 7% NHL) and a lower frequency of breakpoints in the area of bp 3105 among healthy persons (12% vs 23% NHL), clear clustering in the regions of bp 3055, 3105, 3115, and 3165 are evident (Figure 2). This is consistent with the premise that the translocations found in healthy persons are molecularly indistinguishable from those found in patients with FL and may act as precursor lesions for the later development of FL. This would suggest that the t(4;18)-bearing clonal cells are stable and persistent within the patient over a significant period of time, as observed by Liu et al.59 Specific t(14;18)-bearing clones from peripheral blood lymphocytes of 2 healthy persons persisted over a 5-month period. Further, they found no increase in the number of individual clonal sequences with age. Prospective longitudinal follow-up of healthy persons who harbor a bcl-2translocation without FL and of t(14;18)-bearing healthy persons who acquire FL are clearly needed to substantiate this issue.
Comparison of breakpoint sites within MBR forbcl-2 gene rearrangements from healthy persons and patients with FL.
See text for details and references.
Comparison of breakpoint sites within MBR forbcl-2 gene rearrangements from healthy persons and patients with FL.
See text for details and references.
Influence of epidemiologic factors on the incidence ofbcl-2 rearrangement—comparing healthy populations and FL
On the basis of the above premise, it is of interest to examine the putative causal factors in the development of bcl-2rearrangements in healthy persons and in FL patients. Although a large number of epidemiologic factors have been repeatedly studied in FL, the limited data available on bcl-2 incidence in persons without FL restricts our present discussion to an abbreviated list of these factors, including age, sex, and smoking history.34
Age
As with other histologic NHL subtypes, FL incidence increases steadily with age. The relationship between age and frequency ofbcl-2–rearranged cells in healthy populations has been examined in a number of the larger studies. However, it is important to note that all of these have been merely correlative. There are no studies of longitudinal assessment over significant periods of time explore any changes over time. Liu et al59 first observed a higher frequency of bcl-2 positivity in the spleens of people 30 years of age and older (11/19 or 58%) compared with those younger than 30 (0/12 or 0%; P = .001) and further noted a direct correlation between age and the frequency of t(14;18)-containing cells in peripheral blood lymphocyte samples (r = 0.37; P = .0067) and spleens (r = 0.59; P = .0005). Given that there was no change in the number of individual clonal sequences isolated from any given person with increasing age, this was consistent with the progressive accumulation of cells from a single clone rather than the acquisition of additional separate clones with increasing age.
A later study by the same group of investigators54 looking at a larger group of 122 healthy volunteers (all younger than 52) was unable to show any association of bcl-2 frequency with age, but this might have been because of the lack of truly elderly patients within the cohort given that their earlier study found the most significant increase in rates and frequencies of bcl-2rearrangements among those older than 60. Four subsequent studies have also explored this issue55-57,61; only the large study of Ji et al57 found a clear association (r = 0.37; P < .0001). Two of the negative studies were limited in size and power, with 57 and 34 patients only,55,56 but the large study of 137 patients by Rauzy et al61 also failed to find any association. In summary, one possible explanation for these observations is that though there may be no clear association between age and the actual rate of positivity forbcl-2–rearranged cells in the peripheral blood of healthy persons (using assays with sensitivities in the range of 10−5 to 10−6), there appears to be an association between the frequency of such cells with increasing age, at least in persons older than 60. This explanation would also be consistent with the observation that using assays of greater sensitivity (less than 10−7), almost all persons have detectable clonally rearranged cells (88%) regardless of age.56
Sex
There is a uniform sex imbalance in the frequency of FL, with males predominating in all population-based studies. Similarly, in 2 of the 5 studies examining the relationship between sex andbcl-2 rearrangements in healthy persons, males were more frequently positive.54,57 However, there is no intrinsic explanation for this sex imbalance, and it may simply be a surrogate marker for a higher likelihood of exposure to other causative agents, such as tobacco, pesticides, or other environmental factors; however, the one study specifically examining likely pesticide exposure (as indicated by rural residence) found no increased risk forbcl-2 rearrangement.64
Smoking
At least 4 relatively large studies consistently report trends for higher rates of NHL among smokers, with relative risk (RR) values of 1.5,76 1.4,77 2.1,78 and 1.9 (but restricted to women).79 Thus it is highly likely that smoking contributes to NHL risk, but the strength of this association is relatively weak. Some provocative preliminary data suggest that smoking may specifically increase the risk for FL. This evidence is direct and indirect. Herrinton and Friedman80 specifically examined the relationship between smoking habits and histologic subtypes of NHL in the Kaiser Permanente cohort of 252 836 people in California; though smoking was not associated with an increased risk for NHL generally (RR, 1.1; 95% confidence interval [CI], 0.9-1.3), there was a statistically significant increase in the risk for FL among former smokers (RR, 1.9) and current smokers (RR, 1.4). Indirect supporting evidence was provided by Nelson et al,81 who specifically examined risk factors for intermediate-grade NHL and found no association with smoking habits, consistent with the established influence being restricted to other histologic types. Conversely, Brown et al77 found that the RR was greatest for histologically aggressive subtypes (2.3 vs 1.4 overall RR).
In healthy populations the Bell study specifically addressed smoking, finding strong associations in multivariate analysis between indices of tobacco use and the frequency of bcl-2–rearranged cells, whether this was measured by total pack-years (P = .0004), cigarettes smoked per day (P = .008), or total years of smoking (P = .001).54 Given the epidemiologic link between FL and smoking discussed above, the large number of patients studied, and the thorough smoking history data obtained, this study strongly suggests a causative link between a substance contained within tobacco smoke and the generation of bcl-2–IgH rearrangements within peripheral blood lymphocytes. The Rauzy et al61 study also found a greater cumulative cigarette exposure among people with bcl-2 positivity in bone marrow samples (17.5 vs 4.0 pack-years; P < .01461), though a comparison between smokers and nonsmokers in the same study failed to show a difference. Such an association has not been universal, however.64
To summarize the impact of putative epidemiologic factors, there is consistency in the observed association of increasing age, male sex, and smoking history among t(14;18)-bearing healthy persons and patients with FL.
If these sporadic bcl-2 translocations found in healthy persons truly are the precursor lesions for later development of FL, their frequency may vary in geographic regions with different incidence patterns for FL. Unfortunately, few data are available on the frequency of such gene rearrangements in Asian populations. In the Aster study,53 10 excised tonsils from Japanese persons were examined, and 5 (50%) harbored clonally rearranged cells. By comparison, a positivity rate of 33% (5/15) was reported among similarly examined tonsils and follicular hyperplasia lymph nodes from Americans. In a separate report comparing peripheral bloodbcl-2 status between healthy Japanese and German subjects, only 16% (39/241) of the Japanese study population carried the translocation.65 However, this percentage ofbcl-2 positivity is nonetheless relatively high for the incidence of FL in Japan. Although the number of studies is small, derive from a single Asian ethic group, and do not include mcr, these data do suggest that sporadic bcl-2 gene rearrangements are not rare events among Asians.
More data are available on the relative rates of bcl-2rearrangement positivity among European populations, who have an intermediate incidence of FL, and Americans with a high rate. Restricting this analysis to those studies using PCR methods of similar sensitivity,54,55,57-59,61 65 the cumulative incidence of detectable bcl-2 gene rearrangements in the peripheral blood of Europeans is 47% (131/278), and among Americans it is 53% (162/308) (P = .21). Allowing for differences in methodological sensitivity between groups and the inability to match for the age of subjects, sex distribution, and possible confounding factors such as smoking habits, there are clearly no marked differences evident in the rate of detection of bcl-2 rearrangements.
If we accept that the reported bcl-2 rearrangements among healthy persons are the precursor lesions for the subsequent development of FL, this similarity in the frequency of bcl-2rearrangements, despite markedly lower incidence of FL, strongly suggests that additional molecular events are required subsequent to the acquisition of a clonal bcl-2–IgH rearrangement before clinically evident FL can develop. In addition, it appears that such later events, rather than the bcl-2 rearrangements, are rate-limiting for the development of clinical disease. The nature of these putative additional events remains speculative, and the likely causative factors are unknown. Factors that influence the development of subsequent molecular events may in fact vary between geographic regions. Hence, these geographic variations might explain the different incidences of FL.
Geographic variation of bcl-2 in FL
As we have demonstrated, there is marked geographic variation in the incidence of FL. We have also suggested that the background rate ofbcl-2 gene rearrangement in the absence of disease is inadequate to explain the magnitude of observed differences in the incidence of FL between geographic regions. Does FL from different regions share similar underlying molecular defects, or is there evidence of distinct molecular pathogenesis?
This specific issue was addressed in the recent study by Segel et al82 of Jerusalem. They performed PCR for MBRbcl-2 gene rearrangement on FL specimens from 36 patients, finding 61% positive, and reviewed all published studies of the frequency of bcl-2 gene rearrangement in FL up to 1995. To minimize the influence of variability in assay sensitivity between studies, we have limited the following discussion to the comparison of PCR results in which MBR and mcr loci were examined, and we have also included later studies, in particular the recent large series from the MD Anderson Cancer Center in Texas.83 Although there is likely to be some level of variability in the sensitivity of the assays used between laboratories, this will have little impact on the rates of positivity among primary diagnostic tissue samples because such nodes are usually heavily infiltrated by the malignant cells. Conversely, whether fresh or archival material is used as the source of DNA will have a minor influence. As clearly shown by the comparative study of Liu et al,84 the rate of positivity for bcl-2rearrangements will be approximately 10% lower when applied to archival material because of degradation of the DNA. Because PCR products from the mcr breakpoints on average are approximately 3 times larger than MBR breakpoint products, there will be a proportionately greater loss of sensitivity for rearrangements at the mcr locus. Unfortunately, the use of frozen versus archival material was variable between studies and indeed between reports from different geographic regions, with more frequent use of fresh tissue among the North American series. Allowance for such methodological variations may increase the true positivity rate for the samples from Asian patients by 5% to 10% and from European patients by approximately 5%. However, the series of Loke et al,85 which found a 60% rate of bcl-2 rearrangements among Hong Kong Chinese patients, did use frozen tissue in all cases.
Overall, even allowing for such methodological differences, these results (Table 4) demonstrate a significant gradient in the frequency of bcl-2 gene rearrangements from a relatively low rate of 48% to 55% among Asian and European studies to approximately 80% among the series reported from the United States (95% CI, 74%-83%;P < .0001).47,66 83-94 These differences were evident among MBR rearrangements (45%-50% vs 65%, respectively; P = .001 and mcr rearrangements (4%-5% vs 13%, respectively; P = .003).
Frequency of bcl-2 gene rearrangement in follicular NHL from different geographic regions
| Region and author . | No. patients evaluable . | MBR+ (%) . | mcr+ (%) . | Total bcl-2+ (%) . |
|---|---|---|---|---|
| Asia | ||||
| Mitani et al92 | 304-150 | 12 (40) | 1 (3) | 13 (43) |
| Loke et al4-15185 | 16 | 8 (50) | 1 (6) | 9 (56) |
| Subtotal (percentage; 95% CI) | 46 | 20 (43; 29-59) | 2 (4; 0.5-15) | 22 (48; 33-63) |
| Europe | ||||
| Benitez et al86 | 5 | 3 (60) | 0 (0) | 3 (60) |
| Pezzella et al93 | 51 | 18 (35) | 3 (6) | 21 (41) |
| Lee et al91 | 20 | 7 (35) | 1 (5) | 8 (40) |
| Lambrechts et al90 | 21 | 12 (57) | 1 (5) | 13 (62) |
| Poetsch et al66 | 28 | 18 (64) | 0 (0) | 18 (64) |
| Séité et al47 | 64 | 30 (47) | 8 (13) | 38 (59) |
| Johnson et al89 | 50 | 30 (60) | 0 (0) | 30 (60) |
| Subtotal (percentage; 95% CI) | 239 | 118 (49; 43-56) | 13 (5; 3-9) | 131 (55; 48-61) |
| United States | ||||
| Zelenetz et al94 | 40 | 22 (55) | 6 (15) | 28 (70) |
| Liu et al84 | 48 | 24 (50) | 5 (10) | 29 (60) |
| Gribben et al87 | 88 | 56 (64) | 18 (20) | 74 (84) |
| Gulley et al88 | 8 | 4 (50) | 1 (12) | 5 (62) |
| Lopez-Guillermo et al83 | 139 | 105 (70) | 13 (9) | 118 (79) |
| Subtotal (percentage; 95% CI) | 323 | 211 (65; 60-70) | 43 (13; 10-17) | 254 (79; 74-83) |
| Region and author . | No. patients evaluable . | MBR+ (%) . | mcr+ (%) . | Total bcl-2+ (%) . |
|---|---|---|---|---|
| Asia | ||||
| Mitani et al92 | 304-150 | 12 (40) | 1 (3) | 13 (43) |
| Loke et al4-15185 | 16 | 8 (50) | 1 (6) | 9 (56) |
| Subtotal (percentage; 95% CI) | 46 | 20 (43; 29-59) | 2 (4; 0.5-15) | 22 (48; 33-63) |
| Europe | ||||
| Benitez et al86 | 5 | 3 (60) | 0 (0) | 3 (60) |
| Pezzella et al93 | 51 | 18 (35) | 3 (6) | 21 (41) |
| Lee et al91 | 20 | 7 (35) | 1 (5) | 8 (40) |
| Lambrechts et al90 | 21 | 12 (57) | 1 (5) | 13 (62) |
| Poetsch et al66 | 28 | 18 (64) | 0 (0) | 18 (64) |
| Séité et al47 | 64 | 30 (47) | 8 (13) | 38 (59) |
| Johnson et al89 | 50 | 30 (60) | 0 (0) | 30 (60) |
| Subtotal (percentage; 95% CI) | 239 | 118 (49; 43-56) | 13 (5; 3-9) | 131 (55; 48-61) |
| United States | ||||
| Zelenetz et al94 | 40 | 22 (55) | 6 (15) | 28 (70) |
| Liu et al84 | 48 | 24 (50) | 5 (10) | 29 (60) |
| Gribben et al87 | 88 | 56 (64) | 18 (20) | 74 (84) |
| Gulley et al88 | 8 | 4 (50) | 1 (12) | 5 (62) |
| Lopez-Guillermo et al83 | 139 | 105 (70) | 13 (9) | 118 (79) |
| Subtotal (percentage; 95% CI) | 323 | 211 (65; 60-70) | 43 (13; 10-17) | 254 (79; 74-83) |
Restricted to FSC and FM; FLC not included.
Analysis by Southern blotting only performed.
Ideally, it is preferable to have results from simultaneously processed fresh samples representing each geographic region for analysis in a single laboratory, but in the absence of such data, the above comparisons must suffice. Notwithstanding the shortcomings of the limited data set available in published literature, there appears to be a discrepancy in the frequency of bcl-2 gene rearrangements in FL across geographic regions. If these apparent geographic variations are confirmed in larger epidemiologic studies, it suggests the existence of separate pathogenetic processes, each of which culminates in morphologically similar, but molecularly distinct, FL. This is analogous to the recent demonstration that gastric marginal zone B-cell lymphomas appear to consist of 2 pathogenetically discrete entities (with or without t(11;18)) that have different natural histories.95
Although this 2-pathway model remains speculative, previously published results of clinical characteristics and treatment outcomes of patients with FL support this hypothesis (Tables5, 6). For instance, Lopez-Guillermo et al83 found that patients with germline bcl-2 status were older at diagnosis, had more advanced-stage disease, and had higher serum levels of LDH and β2-microglobulin, all suggestive of a higher disease burden, than patients with rearranged bcl-2. A relationship between age and germline bcl-2 gene status was also reported in 2 smaller studies.89 96 More provocatively, though patients with germline bcl-2 had a lower CR rate with therapy, the pattern of relapse was more suggestive of aggressive NHL, with approximately 40% of patients experiencing relapse within 3 years but no relapses observed beyond 3 years. This pattern of relapse appeared distinct from the slow but relentless relapse rate observed for most series of patients with advanced FL.
Clinical characteristics of bcl-2 positive versus germline cases of FL in published series
| Country . | No. FL patients (positive forbcl-2) . | Method of analysis . | Clinical characteristics, bcl-2 positive versus germline cases . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age . | Sex . | Stage . | Histology . | LDH . | β2M . | BM . | Enodal . | |||
| United States113 | 30 (23) | CG | NS | NS | NS | NS | NS | — | — | — |
| United States99 | 20 (13) | CG | NS | NS | NS | — | — | — | — | NS |
| Denmark93 | 70 (39) | PCR | NS | NS | NS | NS | — | — | NS | — |
| Sweden89 | 102 (42) | PCR | Younger5-150 | — | NS | — | NS | — | NS | NS |
| United States83 | 247 (202) | PCR | Younger5-152 | — | Earlier5-151 | NS | Lower5-152 | Lower5-151 | — | NS |
| Canada97 | 212 (164) | CG | NS | NS | Higher5-150 | Lower Gr5-150 | NS | — | Increase5-150 | Lower5-151 |
| United States96 | 46 (22) | PCR | Younger5-151 | — | Earlier5-151 | — | — | — | Decrease5-151 | — |
| Country . | No. FL patients (positive forbcl-2) . | Method of analysis . | Clinical characteristics, bcl-2 positive versus germline cases . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age . | Sex . | Stage . | Histology . | LDH . | β2M . | BM . | Enodal . | |||
| United States113 | 30 (23) | CG | NS | NS | NS | NS | NS | — | — | — |
| United States99 | 20 (13) | CG | NS | NS | NS | — | — | — | — | NS |
| Denmark93 | 70 (39) | PCR | NS | NS | NS | NS | — | — | NS | — |
| Sweden89 | 102 (42) | PCR | Younger5-150 | — | NS | — | NS | — | NS | NS |
| United States83 | 247 (202) | PCR | Younger5-152 | — | Earlier5-151 | NS | Lower5-152 | Lower5-151 | — | NS |
| Canada97 | 212 (164) | CG | NS | NS | Higher5-150 | Lower Gr5-150 | NS | — | Increase5-150 | Lower5-151 |
| United States96 | 46 (22) | PCR | Younger5-151 | — | Earlier5-151 | — | — | — | Decrease5-151 | — |
β2M indicates beta2 microglobulin; BM, bone marrow; Enodal, extranodal; CG, cytogenetics; NS, not significant.
P < .01.
P = .01-.05.
P > .05.
Outcome measures of bcl-2 positive versus germline FL and impact of additional genetic alterations
| Country . | Outcome parameters, bcl-2 versus germline . | Impact of additional genetic changes . | ||
|---|---|---|---|---|
| CR . | PFS . | OS . | ||
| United States113 | NS | NS6-150 | NS | Chromosome 17: decreased CR rate (P < .01) |
| United States99 | — | — | Lower (P = .02) | del17, dup2: trend to worse outcome |
| dup3 or + 3: trend to better outcome | ||||
| Denmark93 | — | — | NS | — |
| France114 | — | — | NS | Abnormal CG: worse outcome, including: 1p21-22 (P < .01), 6q23-6 (P < .001), 17p (P < .001), no. chromosome breaks > 6 (P < .01) |
| Sweden89 | — | — | Higher (P = .011) | — |
| United States132 | — | — | NS | Bcl-611 patients (9 of whom + bcl-2): OS better thanbcl-6 germline |
| United States83 | Lower | Lower | NS | — |
| Canada97 | — | NS | NS | Increased rate trisomy3 in bcl-2 −ve (P < .01), trend del10q in bcl-2 +ve (P = .08) |
| United States96 | — | — | NS | 17p reduced 5-year OS (P = .058), + 21 (P = .013) reduced 5-year OS |
| United States115 | — | — | NS | Any of + 7/+ 12/1p: decreased median survival (P = .028) |
| Country . | Outcome parameters, bcl-2 versus germline . | Impact of additional genetic changes . | ||
|---|---|---|---|---|
| CR . | PFS . | OS . | ||
| United States113 | NS | NS6-150 | NS | Chromosome 17: decreased CR rate (P < .01) |
| United States99 | — | — | Lower (P = .02) | del17, dup2: trend to worse outcome |
| dup3 or + 3: trend to better outcome | ||||
| Denmark93 | — | — | NS | — |
| France114 | — | — | NS | Abnormal CG: worse outcome, including: 1p21-22 (P < .01), 6q23-6 (P < .001), 17p (P < .001), no. chromosome breaks > 6 (P < .01) |
| Sweden89 | — | — | Higher (P = .011) | — |
| United States132 | — | — | NS | Bcl-611 patients (9 of whom + bcl-2): OS better thanbcl-6 germline |
| United States83 | Lower | Lower | NS | — |
| Canada97 | — | NS | NS | Increased rate trisomy3 in bcl-2 −ve (P < .01), trend del10q in bcl-2 +ve (P = .08) |
| United States96 | — | — | NS | 17p reduced 5-year OS (P = .058), + 21 (P = .013) reduced 5-year OS |
| United States115 | — | — | NS | Any of + 7/+ 12/1p: decreased median survival (P = .028) |
CR indicates complete remission; PFS, progression-free survival; and OS, overall survival.
Failure-free survival.
Sehn et al97 from Vancouver analyzed the karyotype of tumor cells in their series of 212 patients with FL. They found patients with t(14;18) translocations to be more likely to have grade 1 histology with a higher likelihood of bone marrow involvement. Contrary to the findings of Lopez-Guillermo et al,83 they found a correlation between the bcl-2 translocation and advanced stage. There are potential discrepancies in results across these reports, which may partly be attributable to the small sample size of the earlier studies, lymphoma histologies included in each study, and differences in technical methods (cytogenetics vs PCR detection of translocations). Nonetheless, these observations are consistent with the proposal that the presence or absence ofbcl-2 gene rearrangements may be associated with distinct disease features and natural history.
Putative secondary or alternative molecular events
As discussed above, molecular events subsequent to the acquisition of the t(14;18) translocation may be necessary and rate-limiting in the development of FL. Indeed, as with the presence or absence of thebcl-2 translocation, these secondary events may similarly be associated with distinct clinical characteristics and outcome parameters despite morphologic equivalence. Numerous nonrandom chromosomal abnormalities in FL are reported in the literature (summarized in Table 6).
Most FL, in addition to the characteristic t(14;18), demonstrate at least one additional karyotypic abnormality.98,99 Earlier work by Yunis et al100 not only demonstrated distinct patterns of these additional chromosomal changes for specific FL histologies, they also found that the number of accumulated additional abnormalities increased from indolent FSC histology to FLC and diffuse large-cell histology. For instance, they defined a group of patients with clinically aggressive FSC on the basis of chemoresistance and shortened survival in whom either loss of chromosome 13 or trisomy of chromosome 2 was consistently detected. In addition, if a large-cell component of FL was present (FMC and FLC), 6 nonrandom defects were common, including +21, which was exclusive to FLC. There is no direct evidence yet available in humans that molecular events accumulate over time to transform a bcl-2–harboring lymphoid cell into clinically evident FL. Indirect evidence that subsequent molecular events are required for disease progression includes the above differences in accumulated genetic mutations between the 3 FL subtypes and the observation that transformation to aggressive lymphoma is also accompanied by cumulative genetic changes.101-104 Specific molecular abnormalities of FL thus predict histologic transformation.
Chromosomal translocations involving the bcl-6 locus are another group of molecular events implicated as a putative mechanism of FL development. The bcl-6 gene on 3q27 generates the corresponding protein that specifically regulates B-cell progression and differentiation within the germinal center. It is postulated that, as a result of bcl-6 translocation, physiologicbcl-6 gene down-regulation following germinal center transition does not occur, preventing subsequent lymphocyte differentiation.105 It is one of the most common mutations in diffuse large B-cell lymphomas.106-109 In FL, abcl-6 translocation is also recognized, but at a much lower frequency.106,107,110 The presence of a bcl-6gene rearrangement in the absence of bcl-2 by cytogenetic analysis in a single patient with FL was reported in 1992.111 However, a more recent analysis of a series of 208 well-characterized FL revealed bcl-6 rearrangement by Southern blotting in the absence of t(14;18) of bcl-2rearrangement in 15 patients (7.2%). These bcl6+/bcl-2− patients infrequently expressed CD10 (28%) or bcl-2 protein (22%) by immunohistochemistry and appeared to have a similar natural history tobcl-2–rearranged FL.112 This establishes that dysregulation of bcl-6 occurs in a subset of bcl-2 germline FL patients and suggests that such dysregulation may be an initiating event in FL, though these cases were not studied for other putative initiating molecular events. The role of bcl-6 dysregulation in the pathogenesis of FL warrants further investigation.
Additional specific chromosomal changes, most commonly involving chromosome 17, did predict for an inferior outcome in the report of Levine et al.113 Similarly, Tilly et al114correlated adverse outcomes with the presence of chromosome 17 and 1p and 6q abnormalities. Offit et al115 reported reduced overall survival in FL patients with cytogenetic abnormalities at 1p or with trisomy of chromosome 12 or 7. None of these reports analyzed prognosis by subtype of FL. Of great interest, Sehn et al97 also found differences in the frequency of cytogenetic abnormalities between bcl-2translocation-positive and translocation-negative patients. This observation lends further support to the 2-pathway model.
The cited results from Sehn et al97 warrant further comment. The lower t(14;18) translocation rate in Asian populations would not of itself be sufficient to explain the reduced absolute incidence rates of FL; there must also be a lower absolute incidence rate of bcl-2 germline FL cases. If the reduction is proportional between bcl-2–rearranged and germline cases, it would be consistent with the hypothesis that there were shared “subsequent” events leading to clinical follicular lymphoma in both groups. However, the differences in cytogenetic characteristic abnormalities between the 2 groups, as reported by Sehn et al, are inconsistent with this suggestion.
These secondary or alternative abnormalities, and not thebcl-2 gene rearrangement, may be the relevant independent indicator of clinical or outcome characteristics. Again, greater numbers of patients and prospectively collected data are required to identify these.
Clinical implications
The relatively high rate of bcl-2 translocation in the absence of FL calls into question its use as an appropriate marker of molecular monitoring. Indeed, in localized FL, a detectablebcl-2 translocation can be found in the peripheral blood or bone marrow of some patients.90,116-118 Moreover, during long-term follow-up, some patients remain in complete remission despite ongoing bcl-2 positivity detectable by sensitive PCR assays.90,119,120 Nonetheless, recent data have demonstrated that the achievement of complete molecular remission (CMR) is associated with improved outcomes.121-123 Historically, the use of conventional alkylating agents or anthracycline-based chemotherapy does not produce CMR, even in patients with complete clinical response rates of more than 80%.57,87,124-126However, novel treatments that include fludarabine-based combination regimens and the monoclonal antibody rituximab have been associated with substantial CMR rates.121,123 Similarly, results of in vitro autologous stem cell purging to produce bcl-2 PCR negativity have also demonstrated encouraging results.127 128 Thus aggressive treatment strategies that maximize the CMR rate may improve clinical endpoints and change the treatment paradigm in advanced FL.
In the absence of available downstream molecular targets, the monitoring of minimal residual disease in FL is based on PCR-detectablebcl-2 gene rearrangement. Assuming that the putative downstream events discussed are rate-limiting and are required for progression to clinical disease, their elucidation may help to better define specific subcategories of FL with respect to clinical characteristics and outcomes. These secondary events may better reflect true minimal residual disease than the presence of PCR-detectablebcl-2 gene rearrangement alone, and their identification may provide clues to more effective therapeutic targets. Gene expression profiling by microarray techniques, a novel technology yet to enter clinical application, offers a potentially powerful method for the characterization of FL.129-131
We have demonstrated, from analysis of the available published data, a significantly lower incidence of bcl-2 translocation in FL between Asian and Western countries, despite similar rates ofbcl-2 positivity in healthy persons for both population groups. This observation supports our hypothesis that molecular pathogenesis of FL may vary across geographic regions and, further, may explain the differences in the incidence rates commonly reported. If these findings were confirmed in prospective studies, it would imply that different etiologic or genetic factors may influence the development of FL across separate regions.
Portions of this paper were presented at the IX Congress of The International Society of Haematology, Asian-Pacific Division, Bangkok, October 1999, and were published in the corresponding Education Programme and Scientific Supplement.
References
Note added in proof
Albinger-Hegyi et al133 sought the presence ofbcl-2 gene rearrangements in a series of 59 Swiss patients with FL. They found 32% (19) of cases to have MBR, and 3% (2) to have an mcr rearrangement that is broadly consistent with the previously published European frequencies. They then sequenced the entire 25-kb stretch of genomic DNA between the MBR and mcr regions, and designed novel PCR primers applicable to formalin-fixed tissue to allow detection of breakpoints in this intervening period. Using these novel methods, they identified an additional 36% (21) of cases with otherwise undetected bcl-2 rearrangements. Importantly 12% (7) of their total sample had breakpoints clustered in an approximately 200-bp region they have called the intermediate cluster region (icr). The geographic variation in frequency of icr breakpoints in FL remains to be explored.
Author notes
John F. Seymour, Leukaemia/Lymphoma Service, The Department of Haematology, The Peter MacCallum Cancer Institute, Locked Bag 1, A'Beckett St, VIC 8006, Australia; e-mail:jseymour@petermac.unimelb.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal